Abstract
The cortex is the outermost region of the cell, comprising all of the elements from the plasma membrane to the cortical actin cytoskeleton that cooperate to maintain the cell’s shape and topology. In eukaryotes without cell walls, this cortex governs the contact between their plasma membranes and the environment and thereby influences cell shape, motility, and signaling. It is therefore of considerable interest to understand how cells control their cortices, both globally and with respect to small sub-domains. Here we review the current understanding of this control, including the regulation of cell shape by balances of outward hydrostatic pressure and cortical tension. The actomyosin cytoskeleton is the canonical regulator of cortical rigidity and indeed many would consider the cortex to comprise the actin cortex nearly exclusively. However, this actomyosin array is intimately linked to the membrane, for example via ERM and PIP2 proteins. Additionally, the lipid membrane likely undergoes rigidification by other players, such as BAR proteins. Recent data also indicates that the septin cytoskeleton may play a formidable and more direct role in stabilization of membranes, particularly in contexts where cells receive limited external stabilization from their environments. Here, we review how septins may play this role, drawing on their physical form, their ability to directly bind and modify membranes and actomyosin, and their interactions with vesicular machinery. Deficiencies and alterations in the nature of the septin cytoskeleton may thus be relevant in multiple disease settings.
Introduction
The rigidity of the cortex is a fundamental property of all cells, and in metazoans, where the nature of each contact between a cell and its neighbor or substrate is critical to complex biology, cortical rigidity plays particularly interesting and important roles. Specialized cellular function is supported by the regulation of interactions between cortical elements, which ensures mechanical stability and flexibility of the cortex. For instance, stable contacts between cells, such as tight junctions, can be encouraged by long-lived, highly rigid cortical structures [Van Itallie et al., 2009]. Alternatively, locally rigid regions can be dynamically coordinated to promote motility. At the simplest level, a critical difference between stable and motile cells is the rapidity with which their membranes are able to locally contort and promote access between receptors and ligands. This deformability may be grossly referred to as ‘cortical rigidity’—the importance of which also extends to include the regulation of cell shape, cytokinesis, development, and the response to a changing external environment. Although cortical rigidity and the cortex is often used to invoke features of the actin cytoskeleton, this review aims not to restrict only to that framework but to consider the cortex as a sum of actin as well as other participants.
A rigid cortex opposes the internal and external forces that push and pull membranes
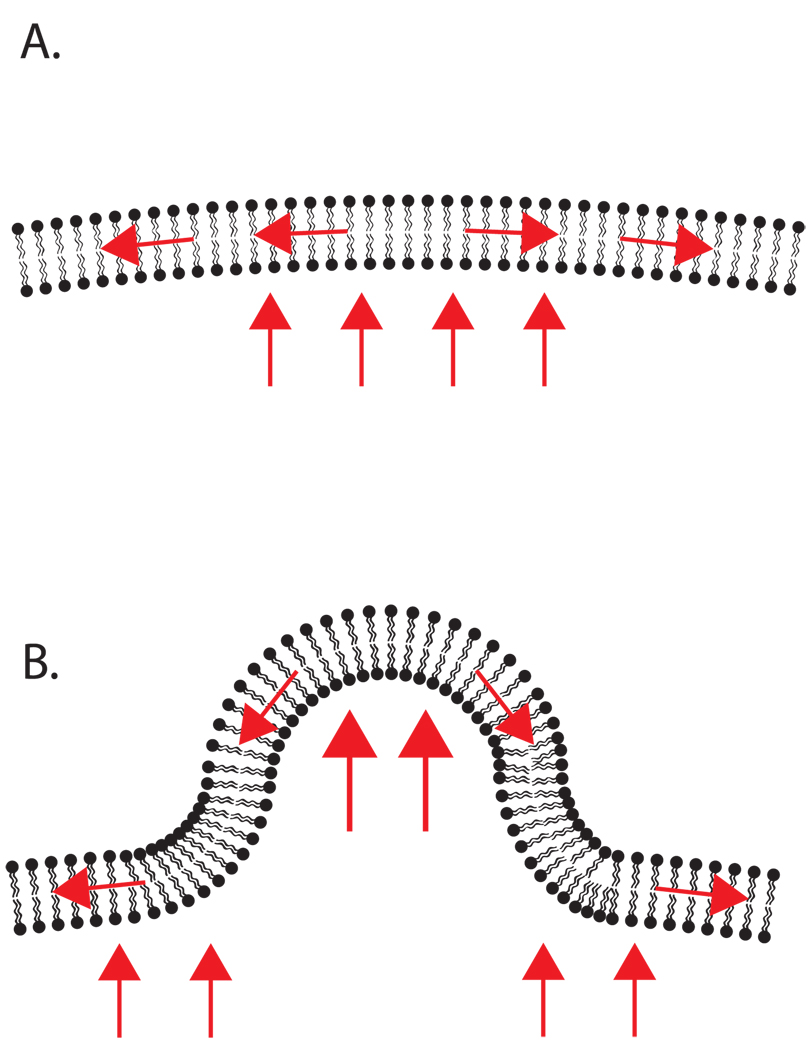
The most simplified view of the cell cortex can be illustrated by analogy with surface tension of a spherical water droplet. The plasma membrane is a laterally inelastic structure, supplying constant tension in the absence of other factors. Meanwhile, hydrostatic pressure within the cell exerts force outward, opposing the tension along the plasma membrane (Figure 1A.) .In reality, of course, cells are not simply spherical water droplets, and many kinds of interactions inside and outside of the cell can cause changes in pressure and tension that influence cell shape. For example, local compression of the cytoplasm, brought about by Myosin II and other motors that contract the actin cytoskeleton results in changes in pressure at the cortex that can cause protrusion; this may be particularly important during motility (Figure 1B) [Charras et al., 2005; Fackler and Grosse, 2008]. A similar pressure gradient may be formed by changes in intracellular ion concentrations that then stimulate protrusions and ultimately motility [Stock and Schwab, 2006] . In either case, it is then clear that the cortex does, in fact, experience hydrostatic pressure generated from the cell’s interior; a model of the cytoplasm as a poroelastic medium has been well studied and reviewed elsewhere [Mitchison et al., 2008] . In a dramatic example of pressure/tension opposition, during mitosis the cortex must resist both internal compression from myosin and localized outward pushing by mitotic spindles. Externally, cell-cell and cell-ECM adhesions are examples of localized forces that can either pull or resist membrane deformation. Leukocytes adhering to vasculature are subject to the additional challenge of shear forces in the blood. The tension along the membrane that opposes both internal and external forces is supplied by interactions between individual membrane lipids and by the linkage of the membrane to rigid internal structures. It is these structures and their connections to each other that is described as the ‘cell cortex’.
Figure 1.
A simple model of pressure and tension on cell membranes. A. Under steady state conditions, hydrostatic pressure pushing outward is balanced by tension in the membrane. (Red arrows indicate force) B. Shape change is achieved when pressure and tension are imbalanced. In this example, additional local outward pressure leads to protrusion.
Cortical rigidity is maintained over many scales and processes
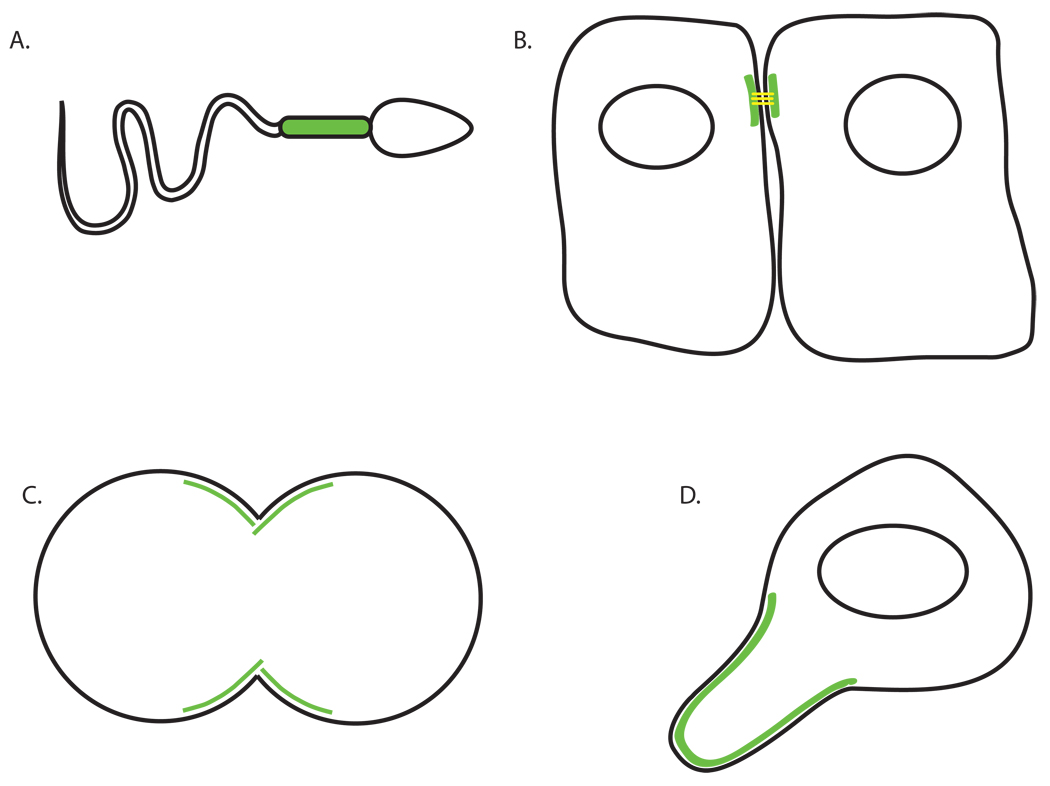
Changes in cell cortex rigidity are important across many length scales. On an organismal scale, during Xenopus development, mesodermic tissue stiffens, causing elongation along the dorsal axis. This process is dependent on the actomyosin-mediated rigidity of the cells themselves, not on fibrillar extracellular matrix proteins [Zhou et al., 2009] . On a single-cell scale, some cells maintain rigid structures over long periods, such as spermatozoa middle pieces or epithelial tight junctions (Figure 2A–B). Under other circumstances, rigidity is a dynamic property. Amoeboid motility relies on a relative loosening of membrane tension at the leading edge and rigidifying of the membrane at the trailing edge for propulsion. These rigid elements and the corresponding ‘amoeboid’ shape are maintained even in the absence of contact with rigid external structures. Similarly, in cells that are relatively soft for most of the cell cycle, tremendous stiffening is observed at the cleavage furrow during cytokinesis (Figure 2C–D). Also dynamic in nature are the very local changes in rigidity required for the formation of protrusive organelles such as filopodia, lamellipodia, and microvilli. At the smallest scale, individual membrane trafficking events occur over as little as 50 nm and require local assembly of rigidity-conferring machinery to bend lipids as well as specific and active machinery that completes the scission or fusion of membranes.
Figure 2.
Cortical rigidity is important in many cellular contexts. Green markings indicate rigid regions of A. the spermatazoa middle piece, B. tight junctions between epithelial cells, C. the cleavage furrow of a mitotic cell, and D. the uropod of a crawling amoeboid cell.
Conventional mechanisms of membrane tension and cortical rigidity
Membranes and Lipids
The most superficial contributor to cellular rigidity is the plasma membrane itself. Since the plasma membrane is the outer layer of the cell and its integrity is essential for many cellular functions, cortical deformations that exceed the rupture point of the membrane are detrimental to cell survival . Indeed, very little lateral stretching of the plasma membrane occurs. It has been estimated that even under optimal in vitro conditions, the maximal deformability of the membrane is only about 4% [Evans and Skalak, 1979; Waugh, 1983] . Due to that limitation, nearly all of the plasma membrane constituting the surface of a protrusion or invagination must be recruited from some other part of the cell, either by the addition of membrane from intracellular stores, or by shifting membrane laterally across the surface of the cell [Sheetz, 2001] . That lateral shifting requires overcoming high-affinity interactions between integral transmembrane proteins and cytoskeletal linkers (to be discussed later) and lower affinity, but more abundant, interactions between the lipids themselves and the cortical cytoskeleton. The deformability of the outer layer of the cortex is therefore dictated more by the availability of additional plasma membrane to be redistributed into that deformation than it is by lateral stretching of the membrane itself.
Cell surfaces are rich with tiny invaginations and protrusions that exist largely independently of attachment to interior structures, and can act as reservoirs of plasma membrane that can be reorganized under tension. Raucher and Sheetz [1999] have shown that these membrane stores can be mobilized during the extension of membrane tethers produced by optical tweezers, a result that has been mathematically modeled as well [Sens and Turner, 2006; Raucher and Sheetz, 1999] . This process depends on release of excess membrane from the cytoskeleton, freeing it to move into the membrane tether. Further, the size of the membrane reservoir can be expanded under conditions of persistent tension [Raucher and Sheetz, 1999] , probably via vesicular addition of new membrane [Togo et al., 2000] . Electron microscopy studies have indicated that cell volume change following exposure to hypotonic media is largely achieved via dissolution of microvilli and redistribution of their membranes into a newly smooth and expanded surface [Grinstein et al., 1984] . Thus, some membrane structures act as lipid sinks, aiding in the maintenance of consistent cortical rigidity in response to changing mechanical pressures.
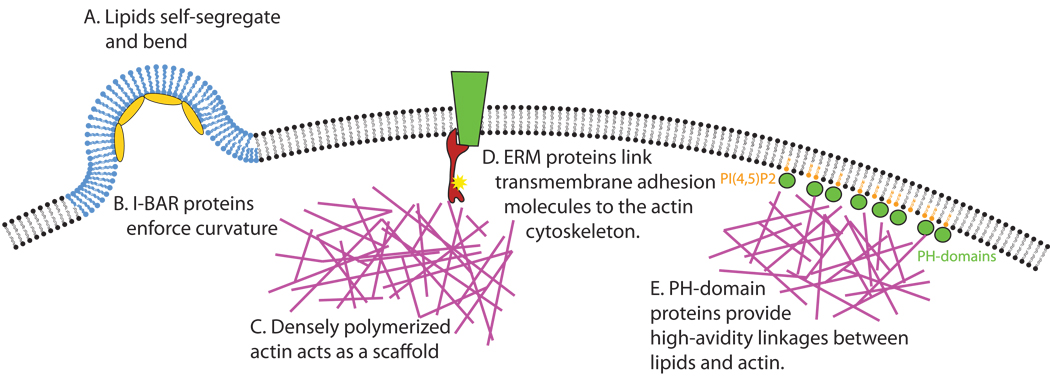
In contrast to limited stretching in the plane of the membrane, there is considerable bending flexibility in lipid membranes; this is highly influenced by the identity and distribution of component lipids. In vitro, membrane curvature emerges in part from segregation into microdomains with different lipid compositions (Figure 3A). Biophysical models and in vitro data from giant unilamellar vesicles show that a heterogeneous lipid bilayer spontaneously undergoes phase separation over time into microdomains of more and less ordered lipids [Baumgart et al., 2003] . The relative concentrations of components such as cholesterol and the synthetic phospholipid DOPC control the precise distribution of these phases [Roux et al., 2005] . Once these phases have been generated, one lipid phase bends, thus minimizing the energy resulting from the phase boundary, known as line tension. Opposing the influence of line tension is the bending rigidity of the lipid microdomain, and the interplay between those two forces determines the final extent of membrane curvature. The interplay between the energy of membrane bending and the energy due to line tension at the phase boundary, as well as the particular structures of the lipids themselves, can control the generation of shapes resembling caveolae and buds in artificial membranes [Sens and Turner, 2004, Jülicher and Lipowsky, 1993] . The relative contribution of lipid segregation on membrane curvature in vivo remains to be seen.
Figure 3.
Cortical rigidity can be regulated by many systems. A. Heterogeneous lipids self-segregate into domains that favor curvature. B. BAR-domain proteins induce or maintain curvature by binding in arrays directly to the plasma membrane. C. Actin forms a thickly polymerized network at the cortex to provide structural support and scaffolding for cortical activities. D. Phosphorylated ERM proteins are important linkers between cortical actin and transmembrane proteins such as CD44. E. Numerous low-affinity interactions between lipids, PH-domain proteins, and cortical actin supply most of the adhesion between the cytoskeleton and the plasma membrane.
Despite the relatively low lateral stretching of the plasma membrane, the dynamic distribution of excess material means that, in sum, the mechanical contribution of the plasma membrane to cortical rigidity is quite small. Rather, the major role of the membrane in resisting cortical deformation comes from its organization of proteins that link the membrane to more rigid structures underneath, many of which are recruited directly by phosphatidylinositols.
BAR-domain proteins
Comprising at least two-dozen members, BAR (Bin–Amphiphysin–Rvs) domain proteins are an emerging family that adds rigidity to the cortex. These lipid-binding domains are sufficient to cause curvature in the cell membrane, and in cell-free systems, BAR domains self-aggregate on liposomes to form tubular structures [Mattila et al., 2007; Peter et al., 2004] . Whether these curvatures arise from stabilization of stochastically curved membranes or whether BAR domains direct membrane deformation is unclear. Membrane structures formed by BAR domains are highly variable. Both the radius and direction of curvature (i.e. whether the structures are concave or convex) depend on the identity of the BAR-domain protein used to generate it. F-BAR domains are generally banana-shaped, and generate invaginations and tubules, while I-BAR domains are zeppelin-shaped, and are involved in the generation of protrusions such as filopodia [Frost et al., 2008; Saarikangas et al., 2009]. PI(4,5)P2 is sufficient for the recruitment of one key I-BAR family member, IRSp53, which is involved in generating filopodial curvature. IRSp53-induced curvature draws the plasma membrane away from the growing actin cytoskeleton, creating space for new protrusions (Figure 3B). Overexpression of the IRSp53 BAR domain leads to spontaneous generation of long, thin protrusions that lack actin, and in cells that lack actin bundling ability, IRSp53-dependent protrusions take the form of membrane ruffles instead [Suetsugu et al., 2006; Lim et al., 2008] . In addition to their membrane attachments, I-BAR proteins can also link the curved membrane to cortical actin. This linkage is direct, and probably occurs through nonspecific electrostatic interactions [Mattila et al, 2007] . Interactions between the plasma membrane and cortical actin are also regulated by interactions of BAR-protein SH3-domains with the WASP/WAVE complex [Takenawa and Suetsugu, 2007; Doherty and McMahon, 2008; Ahmed et al., 2009] . Through the regulated action of BAR-domain proteins, protrusions can arise and dissolve dynamically and rapidly while maintaining the rigidity and integrity of the plasma membrane. Additionally, BAR domains are quite rigid, and several theoretical models have proposed that their binding to the membrane increases the net cortical rigidity of the structures they induce [Arkhipov et al., 2008; Ayton et al., 2009] .
Cortical actin
Certainly the most essential contributor to cellular rigidity is a densely polymerized array of actin filaments and associated crosslinking proteins just beneath the plasma membrane. The patterning of the generalized cortical actin cytoskeleton is still a matter of debate. Several electron microscopy studies have shown dense networks of criss-crossing actin filaments [Charras et al., 2006; Morone et al., 2006] . Those images, along with the observation that the actin-branching complex Arp2/3 is often present at the cortex of cells, have led many to assume that cortical actin is branched [Svitikina, 2003; Svitkina and Borissy, 1999; Johnston et al., 2008] . Recently, a detailed electron tomography study of individual actin filaments in lamellipodia failed to find evidence of substantial branched actin in that structure [Urban et al., 2010] . It is as yet unknown whether the results of that study also represent the architecture of actin filaments outside the lamellipodium. The mechanical properties of cortical actin differ from that of highly bundled actin arrangements and the cell’s interior, as contributions from branching, crosslinking, and bundling can all contribute to the overall rigidity of actin structures [Chaudhuri et al., 2007; Gardel, 2004; Hoffman, 2006].
While the existence of polymerized actin at the cell cortex is certainly necessary for rigidity, it is not sufficient for rigidity at all levels as has been demonstrated in studies in neutrophils. Treatment with Cytochalasin B to depolymerize actin reduced both the cortical tension and the total cellular viscosity of cells subject to an assay in which they were drawn against a micropipet, showing that assembled actin is an essential structure for both membrane tension and the total rigidity of the cell [Charras et al., 2006]. Conversely, Jasplakinolide treatment, which stabilizes some filamentous actin, prevented the cells from being drawn through a 5 um pipet, demonstrating that cellular viscosity was increased when conditions favored polymerization of actin.. However, even in the presence of Jasplakinolide, ‘tongues’ of membrane were still observed being sucked into the micropipet, showing that the pool of polymerized actin induced by that drug did not control the most membrane-proximal components of the cortex, and suggesting that additional components are required for full cortical rigidity [Sheikh et al., 1997]. Specific attachment factors (discussed below) are necessary for connecting the plasma membrane to cortical actin and non-actin structures may also rely upon dynamic actin assemblies in order to form or persist.
Actin-membrane linkages
Spectrin
A network of spectrin lies immediately beneath the plasma membrane in parts or all of many cells and forms functional complexes with F-actin, ankyrin, and 4.1. Its importance was first recognized in red blood cells where it is responsible for maintaining the shape and structural integrity of the cortex . Humans with spectrin mutations suffer from hereditary hemolytic anemia, and their red blood cells are misshapen and highly susceptible to osmotic stress, often losing membrane spontaneously [Perotta et al., 2008]. This phenotypes are probably most evident in red blood cells because, in contrast with most nucleated cells, they lack a cortical actin cytoskeleton. Spectrin also plays mechanical roles in cells that have cortical actin, as spectrin-ankyrin-4.1 complexes bind a wide variety of transmembrane proteins, from ion channels to adhesion molecules (Jenkins and Bennett, 2001; Leshchyns'ka, 2003]. In doing this, these complexes can serve as bridges between cortical F-actin and the plasma membrane and participate in many processes, such as generating epithelial polarity, assembly of cortical actin at cell-cell contacts, and modulating cortical rigidity for tissue-specific processes [Prasain and Stevens, 2009; Itoh et al., 1991, 1993]. For example, in auditory hair cells, spectrin interacts with cortical F-actin to maintain the proper rigidity for sound transduction [Legendre et al., 2008]. Spectrin deletion in C. elegans resulted in unusually fragile cells of several types. Muscle cells were unable to withstand the pressures resulting from internal contractions of myosin, and axons were fractured by the mechanical force of body movement [Hammarlund et al., 2000, 2007; Moorthy et al., 2000].
ERM proteins
Another type of bridge between cortical actin and the plasma membrane is formed by ezrin, radixin, and moesin (collectively, the ERM proteins). ERM proteins contain an actin-binding domain at their C-terminus, and a conserved FERM (4.1, ezrin, radixin, moesin) domain at their N-terminus, which binds transmembrane proteins such as integrins and CD44 (Figure 3D). ERM activity is regulated in several ways, the best studied of which is phosphorylation of the C-terminal domain [Ivetic and Ridley, 2004]. Phosphorylation causes the proteins to unfold, revealing the FERM and actin-binding domains and allowing crosslinking of transmembrane proteins to the actin cytoskeleton. The phosphorylation of ERM proteins is potentiated by PI(4,5)P2 binding [Fievet et al., 2004]. PI(4,5)P2 also plays a role in protein-protein interactions after ERM activation, as it has been shown to be essential for in vitro binding between purified FERM domains and CD44 at physiological ionic strengths [Hirao et al., 1996].
ERM proteins are essential for the control of several kinds of protrusive structures. Overexpression in fibroblasts of dominant-negative FERM domain-GFP fusions resulted in the generation of long, thin, actin-poor protrusions all over the surfaces of cells. This was perhaps due to defects in retraction by the endogenous ERM proteins, which would normally bind to transmembrane proteins and link them to retraction machinery [Amieva et al., 1999]. Similarly, bleb retraction is mediated by ezrin, which is recruited to the plasma membrane concomitantly with the reestablishment of cortical actin on the bleb [Charras et al., 2006]. In T lymphocytes, ERM proteins have been suggested to contribute to overall cortical rigidity. ERM proteins are rapidly dephosphorylated following T cell receptor stimulation, and expression of dominant-negative FERM-GFP made the cells more deformable than controls. It was postulated that during the formation of a synapse with an antigen-presenting cells, ERM protein deactivation is necessary to relax the cell cortex and permit full engagement between the two cells [Faure et al., 2004].
Low-affinity linkages
Individual complexes linking transmembrane proteins to the cytoskeleton are most commonly associated with small areas of strong adhesion, and are therefore not very abundant across the cell. The highest estimates for these structures put them 0.2 um apart [Sheets, 2001]. Cortical rigidity, however, is present across the cell, so there must be other kinds of interactions that provide adhesion between the plasma membrane and cytoskeleton. The lipid PI(4,5)P2 may be as much as 1000x more abundant in the membrane than strong adhesions. It is likely that the wide array of phosphatidylinositol-binding proteins with PH or polybasic domains could assemble between cortical actin and the plasma membrane to form a high-avidity interface between the two surfaces (Figure 3E, [Sheets, 2001]). Indeed, depletion of PI(4,5)P2 by several methods produces measurable decreases in the adhesion energy between the plasma membrane and cytoskeleton, indicating its importance as a mediator of cortical rigidity [Raucher et al., 2000]. Arrays of septin proteins are likely participants in the high-avidity membrane-actin interface and will be the subject of the next section.
Septins as rigidity players at the cell cortex
Properties of septin polymers
Septins are a family of GTP-binding proteins that assemble into filaments and constitute an emerging non-canonical cytoskeleton [Barral and Kinoshita, 2008]. All septin proteins contain a conserved GTP-binding domain, and N-terminal proline-rich and C-terminal coiled coil domains that vary between family members. There are 13 septin genes in the mouse genome and Septins proteins have been divided into four groups on the basis of homology in the proline-rich and coiled-coil regions. Members of these four groups can partially compensate for one another functionally [Kinoshita, 2003]. A member from each of three groups is required to build the minimal hexameric septin complex. A crystal structure exists for one such minimal hexamer, a rod-shaped complex of septins 2, 6, and 7 in a 2:2:2 ratio [Sirajuddin et al., 2007]. These hexamers arrange end-to-end into higher order structures both in cells and in vitro, resulting in elaborate networks of rings, gauzes, and filaments. As will be described in the following sections, these diverse septin superstructures can associate with actin, microtubules, and membranes, or can be free-floating in the cytoplasm.
Septins were initially identified as cell division mutants in yeast, where they play a role in scaffolding cytokinetic machinery at the bud neck and in segregation of material between the mother and daughter cells. Septins appear to function in animal cell division as well [Kinoshita et al., 2002; Joo et al., 2007; Nagata et al., 2003; Surka et al., 2002; Nguyen et al., 2000], but septins are also highly expressed in post-mitotic cells such as spermatozoa and neurons, suggesting septin functions beyond cytokinesis. Recent studies have demonstrated roles for septins in motility, polarity, and exocytosis; some of these may be directly related to regulation of cortical rigidity by septins.
Control of cortical rigidity by septins
One indication that septins regulate the mechanical properties of cells is found in the highly dynamic setting of sperm motility. Septins localize to a small structure in the middle piece of spermatozoa called the annulus, and are required for the structural integrity of that region [Ihara et al., 2005; Steels et al., 2007]. In particular, spermatozoa lacking SEPT4 become fragile through their middle piece and bend sharply or break as they develop and become motile [Ihara et al., 2005]. In another highly motile system, we showed that septin filaments assemble along the cortex of amoeboid T cells in a collar-like distribution that bridges the region between the cell body and the trailing uropod [Tooley et al., 2009]. shRNA knockdown of septin complexes in T cells resulted in dramatic increases in cortical protrusions and blebbing, elongation of the uropod, and the loss of persistent motility. These membrane instabilities could be mimicked by other methods of cortical relaxation and could be suppressed by treatment with taxol, which made the cells appear generally more rigid. Those data support the hypothesis that cortical rigidification is also a role played by septins. Intriguingly, septin-deficient T cells were more apt than control cells to pass through very small pores in a transwell migration assay, supporting the hypothesis that loss of septins leads to increased cortical flexibility [Tooley et al., 2009].
T cells and sperm are similar in that both maintain highly specialized shapes in the absence of external adhesive structures. It is tempting to speculate that septins play a particularly important role in maintaining cortical rigidity under conditions of low adhesion. Another adhesion-independent structure that requires the septin cytoskeleton is the dendritic spine. Septins assemble at the junction of dendrites with neurons and loss of septins results in defective development of the dendritic arbor [Tada et al., 2007]. It may thus be that the septin cytoskeleton also plays a discrete role in this structure in much the same way as it mechanically reinforces the junction between front and back in sperm and lymphocytes.
Studies of septin expression in cancers are consistent with the hypothesis that septins function to maintain the cell cortex. For example, SEPT9_v4, an isoform of SEPT9, is frequently overexpressed in human ovarian and breast tumors, as well as in some leukemias [Montagna et al., 2003; Osaka et al., 1999]. This short isoform does not localize to the cortex as does full-length SEPT9 , and its overexpression can lead to mislocalization of the full-length form. Experimental overexpression of SEPT9_v4 in MCF7 cells led to increased actin-containing protrusions that resembled leading edges, but were not dependent on Cdc42 activity. Further, these cells had slightly increased motility, but lacked directional guidance in 2- and 3-dimensional systems [Chacko et al., 2005].
Mode of action of septins
Direct binding to membranes
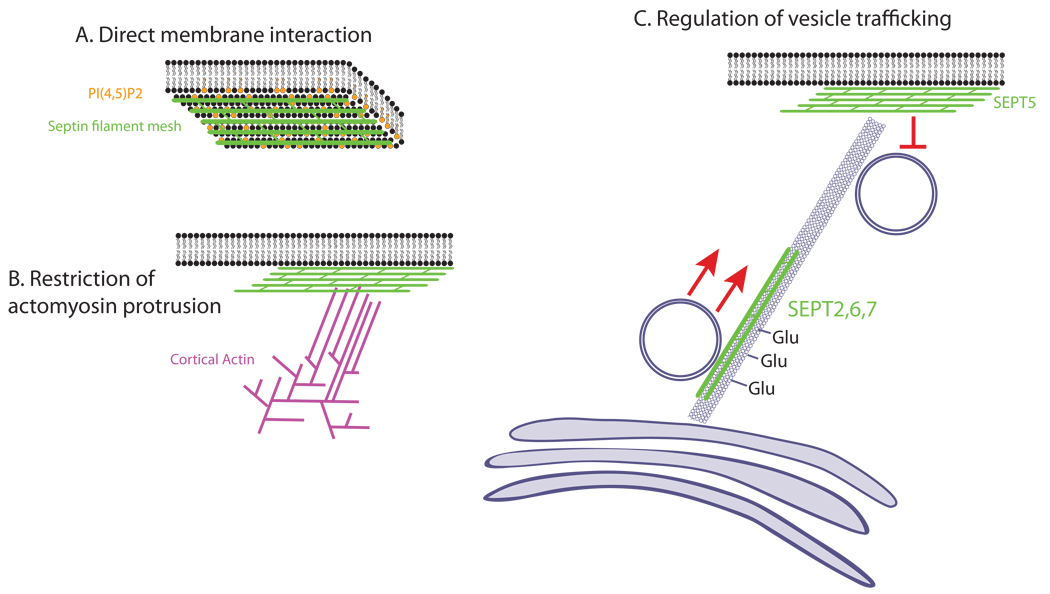
The association of septins with lipids was suggested both by the cortical localization of septins observed by immunofluorescence in some cells and by subcellular fractionation experiments that found septins extracted with cell membranes [Xie et al., 1999]. In vitro studies have supported this. Purified septins have been demonstrated to bind lipids directly, with particular affinities for PIP, PIP2, and PIP3 [Zhang et al., 1999; Tanaka-Taniguchi et al., 2009], which they bind through a polybasic region just N-terminal to the GTP-binding domain. Septin filaments can be disrupted in 3T3 cells by sequestration or depletion of PIP2 and PIP3 [Zhang et al., 1999]. Just as lipids can influence septin structures, septins can assemble in arrays on membranes and dramatically influence their shapes (Figure 4A.) Addition of septins or septin-containing brain extracts to liposomes containing PIP and PIP2 changed spherical giant liposomes into tubular structures in vitro, with properties distinct from those formed by BAR proteins. The authors of that study suggest that despite the relatively weak affinity of individual septin molecules for PIP2, the formation of large septin arrays generates a gently curved, rigid surface with high avidity for the liposome, giving these structures the potential to shape plasma membranes in vivo [Tanaka-Taniguchi et al., 2009]. In this way, the septin cytoskeleton could shape and sequester excess membrane, acting as an effector in regulating the topological plasma membrane reservoir discussed earlier and locally influencing membrane tension.
Figure 4.
Mechanisms of cortical control by septins. A. Structural septin meshworks interact directly with membrane lipids to enforce curvature and may provide contacts with the actin cytoskeleton. B. Septins interacting with Myosin II may control actomyosin-mediated protrusions C. Septins can have a positive or negative role in vesicle trafficking, depending on the location and composition of septin complexes. SEPT2-containing filaments line up on polyglutamated microtubules and promote polarized transport. SEPT5-containing filaments are present on the membrane and negatively regulate vesicle docking.
Association with actomyosin
A second function of cortical septin sheets may be to provide a high-avidity linkage between the plasma membrane and cortical actin. The interactions between actin and septins have been best studied in the context of stress fibers. Principles of the septin-stress fiber interaction can most likely be extended to the septin-cortical actin interaction, since a comparison of cell lines with and without stress fibers demonstrated that in the absence of those structures, septins and actin colocalize at the cortex [Xie et al., 1999]. In both Hela and NIH3T3 cells, septins are dependent on stress fibers for their localization [Kinoshita et al., 1997, 2002]. When the stress fibers were disrupted by a variety of pharmacological treatments, septins became diffuse or formed free-floating cytoplasmic rings. In vitro, septins spontaneously assemble into rings in the absence of polymerized actin and the actin-bundling protein anillin [Kinoshita et al., 2002]. Interestingly, anillin is probably not a necessary cofactor for septin binding in vivo, as it is mostly sequestered in the nucleus during interphase. Just as septins depend on actin for their full assembly in vivo, actin stress fibers are also dependent on septin. Direct disruption of actin-septin binding by the expression of dominant-negative anillin or Borg3 fragments, or of anti-septin RNAi caused the loss of stress fibers [Kinoshita et al., 2002].
Septins may also regulate the actomyosin cytoskeleton via direct binding to Myosin II (MyoII). MyoII has been shown in CHO cells to interact directly with SEPT2 through a small portion of its coiled-coil domain [Joo et al., 2007]. Overexpression of this domain blocks the interaction between SEPT2 and MyoII, and results in decreased MyoII activation. The authors suggest that one function of septin filaments is to act as scaffolds for myosin-activating proteins such as ROCK, CRIK and MLCK [Joo et al., 2007]. Septins could therefore control the cortex by restricting myosin activity and actin-based protrusions (Figure 4B.)
Interaction with vesicle machinery
In addition to their direct mechanical effects on cortical rigidity, septins are likely involved in the regulation of cell shape, polarity, and membrane trafficking through their interactions with microtubules (Figure 4C). Septins may exert these effects by participating in the regulation of membrane deposition or by adjusting the arrangement of compression-bearing microtubules. That septins partially colocalize with microtubules in neurons has been appreciated for many years and direct interactions between septins and tubulin have been observed in vitro [Nuefeld and Rubin, 1994; Sisson et al., 2000]. It is as yet unclear whether septins pattern microtubules or vice versa, since disruption of microtubules displaces SEPT9 in some cells, and depletion of septins alters microtubule patterning in others [Neufeld and Rubin, 1994; Sisson et al., 2000; Spiliotis et al., 2008; Kremer et al., 2005] .
An important cofactor in the microtubule-septin system is the microtuble-stabilizing protein MAP4. In the initial identification of MAP4’s role in this system, Kremer et al. showed that the SEPT2–6–7 complex competes with tubulin for MAP4 binding in HeLa cells. Depletion of septins resulted in thickening of microtubule bundles and a general increase in the number and stability of microtubules present in the cell [Kremer et al., 2005]. Spiliotis et al. [2008] identified a more specific role for the septin-MAP4 interaction in generating polarity in MDCK cells. Here, septins bound poly-glutamated microtubules directly, displacing MAP4 and allowing for ‘fast-tracking’ of vesicles for polarized transport. Interestingly, depletion of septins in this system caused the loss of the poly-glutamated subset of microtubules and the loss of epithelial polarity. Clearly, the interplay between septins, microtubules, and MAP4 is more complex or diverse than is currently appreciated, and an integrated model of how these systems interact remains elusive.
Finally, septins also play a role in trafficking of membrane components via direct interactions with the exocyst complex. In neurons, SEPT5 is associated with synaptic vesicles, and has been shown to modulate exocytosis by directly inhibiting the vesicle release machinery [Beites et al., 1999, 2005, Amin et al., 2008]. This suggests a potential role for septins not only in secretion, but in localized deposition of membrane. Such deposition could relieve local strain on the membrane and effectively reduce cortical rigidity.
Summary
Rigidity of the cell cortex is an essential property of cells that must be properly controlled. Overly rigid cells could be brittle, causing them to fracture with stress, or simply unable to move and adapt to their surroundings. Conversely, overly flexible cells would be unable to maintain their shapes or generate the forces required for processes like mitosis and motility. While an array of systems can regulate the rigidity of the cell cortex, an outstanding question is how these systems cooperate and combine to coordinate local rigidity changes at specific cortical domains, or to respond to changes over the natural history of a cell. Septin filaments interact with many well-established rigidity-regulating systems, and may be essential for integrating and fine-tuning their functions. Determining the cross-regulation of these systems constitutes the important next-step in this rapidly evolving field.
Acknowledgements
We apologize to the authors of the many relevant studies omitted here due to space limitations. We thank Jordan Jacobelli and Matthew Paszek for critical reading of the manuscript and the reviewers for their helpful comments. Supported by National Institutes of Health grants AI52116 (MFK) and the Leukemia and Lymphoma Foundation (MFK).
References
- Ahmed S, Goh WI, Bu W. I-BAR domains, IRSp53 and filopodium formation [Internet] Semin Cell Dev Biol. 2009 Nov; doi: 10.1016/j.semcdb.2009.11.008. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19913105. [DOI] [PubMed]
- Amieva MR, Litman P, Huang L, Ichimaru E, Furthmayr H. Disruption of dynamic cell surface architecture of NIH3T3 fibroblasts by the N-terminal domains of moesin and ezrin: in vivo imaging with GFP fusion proteins. J. Cell Sci. 1999;112(Pt 1):111–125. doi: 10.1242/jcs.112.1.111. [DOI] [PubMed] [Google Scholar]
- Amin ND, Zheng Y, Kesavapany S, Kanungo J, Guszczynski T, Sihag RK, Rudrabhatla P, Albers W, Grant P, Pant HC. Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis. J Neurosci. 2008;14:3631–3643. doi: 10.1523/JNEUROSCI.0453-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkhipov A, Yin Y, Schulten K. Four-scale description of membrane sculpting by BAR domains. Biophys J. 2008;95(6):2806–2821. doi: 10.1529/biophysj.108.132563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton GS, Lyman E, Krishna V, Swenson RD, Mim C, Unger VM, Voth GA. New Insights into BAR Domain-Induced Membrane Remodeling. Biophys J. 2009;97(6):1616–1625. doi: 10.1016/j.bpj.2009.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines AJ. Evolution of spectrin function in cytoskeletal and membrane networks. Biochem Soc Trans. 2009;37(Pt 4):796–803. doi: 10.1042/BST0370796. [DOI] [PubMed] [Google Scholar]
- Barral Y, Kinoshita M. Structural insights shed light onto septin assemblies and function. Curr Opin Cell Biol. 2008;20(1):12–18. doi: 10.1016/j.ceb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425(6960):821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- Beites CL, Campbell KA, Trimble WS. The septin Sept5/CDCrel-1 competes with alpha-SNAP for binding to the SNARE complex. Biochem J. 2005;385(Pt 2):347–353. doi: 10.1042/BJ20041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci. 1999;2(5):434–439. doi: 10.1038/8100. [DOI] [PubMed] [Google Scholar]
- Chacko AD, Hyland PL, McDade SS, Hamilton PW, Russell SH, Hall PA. SEPT9_v4 expression induces morphological change, increased motility and disturbed polarity. J Pathol. 2005;206(4):458–465. doi: 10.1002/path.1794. [DOI] [PubMed] [Google Scholar]
- Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435(7040):365–369. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charras GT, Hu C, Coughlin M, Mitchison TJ. Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175(3):477–490. doi: 10.1083/jcb.200602085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature. 2007;445(7125):295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu Rev Biophys. 2008;37:65–95. doi: 10.1146/annurev.biophys.37.032807.125912. [DOI] [PubMed] [Google Scholar]
- Evans EA, Skalak R. Mechanics and thermodynamics of biomembranes: part 1. CRC Crit Rev Bioeng. 1979;3(3):181–330. [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181(6):879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Salazar-Fontana LI, Semichon M, Tybulewicz VLJ, Bismuth G, Trautmann A, Germain RN, Delon J. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5(3):272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164(5):653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132(5):807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML. Elastic Behavior of Cross-Linked and Bundled Actin Networks. Science. 2004;304(5675):1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Rothstein A, Sarkadi B, Gelfand EW. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Hammarlund M, Jorgensen EM, Bastiani MJ. Axons break in animals lacking beta-spectrin. J Cell Biol. 2007;176(3):269–275. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Davis WS, Jorgensen EM. Mutations in beta-spectrin disrupt axon outgrowth and sarcomere structure. J. Cell Biol. 2000;149(4):931–942. doi: 10.1083/jcb.149.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996;135(1):37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BD. The consensus mechanics of cultured mammalian cells. Proceedings of the National Academy of Sciences. 2006;103(27):10259–10264. doi: 10.1073/pnas.0510348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, Goto M, Okubo K, Nishiyama H, Ogawa O, Takahashi C, Itohara S, Nishimune Y, Noda M, Kinoshita M. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8(3):343–352. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Itoh M, Yonemura S, Nagafuchi A, Tsukita S, Tsukita S. A 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell-cell adhesion sites. J Cell Biol. 1991;115(5):1449–1462. doi: 10.1083/jcb.115.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121(3):491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112(2):165–176. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155(5):739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SA, Bramble JP, Yeung CL, Mendes PM, Machesky LM. Arp2/3 complex activity in filopodia of spreading cells. BMC Cell Biol. 2008;9(1):65. doi: 10.1186/1471-2121-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E, Surka MC, Trimble WS. Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell. 2007;13(5):677–690. doi: 10.1016/j.devcel.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Jülicher F, Lipowsky R. Domain-induced budding of vesicles. Phys Rev Lett. 1993;70(19):2964–2967. doi: 10.1103/PhysRevLett.70.2964. [DOI] [PubMed] [Google Scholar]
- Kinoshita M. Assembly of mammalian septins. J. Biochem. 2003;134(4):491–496. doi: 10.1093/jb/mvg182. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Kumar S, Mizoguchi A, Ide C, Kinoshita A, Haraguchi T, Hiraoka Y, Noda M. Nedd5, a mammalian septin, is a novel cytoskeletal component interacting with actin-based structures. Genes Dev. 1997;11(12):1535–1547. doi: 10.1101/gad.11.12.1535. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ. Self- and actin-templated assembly of Mammalian septins. Dev Cell. 2002;3(6):791–802. doi: 10.1016/s1534-5807(02)00366-0. [DOI] [PubMed] [Google Scholar]
- Kremer BE, Haystead T, Macara IG. Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol Biol Cell. 2005;16(10):4648–4659. doi: 10.1091/mbc.E05-03-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre K, Safieddine S, Küssel-Andermann P, Petit C, El-Amraoui A. alphaII-betaV spectrin bridges the plasma membrane and cortical lattice in the lateral wall of the auditory outer hair cells. J Cell Sci. 2008;121(Pt 20):3347–3356. doi: 10.1242/jcs.028134. [DOI] [PubMed] [Google Scholar]
- Leshchyns'ka I. Neural cell adhesion molecule (NCAM) association with PKCbeta2 via betaI spectrin is implicated in NCAM-mediated neurite outgrowth. J Cell Biol. 2003;161(3):625–639. doi: 10.1083/jcb.200303020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KB, Bu W, Goh WI, Koh E, Ong SH, Pawson T, Sudhaharan T, Ahmed S. The Cdc42 Effector IRSp53 Generates Filopodia by Coupling Membrane Protrusion with Actin Dynamics. J Biol Chem. 2008;283(29):20454–20472. doi: 10.1074/jbc.M710185200. [DOI] [PubMed] [Google Scholar]
- Mattila PK, Pykäläinen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, Lappalainen P. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol. 2007;176(7):953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Charras GT, Mahadevan L. Implications of a poroelastic cytoplasm for the dynamics of animal cell shape. Semin Cell Dev Biol. 2008;19(3):215–223. doi: 10.1016/j.semcdb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna C, Lyu M, Hunter K, Lukes L, Lowther W, Reppert T, Hissong B, Weaver Z, Ried T. The Septin 9 (MSF) gene is amplified and overexpressed in mouse mammary gland adenocarcinomas and human breast cancer cell lines. Cancer Res. 2003;63(9):2179–2187. [PubMed] [Google Scholar]
- Moorthy S, Chen L, Bennett V. Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J Cell Biol. 2000;149(4):915–930. doi: 10.1083/jcb.149.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morone N, Fujiwara T, Murase K, Kasai RS, Ike H, Yuasa S, Usukura J, Kusumi A. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J Cell Biol. 2006;174(6):851–862. doi: 10.1083/jcb.200606007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Kawajiri A, Matsui S, Takagishi M, Shiromizu T, Saitoh N, Izawa I, Kiyono T, Itoh TJ, Hotani H, Inagaki M. Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem. 2003;278(20):18538–18543. doi: 10.1074/jbc.M205246200. [DOI] [PubMed] [Google Scholar]
- Neufeld TP, Rubin GM. The Drosophila peanut gene is required for cytokinesis and encodes a protein similar to yeast putative bud neck filament proteins. Cell. 1994;77(3):371–379. doi: 10.1016/0092-8674(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Sawa H, Okano H, White JG. The C. elegans septin genes, unc-59 and unc-61, are required for normal postembryonic cytokineses and morphogenesis but have no essential function in embryogenesis. J Cell Sci. 2000;113(Pt 21):3825–3837. doi: 10.1242/jcs.113.21.3825. [DOI] [PubMed] [Google Scholar]
- Osaka M, Rowley JD, Zeleznik-Le NJ. MSF (MLL septin-like fusion), a fusion partner gene of MLL, in a therapy-related acute myeloid leukemia with a t(11;17)(q23;q25) Proc Natl Acad Sci USA. 1999;96(11):6428–6433. doi: 10.1073/pnas.96.11.6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta S, Gallagher PG, Mohandas N. Hereditary spherocytosis. Lancet. 2008;372(9647):1411–1426. doi: 10.1016/S0140-6736(08)61588-3. [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent H, Mills I, Vallis Y, Butler P, Evans P, McMahon H. BAR domains as aensors of membrane curvature: The amphiphysin BAR structure. Science. 2004;303(5657):495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77(1):53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Sheetz MP. Characteristics of a membrane reservoir buffering membrane tension. Biophys J. 1999;77(4):1992–2002. doi: 10.1016/S0006-3495(99)77040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100(2):221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 2005;24(8):1537–1545. doi: 10.1038/sj.emboj.7600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, Pykäläinen A, Laurinmäki P, Mattila PK, Kinnunen PKJ, Butcher SJ, Lappalainen P. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr Biol. 2009;19(2):95–107. doi: 10.1016/j.cub.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Sens P, Turner MS. Theoretical model for the formation of caveolae and similar membrane invaginations. Biophys J. 2004;86(4):2049–2057. doi: 10.1016/S0006-3495(04)74266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sens P, Turner MS. Budded membrane microdomains as tension regulators. Phys Rev E Stat Nonlin Soft Matter Phys. 2006;73(3 Pt 1) doi: 10.1103/PhysRevE.73.031918. 031918. [DOI] [PubMed] [Google Scholar]
- Sheikh S, Gratzer WB, Pinder JC, Nash GB. Actin polymerisation regulates integrin-mediated adhesion as well as rigidity of neutrophils. Biochem Biophys Res Commun. 1997;238(3):910–915. doi: 10.1006/bbrc.1997.7407. [DOI] [PubMed] [Google Scholar]
- Sheetz MP. Cell control by membrane-cytoskeleton adhesion. Nat Rev Mol Cell Biol. 2001;2(5):392–396. doi: 10.1038/35073095. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kühlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449(7160):311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava Lamp, a novel peripheral Golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151(4):905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ. Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol. 2008;180(2):295–303. doi: 10.1083/jcb.200710039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steels JD, Estey MP, Froese CD, Reynaud D, Pace-Asciak C, Trimble WS. Sept12 is a component of the mammalian sperm tail annulus. Cell Motil Cytoskeleton. 2007;64(10):794–807. doi: 10.1002/cm.20224. [DOI] [PubMed] [Google Scholar]
- Stock C, Schwab A. Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol (Oxf) 2006;187(1–2):149–157. doi: 10.1111/j.1748-1716.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Murayama K, Sakamoto A, Hanawa-Suetsugu K, Seto A, Oikawa T, Mishima C, Shirouzu M, Takenawa T, Yokoyama S. The RAC binding domain/IRSp53-MIM homology domain of IRSp53 induces RAC-dependent membrane deformation. J Biol Chem. 2006;281(46):35347–35358. doi: 10.1074/jbc.M606814200. [DOI] [PubMed] [Google Scholar]
- Surka MC, Tsang CW, Trimble WS. The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell. 2002;13(10):3532–3545. doi: 10.1091/mbc.E02-01-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160(3):409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145(5):1009–1026. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M. Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol. 2007;17(20):1752–1758. doi: 10.1016/j.cub.2007.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8(1):37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tanaka-Takiguchi Y, Kinoshita M, Takiguchi K. Septin-mediated uniform bracing of phospholipid membranes. Curr Biol. 2009;19(2):140–145. doi: 10.1016/j.cub.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Togo T, Krasieva TB, Steinhardt RA. A decrease in membrane tension precedes successful cell-membrane repair. Mol Biol Cell. 2000;11(12):4339–4346. doi: 10.1091/mbc.11.12.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley AJ, Gilden J, Jacobelli J, Beemiller P, Trimble WS, Kinoshita M, Krummel MF. Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat Cell Biol. 2009;11(1):17–26. doi: 10.1038/ncb1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban E, Jacob S, Nemethova M, Resch GP, Small JV. Electron tomography reveals unbranched networks of actin filaments in lamellipodia. Nat Cell Biol. 2010;12(5):429–435. doi: 10.1038/ncb2044. [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Fanning AS, Bridges A, Anderson JM. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell. 2009;20(17):3930–3940. doi: 10.1091/mbc.E09-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh RE. Effects of abnormal cytoskeletal structure on erythrocyte membrane mechanical properties. Cell Motil. 1983;3(5–6):609–622. doi: 10.1002/cm.970030526. [DOI] [PubMed] [Google Scholar]
- Xie H, Surka M, Howard J, Trimble WS. Characterization of the mammalian septin H5: distinct patterns of cytoskeletal and membrane association from other septin proteins. Cell Motil Cytoskeleton. 1999;43(1):52–62. doi: 10.1002/(SICI)1097-0169(1999)43:1<52::AID-CM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol. 1999;9(24):1458–1467. doi: 10.1016/s0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136(4):677–688. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]