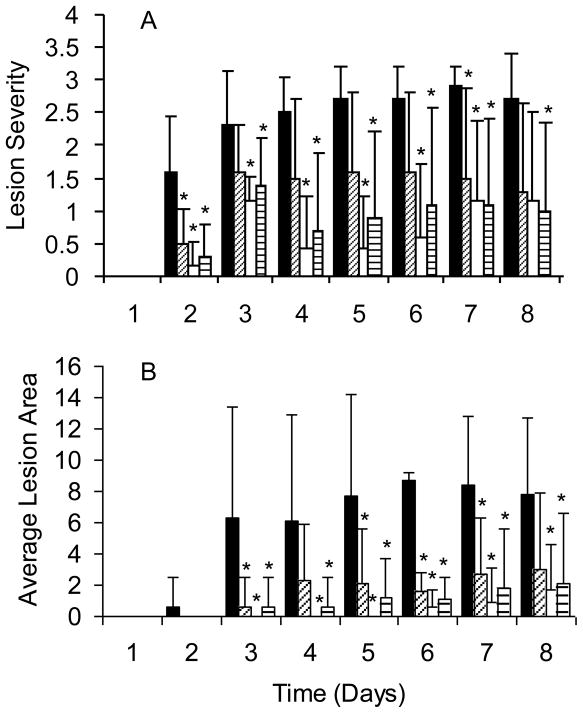
Fig. 7.
Protection of ICR mice immunized i.m. at time 0, 3, 6 and 10 weeks with alum (■, N=10), 30μg J14-KLH (▨, N=10), 30μg hexavalent (□, N=7), or 30μg hexavalent+ 30μg J14 (▤, N=10). Four weeks after the final immunization, the mice were challenged s.c. with 1.2 × 107 CFU type 3 GAS. (A) Average lesion severity scores: 0=normal skin, 1=swelling, 2=swelling and discoloration, 3=skin breakdown, 4=necrotic ulcer and (B) average lesion size (sq. mm) were recorded daily post challenge. *Indicate p ≤ 0.05.