Abstract
We show that 1alpha, 25-Dihydroxyvitamin D3 (1,25(OH)2D3) and a synthetic non-genotropic vitamin D analog agonist, 1a,25(OH)2-lumisterol (JN), exhibit similar rapid effects on sarcomere shortening (contraction) of isolated adult cardiomyocyte. We also report that the vitamin D receptor (VDR) specifically interacts with Caveolin-3 in the t-tubules and sarcolemma of isolated adult rat cardiac myocytes. Confocal immunofluorescence microscopy analysis showed co-localization of VDR and Caveolin-3 in the t-tubules and sarcolemma of cardiomyocytes. Co-Immunoprecipitation studies using VDR antibodies revealed that Caveolin-3 specifically co-precipitates with the VDR and similarly the VDR is co-precipitated with Caveolin-3 antibody. VDR is also in association with Serca-2, the sarcoplasmic reticulum Ca2+-ATPase, as demonstrated by co-immunoprecipitation, suggesting a role of VDR in regulating cardiac contractility by direct interaction with Serca-2. Treatment of isolated adult rat cardiomyocytes with 10nM 1,25(OH)2D3 for 1 hr caused decreased association between VDR and Caveolin 3. These discoveries of the association between VDR and Caveolin-3 and the regulation of this interaction by 1,25(OH)2D3 are fundamentally important in understanding 1,25(OH)2D3 signal transduction in heart cells and suggest a novel mechanism for VDR in the regulation of heart structure and function.
Keywords: VDR, caveolin-3, cardiomyocyte, heart, vitamin D
1. Introduction
1,25-Dihydroxyvitamin D3 (1,25(OH)2D3) exerts its effects through the vitamin D receptor (VDR), an extensively characterized ligand-activated transcription factor that is expressed in a wide array of tissues and cell types including heart [1,2,9]. Vitamin D3 deficiency and reduced levels of the active Vitamin D metabolite 1,25(OH)2D3 have been associated with the etiology and pathogenesis of congestive heart failure (CHF) [1–3]. Studies from our lab demonstrated that Vitamin D3 deficiency alters myocardial function, morphology and extracellular matrix (ECM) [2, 4, 5]. We have also shown that ablation of the VDR signaling system in VDR knockout mice results in profound changes in heart structure and function [6].
The VDR has been extensively characterized as a nuclear steroid receptor that modulates gene transcription upon binding of its ligand 1,25(OH)2D3 [9]. 1,25(OH)2D3 has also been shown to exert fast, non-genomic responses involving stimulation of signal transduction pathways through putative membrane associated receptors [7–9]. The VDR has been found in isolated membrane fractions of both chick intestinal cells, and chick embryonic skeletal muscle cells [10, 11]. Our lab has reported that in adult rat and mouse cardiomyocytes VDR is located in the t-tubular membrane structures [12]. However, a specific interaction between VDR and t-tubular membrane proteins was not determined.
The t-tubules of mammalian cardiac ventricular myocytes are invaginations of the plasma membrane. The development of t-tubules appears to depend on proteins and lipids and shows properties that are similar to the development of caveolae, which requires cholesterol and caveolins [13]. Caveolin is the principle structural protein that is both necessary and sufficient for the formation of caveolae membrane domains, which functions both in protein trafficking, signal transduction and membrane cholesterol homeostasis [14, 15]. Caveolae-associated VDR has been observed in various tissues. However, the interaction between VDR and t-tubules and its function in cardiomyocytes has not been characterized. In this report, we show that the VDR localizes to the t-tubule and sarcolemma in rat cardiomyocytes and co-immunoprecipitates and localizes with the membrane protein Caveolin-3. Moreover, we report that 1,25(OH)2D3 rapidly (within minutes) modulates the contraction of isolated cardiomyocytes and affects the interaction of Caveolin-3 and the VDR.
2. Materials and Methods
2.1 Animals
All procedures involving animals were executed in accordance with the guidelines of the University Committee on the Use and Care of Animals (UCUCA) of the University of Michigan. Three to six-month-old male Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA) were used in this study. These rats were housed in the University of Michigan Laboratory Animal Facility in standard cages with a 12-h light, 12-h dark cycle. Rats were fed standard rodent chow and water.
2.2 Isolation of rat ventricular myocytes
Ventricular myocytes were isolated from rat hearts as previously described [16]. Male Sprague-Dawley rats (250–300g) were pre-treated with 0.01 U/kg heparin IP followed 10 minutes later by a lethal dose of pentobarbital. The heart was quickly removed and mounted on a Langendorff apparatus, and retrograde perfused with Krebs-buffered solution (KREBS) containing (in mmol/l): 118 NaCl, 4.8 KCl, 1.2 MgSO4·7H2O, 1.0 CaCl2·2H2O, 25 HEPES, 1.2 KH2PO4, and 11 glucose (pH set to 7.4 using HCl). When the coronary circulation had cleared of blood, perfusion was continued with Ca2+-free KREBS isolation solution for 3 min, followed by perfusion for a further 30 min with Ca2+-free KREBS isolation solution containing 6750 U of collagenase type 2 (Worthington Biochemical Corporation, Lakewood, NJ) and 12 mg of hyaluronidase (Sigma, St. Louis, MO). Calcium concentration was gradually increased to 0.75 mM during the digestion. The ventricles were then excised, minced, and gently shaken at 37°C in the collagenase-containing solution. Ventricular cells were collected from this solution at 5 min intervals and re-suspended in KREBS containing 1% BSA and 1.75 mM CaCl2. Cells were then plated onto laminin-coated coverslips in DMEM supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin (Pen/Strep, Life Technologies), and 5% serum. Cells were incubated at 37°C for 2 hrs in 2% CO2.
2.3 Isolated cardiac ventricular myocytes contraction studies
Cells were plated as above. After 2-hr incubation the plating media was changed to media 199 supplemented with pen/strep (M199), 10 mM HEPES, 0.2 mg/ml bovine serum albumin, and 10 mM glutathione (M199+). Individual cover slips were transferred to a temperature-controlled stimulation chamber containing platinum electrodes mounted to the sides of each well and a glass bottom mounted on a Nikon microscope. Myocytes were electrically stimulated (Myopacer, 2.5 ms pulse, 0.5 Hz., 4 V) and the chamber was perfused with M199+. Sarcomere shortening was detected using a video-based detection system (Ionoptix, Milton, MA) on intact myocytes. Recordings were made before and 1, 2.5, 5, 10, and 15, 30 min after addition of calcitriol (10−9 M; Sigma) or JN (10−8M, provided by Dr. Norman, University of California, Riverside). For JN+HL study, cells were pretreated with HL (10−8M, provided by Dr. Norman) for 1 hr before addition of JN (10−8M). Ten twitches per myocyte were collected for each sample. The shortening transient was indistinguishable over this same time interval in non-stimulated myocytes. Signal averaged data were analyzed to determine resting sarcomere length, peak shortening normalized for resting sarcomere length (% peak height), time to peak shortening (TTP), time to 25, 50, and 75% relaxation (TTR25, TTR50, and TTR75, respectively), and maximum normalized shortening and relaxation velocities (+dl/dt-max, −dl/dt-max, respectively).
2.4 Immunocytochemistry
Immunofluorescent staining of cells was carried out in a method similar to that previously described [17]. Briefly, laminin-coated cover slip-plated cells were washed in PBS and then fixed in 10% neutral buffered formalin (Sigma, St Louis, MO) for 10 min, rinsed in PBS, and blocked in 10% Donkey serum in PBS-0.05% Triton-X 100 (PBS-T). Cells were incubated with rabbit VDR antibody (sc-1008; 1:100; Santa Cruz, CA) and mouse caveolin-3 antibody (sc-5310; 1:200; Santa Cruz) diluted in 5% Donkey serum/PBS-T at 4°C overnight. After incubation, cells were rinsed in PBS and then incubated with donkey anti-rabbit Alexa488, donkey anti-mouse Alexa594 (Invitrogen, Carlsbad, CA) secondary antibody diluted in 5% Donkey serum in PBS-T for 1 hour at room temperature. Cells were then washed in PBS, and mounted in ProLong Gold (Invitrogen). Slides were analyzed with Olympus FV-500 imaging software on an Olympus iX 81 confocal microscope and finalized using Adobe Photoshop (Adobe Systems Inc).
2.5 Co-Immunoprecipitation of Cav-3 and VDR
Polyclonal VDR antibody (100 ug; sc-1008 from Santa Cruz), monoclonal VDR antibody (100 ug, MA1-710 from Affinity Bioreagents), or monoclonal caveolin-3 antibody (100 ug, sc-5310 from Santa Cruz) were covalently immobilized overnight at room temperature with gentle end-over-end mixing to 50 ul of Antibody Coupling Gel (AminoLink Plus Gel, Pierce, Rockford, IL) respectively into a Handee Spin Cup column as recommended by the manufacturer (Pierce, Rockford, IL). Isolated rat cardiomyocytes and intestinal mucosa were prepared as previously described [12]. For treated cardiomyocytes, cells were treated with 10−9M of 1,25(OH)2D3 for 1 hour. Control cells were treated with same amount of ethanol for 1 hour. To extract protein, both isolated cardiomyocytes and intestinal mucosa were incubated with 10 volumes (v/v) of Pierce M-PER® extraction reagent for 10 min at room temperature with rocking, then sonicated on ice 6 times at 50% power, 12 bursts each time. After centrifuging at 27000 g for 15 min to get the protein supernatant, 600 ug of cardiomyocyte and intestinal lysate were incubated with the VDR gel resin or caveolin-3 gel resin and “quenched control” resin for 2 hr at room temperature, then washed and eluted off in 50 ul fractions 4 times as recommended by the manufacturer (Pierce). 20 ul of eluted samples from the first 3 fractions was subjected to Western Blot analysis by running on a 12.5% Criterion SDS-PAGE Tris HCl gel (Bio-Rad Laboratories, Hercules, CA) and transferred to an Immobilon-P polyvinyl difluoride membrane (Millipore, Bedford, MA). Membrane was blocked for 1 hr in 5% nonfat dry milk and then incubated with primary VDR antibody (sc-1008, rabbit polyclonal, 1:200, Santa Cruz) diluted in 5% milk/Tris-buffered saline containing 0.05% Tween 20 (TBST) for 1 hr at room temperature. After four 5-min washes with TBST, membrane was incubated with a secondary antibody conjugated with horseradish peroxidase. The blot was repetitively stripped and reprobed with other primary antibodies: a 1:200 dilution of the caveolin-3 antibody (sc-5310 from Santa Cruz), a 1:200 dilution of serca 2 antibody (sc-8094). Secondary antibodies used were: a 1:1000 dilution of NA934 anti-rabbit for VDR (GE Healthcare), a 1:1000 dilution of NA931 anti-mouse for Cav-3 (GE Healthcare), and a 1:1000 dilution of anti-goat (sc-2020) for serca 2.
2.6 Statistical analysis
In all experiments significant differences between data sets was determined using the unpaired student’s t-test. A two-sided P value of <0.05 was considered significant. Data are presented as means ± SE unless otherwise stated.
3. Results
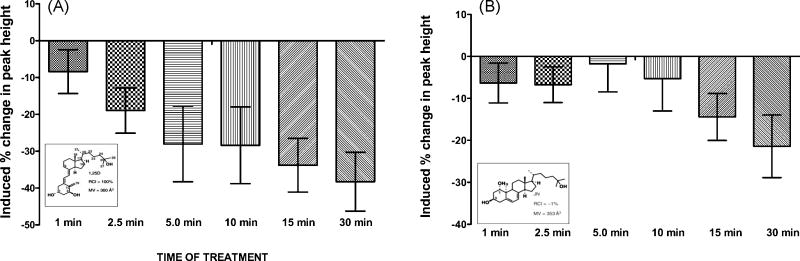
1,25(OH)2D3 exerts a genomic response by regulating gene transcription through its classical nuclear vitamin D receptor (VDR) [9]. It has also been shown to elicit rapid responses putatively through a membrane-associated VDR [9]. To further investigate VDR dependent responses of isolated adult rat cardiomyocyte contractility studies were performed following in culture treatment with 1,25(OH)2D3 and a non-genotropic agonist vitamin D analog, 1a,25(OH)2-lumisterol (JN). Similar responses in sarcomere shortening and change in peak contraction height was observed for both 1,25(OH)2D3 and JN treatment (figure 1). Decreased sarcomere shortening after 15 min of incubation (figure 1A, B) and decreased sarcomere peak height (peak shortening) in minutes (figure 1C, D) was observed in 1,25(OH)2D3 and JN treated cardiomyocytes. JN has been shown to act as a specific non-genotropic agonist, and activate the membrane-associated VDR (18). Thus, the data support the possibility that 1,25(OH)2D3 binds to a membrane associated VDR and thereby elicits the rapid effects on cardiomyocyte sarcomere shortening. Additionally, 1b,25(OH)2D3 (HL), a synthetic non-genotropic antagonist of 1,25(OH)2D3, attenuated the effect of JN on sarcomere shortening and peak shortening (data not shown).
Figure 1.
A, Percent change in cardiomyocyte contraction [peak of cell shortening] in response to treatment with 10− 9M 1,25(OH)2D3 [Insert: Chemical structure of 1,25(OH)2D3; RB From Mizwicki et al. Sci Signal. 2, re4 (2009) RCI= relative competitive index ; MV=molecular volume]. Isolated individual rat cardiomyocytes were electrically stimulated and contraction recorded before and after 1,25(OH)2D3 treatment. Data shown are percent change in peak height after treatment compared to peak height at time zero (before treatment). Data shown were average of 4 cells. B, Percent change in cardiomyocyte contraction [peak shortening] in response to treatment 10−8M JN [Insert: chemical structure. From Mizwicki et al. Sci Signal. 2, re4 (2009) RCI= relative competitive index ; MV=molecular volume]. Data shown were average of 6 cells. JN (1,25(OH)2-lumisterol), is a non-genotropic 1,25(OH)2D3 analog with poor transcriptional activity. Thus, the effects of JN are reportedly through the membrane-associated VDR. The similarity of the effects between 1,25(OH)2D3 and JN in this figure suggests that vitamin D analogs can elicit non-genomic effects through a membrane-associated VDR.
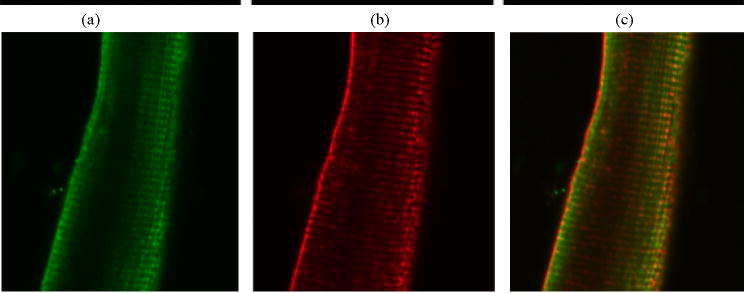
Since caveolae-associated VDR in the plasma membrane has been reported in other cells we checked whether VDR is also associated with caveolae like t-turbules in cardiomyocytes. Immunocytochemistry and confocal microscopy was used to identify the co-localization of VDR with the heart-specific caveolin protein, Caveolin-3 (Figure 3). Consistent with previous findings, a strong immunoreactivity of VDR was observed in isolated adult rat ventricular cells in a repeating pattern of transverse lines throughout the cell (figure 2). Caveolin-3 is a major component of cardiac caveolae/t-tubule complex. Single and double labeling with VDR and caveolin 3 antibodies showed similar patterns of immunoreactivity, suggesting that these two proteins are closely associated (figure 2). A higher (110X) resolution photomicrograph of cardiomyocyte cells (figure 2) showed a clear pattern of association between VDR and Caveolin-3 in the t-tubules and also the sarcolemma, these data demonstrated that in ventricular cardiac myocytes, membranous VDR is also closely associated with Caveolin-3 complexes.
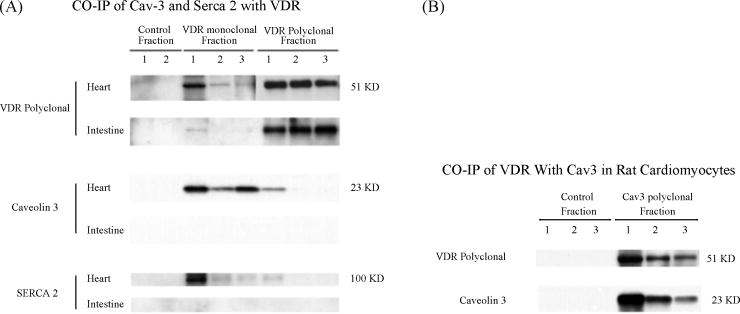
Figure 3.
A, Co-immunoprecipitation of Caveolin-3 and serca 2 with VDR antibodies. 600 ug of cardiomyocyte and intestinal protein lysates were incubated with VDR monoclonal and polyclonal antibody-coupled gel resins, and control gel resin respectively. Eluted fractions of IPed protein samples (20 ul each) were subjected to Western blot analysis. The blot was first probed with Caveolin-3 antibody (sc-5310) and then striped, and reprobed with Serca 2 antibody (sc-8094) and VDR antibody (sc-1008), respectively. B, Co-immunoprecipitation of VDR with caveolin-3 antibody. 600 ug of cardiomyocyte lysates were incubated with caveolin-3 antibody-coupled gel resin and control resin. Eluted fractions of immuno-precipitated samples (20 ul each) were subjected to Western blot analysis. The blot was first probed with caveolin-3 antibody (sc-5310) and then striped, and reprobed with VDR antibody (sc-1008).
Figure 2.
Immunofluorescent confocal microscopy of adult rat cardiac myocytes. Double labeling using an antibody against VDR (sc-1008, rabbit polyclonal, a) and an antibody against caveolin-3 (sc-5310, mouse monoclonal, b) show similar transverse staining pattern, whereas a merge (c) shows association.
To better characterize the interactions between VDR and t-tubule proteins, polyclonal (SC-1008, Santa Cruz), and monoclonal (MA1-710, Affinity Bioreagent) VDR antibodies were immobilized to antibody coupling gel (AminoLink Plus gel, Pierce), to precipitate VDR-associated proteins. The eluted samples were run on SDS-PAGE gel and transferred to PVDF membrane. Western blot analyses were performed. Figure 3A shows that the heart specific caveolin, Caveolin-3, is co-precipitated with VDR, as is SERCA2, the sarcoplasmic reticulum Ca2+-ATPase, which is responsible for calcium from cell cytoplasm and causing relaxation in cardiac myocytes. Additionally, Caveolin-3 Ab (PA1-066, Affinity Bioreagent) immobilized to coupling gel can also bring down VDR (figure 3B), suggesting that the interaction between VDR and caveolin-3 is specific.
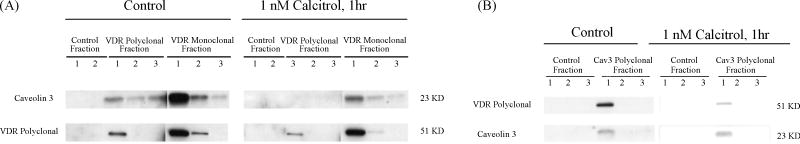
Caveolin proteins are known to directly interact with a number of signaling molecules and modify their functions [19]. In many examples Caveolins hold these signal transducing molecules in the inactive conformation until activation by an appropriate stimulus. To investigate the effect of 1,25(OH)2D3 on the interaction between VDR and Caveolin-3, isolated adult rat cardiomyocytes were treated with 10nM 1,25(OH)2D3 for 1 hour before protein was extracted. Control cells were treated with ethanol vehicle. Co-immunoprecipitation and Western blot analyses were performed. As shown in figure 4A, 1,25(OH)2D3 resulted in a significant decrease in caveolin-3 amount when immunoprecipitated by VDR antibodies, indicating that a dissociation occurred between VDR and caveolin-3 when 1,25(OH)2D3 binds to VDR. A similar result was observed when VDR was immunoprecipitated with Caveolin-3 antibody (figure 4B) and normalized to precipitated Caveolin-3 levels.
Figure 4.
Effect of 1,25(OH)2D3 on interaction between VDR and Caveolin-3. Isolated cardiomyocytes from adult rat heart were treated in culture with 1 nM 1,25(OH)2D3 or ethanol (control) for 1 hour before protein extraction. 600 ug of protein lysates each were incubated with VDR monoclonal (MA1-710) and polyclonal (sc-1008) antibody-coupled gel resins, caveolin-3 polyclonal (PA1-066) antibody-coupled gel resin, and control gel resin respectively. Eluted fractions of IPed protein samples (20 ul each) were subjected to Western blot analysis. A, Western blot of VDR antibody IPed samples. The blot was first probed with Caveolin-3 antibody (sc-5310), then striped and reprobed with VDR antibody (sc-1008). B, Western blot of caveolin-3 antibody IPed samples. The blot was first probed with VDR antibody (sc-1008), then striped and reprobed with caveolin-3 antibody (sc-5310).
4. Discussion
The VDR is traditionally considered a member of the nuclear receptor family and a ligand-activated transcription factor, which mediates the genomic responses of 1,25(OH)2D3. However it has become clear that VDR, along with other steroid hormone receptors, elicit a variety of non-genomic, rapid responses via a localization to, or association with, the plasma membrane. These non-genomic responses involve stimulation of trans-membrane signal transduction pathways acting through membrane-associated receptors [9]. Notable non-genomic responses of VDR include activation of MAP kinase in human leukemia NB4 cells [20], release of insulin from pancreatic b-cells [21], and stimulation of intestinal calcium transport [22]. It has been shown that VDR is associated with caveolae-1 microdomains in chick intestinal cells, suggesting a possible mechanism of membrane localization. Our lab has previously shown that the t-tubule fraction of rat hearts to be enriched in VDR and caveolin-3, the predominant caveolin subtype in cardiac myocytes [12]. In this study, we demonstrated that VDR is indeed physically associated with Caveolin-3 in the t-tubules and sarcolemma of the heart by confocal immunofluorescent microscopy studies and co-immunoprecipitation studies. We have also shown a rapid direct effect of 1, 25(OH)2D3 on contraction in isolated individual cardiomyocyte, which is to modulate the rate and magnitude of contraction. Data presented here support the concept that this effect can be attributed to a membrane protein associated (Caveolin-3) VDR. The fact that the effect on isolated cardiomyocyte contractility was seen within minutes of 1,25(OH)2D3 treatment, and also the fact that similar results were obtained using a nongenotropic agonist D3 analog JN, which preferentially acts through the membrane-associated VDR further supports this conclusion.
It is the rate of calcium influx through calcium channels that is responsible for the rate of cardiomyocyte contraction. These calcium channels are located primarily at the t-tubules. T-tubules are composed of interconnected caveolae-like elements, and repeated caveolae formation in the absence of fission leads to the generation of t-tubules [23]. Many of the proteins involved in excitation-contraction coupling are concentrated at the t-tubules, including L-type calcium channels (LTCC), which play a critical role in regulating calcium-dependent signaling in cardiac myocytes [24]. The β2-adrenergic receptor, associated with Caveolin-3 in cardiac myocytes, is important in the regulation of a number of key proteins in the heart, in particular, the LTCC, the SR Ca2+-ATPase, and the contractile proteins (via troponin I) [25]. Recently it has been shown that sub-cellular localization of LTCC to caveolar macromolecular signaling complexes is essential for regulation of the channels by the β2-adrenergic receptor [26]. Serca-2, the sarcoplasmic reticulum ca2+-ATPase, is responsible for removing calcium from cell cytoplasm and causing relaxation in cardiac myocytes. Here we show that VDR interacts with Serca 2 (figure 3A), which may partially explain the decreased rate of contraction in 1,25(OH)2D3-treated cardiomyocytes observed in this study.
The finding of membrane-associated VDR interacting with Caveolin-3 in cardiac myocytes is important. Caveolin is the principle structural protein that is both necessary and sufficient for the formation of caveolae membrane domain. Caveolae function both in protein trafficking and signal transduction, as well as in cholesterol homeostasis [14, 15]. In addition to a structural role of caveolins in the formation of caveolae, caveolin proteins interact directly with a number of signaling molecules involved in various signaling pathways and modify their functions. These include the Src-family tyrosine kinase, MAP kinase cascades, adenylyl cyclase, PKA, PKC, TGF receptor pathway, and endothelial nitric oxide synthase (eNOS) [19]. Caveolin holds these signal transducers in the sensitive or inactive conformation until activation by an appropriate stimulus. Thus, part of the functions of membrane-associated VDR could well be through its interaction with caveolin-3 and by affecting caveolin-associated signal transduction pathways. It is reasonable to hypothesize that 1,25(OH)2D3 could cause changes in the interaction between VDR and Caveolin-3, thus causing a cascade effect on caveolin-associated signal transducers and thus affect the contraction of myocytes. We investigated the effect of 1,25(OH)2D3 on the interaction between VDR and caveolin-3 by treating the cardiomyocytes with 10nM 1,25(OH)2D3. The co-immunoprecipitation studies clearly showed that there is a dissociation of VDR from Caveolin-3 after 1 hour of 1,25(OH)2D3 treatment (figure 4). This dissociation could inactivate or activate caveolin-associated signal transduction pathways, which may account for the non-genomic effects of VDR. If this process is physiologically relevant it may well be that the processes that initiates the translocation of the VDR to the nucleus to affect gene transduction and the genomic actions of 1,25(OH)2D3 are directly responsible for the non-genomic effects of the hormone. Our study revealed that understanding the mechanism of non-genomic actions of VDR in cardiomyocytes is important for our future understanding of the role of VDR in heart physiology and disease.
Acknowledgments
The authors wish to acknowledge the expert technical assistance of Dr. Daniel Tishkoff. This work was supported by NIH grant 5R01-HL074894 (R.U. Simpson) and the NIH funded MICHR center, Cancer Center, Organogenesis Center and the Diabetes Center at the University of Michigan Medical School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy. 2009 Jun;29(6):691–708. doi: 10.1592/phco.29.6.691. [DOI] [PubMed] [Google Scholar]
- 2.Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79:1706–12. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shane E, Mancini D, Aaronson K, Silverberg SJ, Seibel MJ, Addesso V, et al. Bone mass, vitamin D deficiency, and hyperparathyroidism in congestive heart failure. Am J Med. 1997;103:197–207. doi: 10.1016/s0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 4.O’Connell TD, Simpson RU. 1,25-Dihydroxyvitamin D3 regulation of myocardial growth and c-myc levels in the rat heart. Biochem Biophys Res Commun. 1995;213:59–65. doi: 10.1006/bbrc.1995.2098. [DOI] [PubMed] [Google Scholar]
- 5.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258:E134–42. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 6.Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol. 2007;103:521–4. doi: 10.1016/j.jsbmb.2006.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Boland AR, Boland RL. Non-genomic signal transduction pathway of vitamin D in muscle. Cell Signal. 1994;6:717–24. doi: 10.1016/0898-6568(94)00042-5. [DOI] [PubMed] [Google Scholar]
- 8.Morelli S, Buitrago C, Boland R, de Boland AR. The stimulation of MAP kinase by 1,25(OH)(2)-vitamin D(3) in skeletal muscle cells is mediated by protein kinase C and calcium. Mol Cell Endocrinol. 2001;173:41–52. doi: 10.1016/s0303-7207(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 9.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–8. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 10.Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem. 2002;86:128–35. doi: 10.1002/jcb.10191. [DOI] [PubMed] [Google Scholar]
- 11.Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1 alpha,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–71. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- 12.Tishkoff DX, Nibbelink KA, Holmberg KH, Dandu L, Simpson RU. Functional Vitamin D Receptor in the T-Tubules of Cardiac Myocytes. Endocrinology. 2008;149(2):558–64. doi: 10.1210/en.2007-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–67. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- 14.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–9. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 15.Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–11. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 16.Green JJ, Robinson DA, Wilson GE, Simpson RU, Westfall MV. Calcitriol modulation of cardiac contractile performance via protein kinase C. J Mol Cell Cardiol. 2006;41:350–9. doi: 10.1016/j.yjmcc.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Nibbelink KA, Tishkoff DX, Hershey SD, Rahman A, Simpson RU. 1,25(OH)2-vitamin D3 actions on cell proliferation, size, gene expression, and receptor localization, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103:533–7. doi: 10.1016/j.jsbmb.2006.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vertino AM, Bula CM, Chen JR, Almeida M, Han L, Bellido T, et al. Nongenotropic, anti-apoptotic signaling of 1alpha,25(OH)2-vitamin D3 and analogs through the ligand binding domain of the vitamin D receptor in osteoblasts and osteocytes. Mediation by Src, phosphatidylinositol 3-, and JNK kinases. J Biol Chem. 2005;280:14130–7. doi: 10.1074/jbc.M410720200. [DOI] [PubMed] [Google Scholar]
- 19.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–95. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 20.Song X, Bishop JE, Okamura WH, Norman AW. Stimulation of phosphorylation of mitogen-activated protein kinase by 1alpha,25-dihydroxyvitamin D3 in promyelocytic NB4 leukemia cells: a structure-function study. Endocrinology. 1998;139:457–65. doi: 10.1210/endo.139.2.5747. [DOI] [PubMed] [Google Scholar]
- 21.Kajikawa M, Ishida H, Fujimoto S, Mukai E, Nishimura M, Fujita J, et al. An insulinotropic effect of vitamin D analog with increasing intracellular Ca2+ concentration in pancreatic beta-cells through nongenomic signal transduction. Endocrinology. 1999;140:4706–12. doi: 10.1210/endo.140.10.7025. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz Z, Ehland H, Sylvia VL, Larsson D, Hardin RR, Bingham V, et al. 1alpha,25-dihydroxyvitamin D(3) and 24R,25-dihydroxyvitamin D(3) modulate growth plate chondrocyte physiology via protein kinase C-dependent phosphorylation of extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase. Endocrinology. 2002;143:2775–86. doi: 10.1210/endo.143.7.8889. [DOI] [PubMed] [Google Scholar]
- 23.Franzini-Armstrong C. Simultaneous maturation of transverse tubules and sarcoplasmic reticulum during muscle differentiation in the mouse. Dev Biol. 1991;146:353–63. doi: 10.1016/0012-1606(91)90237-w. [DOI] [PubMed] [Google Scholar]
- 24.Takagishi Y, Yasui K, Severs NJ, Murata Y. Species-specific difference in distribution of voltage-gated L-type Ca(2+) channels of cardiac myocytes. Am J Physiol Cell Physiol. 2000;279:C1963–9. doi: 10.1152/ajpcell.2000.279.6.C1963. [DOI] [PubMed] [Google Scholar]
- 25.Ostrom RS, Violin JD, Coleman S, Insel PA. Selective enhancement of beta-adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol Pharmacol. 2000;57:1075–9. [PubMed] [Google Scholar]
- 26.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–5. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]