Abstract
Erythrocytes infected with malaria parasites have increased permeability to various solutes. These changes may be mediated by an unusual small conductance ion channel known as the plasmodial surface anion channel (PSAC). While channel activity benefits the parasite by permitting nutrient acquisition, it can also be detrimental because water-soluble antimalarials may more readily access their parasite targets via this channel. Recently, two such toxins, blasticidin S and leupeptin, were used to select mutant parasites with altered PSAC activities, suggesting acquired resistance via reduced channel-mediated toxin uptake. Surprisingly, although these toxins have similar structures and charge, we now show that reduced permeability of one does not protect the intracellular parasite from the other. Leupeptin accumulation in the blasticidin S-resistant mutant was relatively preserved, consistent with retained in vitro susceptibility to leupeptin. Subsequent in vitro selection with both toxins generated a double mutant parasite having additional changes in PSAC, implicating an antimalarial resistance mechanism for water-soluble drugs requiring channel-mediated uptake at the erythrocyte membrane. Characterization of these mutants revealed a single conserved channel on each mutant, albeit with distinct gating properties. These findings are consistent with a shared channel that mediates uptake of ions, nutrients and toxins. This channel's gating and selectivity properties can be modified in response to in vitro selective pressure.
Keywords: ion channel mutants, gating, selectivity, noise analysis, Plasmodium falciparum, PSAC
1. Introduction
Invasion and growth within erythrocytes is a hallmark of plasmodium species; it permits these parasites to evade host immune responses and provides access to hemoglobin as a nutritive source. In the case of Plasmodium falciparum, the cause of the most severe form of human malaria, growth within erythrocytes also accounts for most of the clinical sequelae. Intracellular growth is associated with a dramatic remodeling of the host cytosol, with export of a large number of proteins to this compartment [1, 2], generation of a specialized membranous network [3], and changes in erythrocyte membrane properties including increased permeability to various organic and inorganic solutes [4–6].
Electrophysiological studies have now established that the permeability changes result from one or more defined ion channels and cannot be attributed to non-specific membrane leaks [7]. While several other ion channels have been reported, a number of studies now point to the plasmodial surface anion channel (PSAC) as the primary uptake mechanism for most, if not all, small uncharged solutes and monovalent ions [8–10]. PSAC activity is conserved on erythrocytes infected with other malaria parasites [11] and is absent on cells infected with Babesia divergens, another apicomplexan parasite that invades human erythrocytes [12]. PSAC also has a number of unusual functional properties that distinguish it from known ion channels in higher organisms [13–15].
In vitro selection has recently been used to generate two separate parasite mutants that carry altered PSAC activity [9, 16]. One mutant was generated after selection with blasticidin S, a peptidyl nucleoside antibiotic presumed to kill parasites by inhibiting protein translation on ribosomes within the intracellular parasite [17]. Although blasticidin S has been used in basic malaria research to select for transfected parasites expressing the deaminase BSD [18–20], a resistant mutant was spontaneously selected by continuous application of blasticidin S pressure to a specific parasite isolate (FCB) in the absence of the BSD resistance gene. The second channel mutant was selected with leupeptin, a cysteine and serine protease inhibitor that has multiple intracellular parasite targets [21–23]. This mutant did not have measurable changes in cellular protease activity or leupeptin sensitivity of parasite proteases; there were also no detectable changes in sequence or expression levels of key parasite protease genes.
What is the mechanism of acquired resistance in these two mutants? Because biochemical characterization revealed altered PSAC activity, both studies proposed that in vitro resistance results from changes in the channel that reduce toxin uptake at the host erythrocyte membrane and thereby prevent access to intracellular targets. However, parasite survival and expansion under selective pressure are complex and there are alternative explanations that deserve examination. Moreover, a number of other ion channels have been proposed for the infected erythrocyte membrane [7], further complicating interpretation of the macroscopic measurements in the previous reports.
To address these concerns, we have now undertaken more rigorous characterization of these mutants. First, we determined that resistance to either leupeptin or blasticidin S confers minimal protection against the other agent. We then used in vitro selection to generate a new parasite mutant resistant to both blasticidin S and leupeptin. Cell-attached patch-clamp revealed distinct changes in single channel gating and conductance that were strictly associated with each of the three mutant phenotypes, supporting the proposal of resistance acquired by selection of changes in a single ion channel type. Our study also provides insights into the PSAC's selectivity filter, which appears to have a surprising ability to allow permeation of a broad range of charged and uncharged solutes while maintaining the ability to distinguish between solutes of similar size, geometry, and charge.
2. Materials and methods
2.1 Growth inhibition assays
Isobologram analysis of growth inhibition by combinations of compound 2 with blasticidin S or leupeptin were performed using a SYBR green I-based fluorescence assay for parasite nucleic acid in 96-well format. Wild-type parasite cultures were synchronized in 5% D-sorbitol before seeding at 0.2 to 0.5% parasitemia and 5% hematocrit in RPMI 1640 supplemented with 25 mM HEPES, 2% serum, 50 mg/liter hypoxanthine, and defined dilutions of PSAC antagonist with toxin. Cultures were maintained for 3 days at 37°C in 5% O2–5% CO2. The plates were then subjected to freezing-thawing before addition of SYBR green I at twice the manufacturer's recommended final concentration, incubation for 30 min, and measurement of fluorescence (excitation and emission wavelengths of 485 and 528 nm, respectively). Background fluorescence was subtracted by use of control cultures killed by 20 μM chloroquine. IC50 values for each ratio of PSAC antagonist and toxin were estimated by linear interpolation. Similar results were obtained with the Indo 1 and HB3 laboratory parasite lines.
2.2 In vitro selection of FCB-2mut
Mutant parasite lines resistant to blasticidin S or leupeptin were selected by continuous cultivation of wild-type parasites as described previously [9, 16]. To select for a parasite resistant to both toxins, we challenged the single mutant lines FCB-br1 and HB3-leuR1 with 2.5 μg/mL blasticidin S and 50 μM leupeptin. After extended continuous cultivation (~ 6 months), a double mutant was generated from FCB-br1 on both of two separate attempts. The clone, FCB-2mut, was then obtained by limiting-dilution cloning. We were unsuccessful in attempts to generate a double mutant from the HB3-leuR1 line.
2.3 Leupeptin accumulation by intact cells
Leupeptin accumulation into erythrocytes infected with various parasite isolates were performed with a semi-quantitative assay that measures papain-mediated hydrolysis of Z-Phe-Arg-AMC and its inhibition by leupeptin from cell lysates [16]. We measured leupeptin content of intact cells after a 30 minute preincubation with 40 μM leupeptin in PBS. This leupeptin concentration and preincubation duration optimally distinguishes between uptake by infected and uninfected cells, permitting sensitive detection of PSAC-mediated uptake. Measurement of uptake entailed rapid washes to reduce extracellular leupeptin to undetectable levels, lysis of the infected cell membrane with 0.05% saponin, addition of the released erythrocyte cytosolic contents to a buffered papain and Z-Phe-Arg-AMC mixture, and detection of fluorescence. ZPhe-Arg-AMC is a peptide substrate that exhibits a marked fluorescence increase when digested by papain, a leupeptin-sensitive protease. Fluorescence measurements were normalized to % leupeptin content by comparison to matched controls incubated without leupeptin, which as defined as 0% content; complete inhibition of papain-mediated hydrolysis was set to 100% content. Control wild-type parasites were included in each experiment that examined parasite mutants, allowing direct comparison of accumulation.
2.4 Osmotic lysis kinetics
Continuous tracking of infected erythrocyte osmotic lysis kinetics in organic solutes was used to examine PSAC activity and was performed as described previously [24]. Trophozoite-infected erythrocytes were enriched by percoll-sorbitol separation, washed in PBS (150 mM NaCl, 20 mM Na2HPO4, pH 7.5),and resuspended at 37 °C and 0.15% hematocrit in permeant solutes (145 mM PhTMA+ chloride or 280 mM sorbitol) buffered with 20 mM Na-HEPES, 0.1 mg/ml BSA, pH 7.4. Osmotic swelling and lysis were then continuously monitored by recording transmittance of 700 nm light through the cell suspension (DU640 spectrophotometer with Peltier temperature control, Beckman Coulter). The resulting transmittance recordings were normalized so that 100% osmotic lysis of infected cells corresponds to the steady-state transmittance measured after extended incubation (typically 2 h). The time to 50% lysis was estimated by interpolation. The dose-response for inhibition by compound 2 was determined using osmotic lysis experiments with sorbitol, as described [24].
2.5 Electrophysiology
Cell-attached patch-clamp recordings of trophozoite-stage infected erythrocytes were obtained as described previously [8]. All recordings used symmetric bath and pipette solutions of 1,000 mM choline chloride, 115 mM NaCl, 10 mM MgCl2, 5 mM CaCl2, 20 mM Na-HEPES, pH 7.4. This hypertonic solution increases the signal-to-noise ratio for single PSAC detection by increasing Cl− flux through open channels and by reducing pipette electrical noise. Pipettes were fabricated from quartz capillaries and had resistances of 1 to 3 MO in the recording solution. Seal resistances were greater than 100 GO. Holding membrane potential was 0 mV; all recordings shown were obtained with pulses to −100 mV. We did not use perfusion of the bath. Voltage clamp recordings were obtained with an Axopatch 200B amplifier (Molecular Devices), filtered at 5 kHz, digitized at 100 kHz, and recorded with Clampex 9.0 software (Molecular Devices).
Dwell time distributions were determined from up to 75 seconds of single channel recordings for each isolate by using home-written code that detects mid-threshold crossings, uses linear interpolation of adjacent sample times, and corrects for a Gaussian filter risetime of 66.4 μs as described in detail previously [14]. Histogram ordinate values were normalized to percent of the total number of events detected under each condition. Histograms are displayed on square root-logarithmic plots because time constants for simple exponentially decaying processes are visible as maxima [25]. Spectral analyses were carried out as described previously [6, 26]. These analyses used continuous single channel recordings of up to 60 s in duration acquired at a membrane potential of −100 mV with minimal filtering (30 kHz Butterworth).
3. Results
3.1 Similar structures and resistance mechanisms for blasticidin S and leupeptin
Both blasticidin S and leupeptin carry a positively charged guanidinium moiety and have relatively high molecular weights (> 420 dal; Fig. 1A), two features that limit membrane permeability and, hence, toxicity against many cell types [27–30]. Both agents nevertheless kill in vitro cultures of P. falciparum and are thought to work against intraerythrocytic targets [17, 31]. How these toxins enter infected cells is therefore an important question and is not well-established. One possibility is that they enter infected erythrocytes via the plasmodial surface anion channel (PSAC), a broad permeability ion channel not present on uninfected cells [6]. Consistent with this possibility, both blasticidin S- and leupeptin-resistant malaria parasites exhibit marked changes in PSAC activity, suggesting acquisition of resistance primarily due to changes in this channel that yield reduced uptake of toxin [9, 16].
Fig. 1.
Toxicity of blasticidin S and leupeptin is reduced by a specific PSAC antagonist. (A) Structures of blasticidin S and leupeptin, two toxins that kill malaria parasites. Both are positively charged, bulky organic solutes that cross membranes poorly. (B) Structure of compound 2, a specific PSAC antagonist identified through a drug discovery project. (C) Dose response for inhibition of sorbitol-mediated osmotic lysis by compound 2. Solid line represents the best fit to y = 1/(1 + (x/K0.5)) with a K0.5 of 84 nM. (D) Single channel recordings obtained without (top trace) or with 500 nM compound 2 (remaining traces) in both bath and pipette. Dashes next to each trace reflect the closed channel level; scale bars beneath the traces represent 1 s (horizontal) and 2 pA (vertical). (E) Isobolograms for parasite growth inhibition by mixtures of compound 2 with blasticidin S or leupeptin (left and right panels, respectively). Each symbol represents concentrations of toxin and compound 2 that produce 50% parasite killing (abscissa and ordinate, respectively) and is the mean of two independent experiments. The solid line represents the line of additivity, expected for non-interacting drug combinations. Note that the symbols are shifted to the right of this line in each panel, indicating that compound 2 antagonizes killing by both toxins.
We began with an independent test of this possibility by examining growth inhibition by blasticidin S or leupeptin in the presence of a recently identified high affinity PSAC antagonist (compound 2, Fig. 1B–D; [32]). Because this and other PSAC antagonists also kill parasites [33], we performed dose responses of combinations of each toxin with compound 2 and quantified parasite killing using SYBR Green I-based detection of parasite DNA. The relative killing by toxin and channel antagonist in each mixture was determined through isobologram analyses as shown in Fig. 1E. In these graphs, the IC50 values for these combinations were significantly shifted to the right of the line of additive effect (solid diagonal), expected for noninteracting combinations. Moreover, the direction of these shifts indicates that blasticidin S and leupeptin toxicities are reduced by the PSAC antagonist. While similar antagonism of leupeptin toxicity has been reported with NPF-1, a known PSAC antagonist [16], it has not been previously demonstrated for blasticidin S. Similar antagonism by two unrelated PSAC inhibitors is strong evidence against the alternate hypothesis that NPF-1 and 2 interfere with leupeptin or blasticidin S toxicity via a mechanism other than inhibited uptake at the erythrocyte membrane. These observations suggest PSAC-mediated uptake of blasticidin S and leupeptin is an essential step in their toxic effects.
3.2 Limited cross-resistance and generation of a double mutant
In light of the similar structures of these toxins and the apparent similarities in their acquiredresistance mechanisms, we next wondered whether these parasite mutants exhibit cross-resistance. The blasticidin S-resistant parasite clone could only be generated from the FCB laboratory isolate and is therefore called FCB-br1; the leupeptin resistant isolate could only be generated from the HB3 wild-type isolate and is here referred to as HB3-leuR1. In light of the requirement of specific parasite genetic backgrounds, we examined growth rates of both wild-type parents and each mutant. In the absence of either toxin, each of these parasites grew well with increasing parasitemia every 48 h, the duration of the intraerythrocytic cycle (Fig. 2A). As reported previously [9], the blasticidin S-resistant parasite, FCB-br1, exhibited smaller increases in parasitemia with each cycle. Addition of either 50 μM leupeptin or 2.5 μM blasticidin S rapidly sterilized cultures of both wild-type parents, but allowed expansion of the corresponding mutant (Fig. 2B and C, respectively). Importantly, these experiments revealed minimal cross-resistance because each single mutant was quickly killed by the other toxin. Simultaneous application of both blasticidin S and leupeptin cleared cultures of either mutant (Fig. 2D).
Fig. 2.
Propagation of wild-type and mutant parasites without toxins (A), with leupeptin or blasticidin S alone (B and C, respectively), or with both toxins (D). In each panel, symbols represent % parasitemia determined by daily examination of Giemsa-stained smears after seeding 0.4% ring-stage synchronous cultures. Parasite isolates are distinguished with different symbols as: FCB (filled triangles), FCB-br1 (white triangles), FCB-2mut (red triangles), HB3 (filled circles), HB3-leuR1 (white circles). Notice that cultures of each single mutant are rapidly killed by the other toxin and that only the double mutant FCB-2mut survives when both toxins are applied.
We next used in vitro selection to generate a parasite mutant resistant to both blasticidin S and leupeptin because it may carry additional distinct changes in PSAC. This parasite, referred to here as FCB-2mut, shares the slow growth of the FCB-br1 single mutant in the absence of drug pressure and can be propagated in the presence of either or both toxins (red symbols, Fig. 2). We also attempted to create a double mutant by challenging the leupeptin resistant HB3-leuR1 isolate with 2.5 μg/mL blasticidin S, but were unable on three separate selections. Wild-type FCB parasites were also challenged with 50 μM leupeptin alone, but these selections failed to generate leupeptin resistance (n = 3 attempts), suggesting that changes in the channel acquired during selection with blasticidin S resistance are permissive for generation of leupeptin resistance. Similarly, we have not been able to generate blasticidin S resistance by in vitro selections using the HB3 isolate (n = 4). These findings, summarized in Fig. 3, suggest a requirement for a certain genetic background in the generation of each mutant. The wild-type isolates used here presumably carry polymorphisms in one or more parasite genes that are permissive for accrual of resistance mutations.
Fig. 3.
Schematic summarizing the selection of each mutant and requirement for permissive genetic backgrounds. (A) HB3 is the only wild-type isolate that has successfully yielded leupeptin-resistant parasites, but it is incapable of acquiring blasticidin S resistance. (B) FCB can acquire blasticidin S resistance, which is lost upon removal of selective pressure (reverse arrow, described below). Although leupeptin resistance can be selected from the blasticidin S mutant (FCB-br1), the wild-type FCB parent appears unable to acquire leupeptin resistance directly. The double mutant parasite does not undergo loss of resistance upon removal of drug pressure. The mutants in panel B are shown as containing fewer merozoite progeny because of their slower growth rates (Fig. 2A), suggesting a fitness cost associated with resistance. Leu, leupeptin; blas, blasticidin S.
3.3 Reduced leupeptin permeability in the double mutant
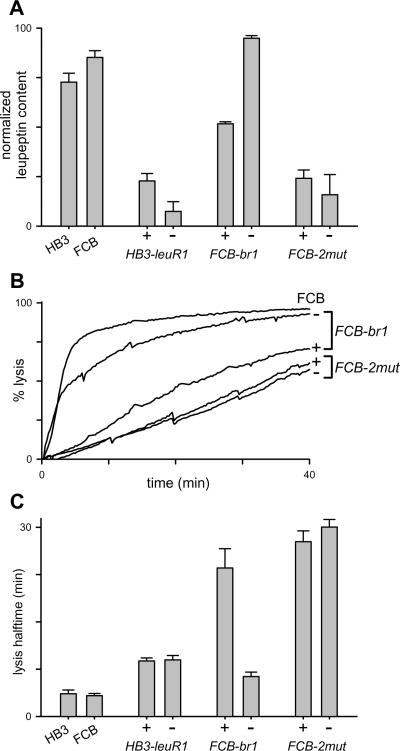
We next used a semi-quantitative permeability assay to measure leupeptin uptake by erythrocytes before and after infection with each of these parasite isolates to examine possible mechanisms of resistance (Fig. 4). Uninfected erythrocytes exhibited low leupeptin uptake in this assay [16]. Erythrocytes infected with either of the two wild-type parasite isolates, FCB and HB3, exhibited high leupeptin permeability that was attributed to PSAC because it could be largely abolished by compound 2, the specific PSAC inhibitor used in Fig. 1. Leupeptin permeability was also affected by selection of blasticidin S- or leupeptin-resistant mutants. Selection with blasticidin S yielded only a modest reduction in leupeptin permeability (FCB-br1 isolate). This intermediate value may produce limited cross resistance between blasticidin S and leupeptin, but is not sufficient to permit continuous propagation of FCB-br1 cultures in the presence of 50 μM leupeptin. Selection of the double mutant from the FCB-br1 background reduced leupeptin permeability further, reaching a level similar to that in the HB3-leuR1 single mutant. These findings are consistent with a threshold level of leupeptin uptake required for parasite killing in vitro. They also suggest that the double mutant isolate may be resistant to leupeptin because of additional changes in this ion channel.
Fig. 4.

Leupeptin accumulation in wild-type and mutant infected cells reveal a pattern consistent with resistance phenotypes. Leupeptin content of cells infected with indicated isolates was measured as described in the Materials and Methods and is represented as normalized mean ± S.E.M. of up to 13 independent trials for each isolate. Wild-type and drug-resistant parasites are shown as grey and black bars, respectively. Uptake is PSAC-mediated because it is inhibited by 5 μM compound 2 (HB3 + 2). This inhibitor also reduced uptake by FCB-infected cells (not shown). Uninfected erythrocytes lack PSAC activity and exhibit much lower uptake (Uninf). While blasticidin S resistance confers some reduction in leupeptin uptake (FCB-br1 relative to FCB, P < 0.001), both leupeptin resistant mutants exhibit significantly lower uptake (HB3-leuR1 and FCB-2mut, P = 0.001 in comparisons to FCB-br1).
3.4 Distinct PSAC single channel properties for each mutant
To examine this and other possibilities, we performed cell-attached patch-clamp of erythrocytes infected with each parasite and obtained single channel recordings. With each isolate, only a single ion channel type was detected under our recording conditions. Inspection of these recordings reveals that while some single channel properties are similar among these five isolates, there are also important differences. Most obviously, the frequency and timing of transitions between open and closed states, commonly referred to as “gating”, differ markedly between isolates (Fig. 5). These differences were reproducible in recordings from multiple separate cells as demonstrated with individual traces from 3 separate cells for each parasite in Fig. 5B.
Fig. 5.
Single channel recordings on erythrocytes infected with each wild-type or mutant parasite, as indicated. (A) Black scale bars beneath traces represent 100 ms (horizontal) and 3pA (vertical). (B) For each parasite, the three traces shown reflect recordings from three separate cells. Scale bars represent 10 ms and 3 pA for all traces in this panel. Red and green dashes flanking each trace represent the closed and open channel states, respectively. FCB-br1 channels also exhibit an intermediate conductance level [9]. Notice that each isolate's characteristic channel gating behavior is reproducible and distinct.
As described previously [9], recordings on erythrocytes infected with the FCB-br1 isolate revealed frequent transitions to an intermediate conductance level. Interestingly, in vitro selection of the double mutant from the FCB-br1 background abolished this subconductance state and reestablished relatively simpler transitions between the open and closed states (FCB-2mut traces). As apparent from the greater amplitude of opening transitions in the FCB-2mut traces, the double mutant exhibits a modest, but statistically significant increased single channel conductance (Fig. 6, P = 10−5, Student's t test).
Fig. 6.

Chord conductances for channels detected on indicated parasites. Conductances were calculated from cell-attached recordings at −100 mV relative to the holding potential of 0 mV. n = 12–22 channel molecules for each parasite.
To allay concerns about biased selection of traces, we analyzed the durations of open and closed channel events, known as dwell times, from extended single channel recordings on the mutant and wild-type infected cells. Fig. 7A shows the resulting all points histograms generated from individual channel molecules from FCB and FCB-2mut; Fig. 7B displays the results for HB3 and HB3-leuR1.
Fig. 7.
Dwell time distributions reveal significantly differing gating properties for the mutants. (A) Comparison of PSAC gating properties for FCB and FCB-2mut (black and red traces, respectively). (B) Comparison of gating properties of channels from HB3 and HB3-leuR1 (black and red traces, respectively). Each histogram in these 4 panels corresponds to all points binning of tallied durations from up to 104,000 channel events. In both (A) and (B), the left and right panels show distributions of open and closed channel durations, respectively. (C) Power spectra of single channel recordings from HB3-leuR1 (green traces), FCB-br1 (black traces), and FCB-2mut (red traces). For each mutant, the three traces reflect analyses from three separate channel molecules. The clustering of each group indicates reproducible differences between these mutant channels. Notice especially, that selection of FCB-2mut from the FCB-br1 background produces marked changes in channel properties. The dashed black line is an arbitrary line with a slope of 1/f and is included to permit comparison to the slope of each mutant channel's power spectrum.
For each isolate, the distributions of open durations revealed a single unambiguous peak (left panels in Figs. 7A and 7B). The open distributions for FCB-2mut and both wild-type isolates were adequately fitted by the probability density function for a single exponentially decaying open channel state as described previously [14], but that of HB3-leuR1 was somewhat more complicated (not shown). The mean open duration of the wild-type isolates were 0.20–0.24 ms, matching a previous estimate for channels on the Indo 1 isolate [14]. These similar values suggest conservation of wild-type channel gating because these three parasites were isolated from patients in different parts of the world. In contrast, channels induced by FCB-2mut and HB3-leuR1 exhibited marked increases in mean open durations with measured values of 0.67 ms and 1.46 ms, respectively (P < 10−4 in comparisons to corresponding wild-type clones, Mann-Whitney U test). The distributions of closed durations were more complex and were not clearly altered in either mutant (right panels in Figs. 7A and 7B). As described previously [14], these closed distributions suggest a minimum of 3 distinct closed states for both wild-type and mutant channels.
Dwell time distributions were not calculated for the FCB-br1 mutant because the subconductance state present in its recordings confounded interpretation. We therefore performed power spectral analyses for all three mutants. As described previously for wild-type PSAC activity [6], this analysis revealed complex 1/f spectra for each mutant. Importantly, spectra from separate channel molecules on each mutant cell line revealed reproducible differences most apparent at low frequencies (Fig. 7C). These findings further substantiate the observed differences in PSAC gating associated with selection of each mutant phenotype. Complex 1/f spectra are consistent with multiple kinetic states for this channel as implicated by the closed dwell time distributions. Fluctuations with this type of spectra have been reported for several other channels but their mechanisms are still debated [26, 34–37].
3.5 Differing abilities of mutants to revert upon removal of selective pressure
FCB-br1 was previously shown to revert to a wild-type phenotype when blasticidin S pressure is removed from in vitro cultures [9]. This reversion process was completed within several weeks and includes restoration of wild-type sensitivity to killing by blasticidin S, organic solute permeability, and single channel gating. In contrast, HB3-leuR1 exhibited stable leupeptin resistance and altered PSAC activity despite removal of leupeptin pressure for 3 months [16]. We therefore wondered about the stability of the double mutant channel phenotype when both leupeptin and blasticidin S are removed. Examination of the phenotype's stability upon removal of drug pressure is important because it can shed light on the molecular mechanisms responsible for resistance.
Figure 8A shows leupeptin accumulation into cells infected by each mutant before and after drug pressure removal. When blasticidin S pressure is removed from FCB-br1 cultures, we found that leupeptin content increased from its intermediate value to wild-type levels within 4 weeks, providing an independent demonstration of this mutant's ability to undergo rapid reversion. HB3-leuR1's low leupeptin permeability did not revert despite measurements after up to 4 months of in vitro culture without leupeptin pressure. Leupeptin uptake by the FCB-2mut isolate also remained low despite 4 months of culture without either blasticidin S or leupeptin.
Fig. 8.
Differing capacities of mutants to revert to wild-type phenotype upon removal of selective pressure. (A) Normalized leupeptin accumulation into cells infected with indicated isolates. In this and subsequent panels, uptake is shown into mutant infected cells obtained from cultures under continuous selective pressure or after extended propagation without either toxin (+ and −, respectively). Notice that FCB-br1 undergoes reversion to a level matching its wild-type parent (FCB) after removal of selective pressure, but that the other two mutants exhibit stable reductions. (B) Osmotic lysis kinetics of cells infected with indicated isolates in PhTMA+ chloride. For each cell type, PhTMA+ uptake is the rate limiting determinant of lysis kinetics. Notice faster kinetics of lysis upon blasticidin S removal for FCB-br1, but sustained slow PhTMA+ uptake in FCB-2mut after culturing without either toxin. (C) Mean ± S.E.M. osmotic lysis halftimes determined from kinetic experiments as in (B). The measured halftime is inversely proportional to PhTMA+ permeability [24]. While reversion from a resistant phenotype is apparent as an increase in leupeptin uptake in (A), it is reflected by a decrease in lysis halftime in (C).
As an independent test, we also examined reversion more quantitatively by tracking osmotic lysis of cells in isotonic solutions of phenyltrimethylammonium (PhTMA+), an organic cation with high PSAC permeability [38]. We selected this solute because it has relatively well preserved permeability in each mutant (Figs. 8B and 8C), permitting accurate estimation of lysis half-time. As found with leupeptin uptake measurements, these experiments revealed that FCB-br1 rapidly loses its reduced PhTMA+ permeability upon removal of drug pressure, but HB3-leuR1 and FCB-2mut do not. Finally, patch-clamp studies have demonstrated that the altered channel gating on FCB-br1-infected erythrocytes is lost when the cultures are maintained without blasticidin S [9]; our single channel recordings with HB3-leuR1 and FCB-2mut did not reveal reversion of altered gating (not shown).
FCB-2mut combines the more marked reduction in PhTMA+ permeability of the blasticidin S single mutant with the greater reduction in leupeptin permeability seen in the leupeptin single mutant. Its lack of restored PhTMA+ permeability after removal of both toxins suggests genome-level changes required for addition of leupeptin resistance stabilize the changes selected by blasticidin S. Consistent with these stable changes in transport properties, FCB-2mut cultures exhibited unchanged growth rates when challenged with both leupeptin and blasticidin S after culturing for 4 months without these toxins (not shown).
4. Discussion
Mutant selection in microbes, as used in the present study, is an important research tool in the study of cellular biology and biochemistry. The primary advantage of single-celled microbes over higher organisms for such studies is the large number of independent wild-type cells that can be screened for desired mutations. Classical studies in viruses, bacteria and yeasts have identified countless mutants under stringent selective conditions [39, 40]. A number of mutant selections have also been carried out in P. falciparum [41–43], despite this organism's significantly slower replication rate and more restrictive growth requirements.
How might leupeptin and blasticidin S select for altered PSAC activity? Because the genetic basis of this activity is presently unknown, we consider several possibilities. First, channel activity may reflect one or more parasite-encoded proteins trafficked to the host membrane. In this scenario, the toxins could select for mutations in the responsible parasite genes to yield channels that produce reduced toxin uptake by altering key residue(s) involved in solute permeation. Second, the channel may be endogenous to the host membrane, but may require activation by one or more parasite-encoded enzymes [44, 45]. Here, mutations in these enzymes may be selected by the toxins to alter activation, yielding modified channels that limit toxin uptake. Third, the channel may consist of both host- and parasite-encoded proteins such that mutations in the parasite component can lead to altered channel activity. Fourth, it is possible that toxin exposure affects parasite metabolism and leads to production of soluble modulators that alter channel activity [46]. This scenario would require that some of these changes in metabolism be irreversible because channels on HB3-leuR1 and FCB-2mut do not revert to wild-type channels despite removal of toxin for many generations. Although functional polymorphisms between geographically divergent parasites not subjected to selection with toxins suggest at least one parasite-encoded channel subunit [8, 10], definitively distinguishing between these possibilities will require identification of the channel's gene(s).
Because some studies have suggested multiple distinct ion channels on the infected erythrocyte membrane [7], we wondered whether leupeptin and blasticidin S might be selecting for changes in separate parasite-induced ion channels. Several observations suggest effects on a single ion channel type. First, we tallied our experience with cell-attached patch-clamp of the isolates presented here. A total of 317 patches with seal resistance > 50 gigaohms were examined from the two wild-type isolates and compared to patches from the three mutants (n between 77 and 196 patches for each mutant). Overall, 26 % of patches yielded one or more channels. Although there was some molecule-to-molecule variation, we never detected the characteristic gating behavior of any of the three mutant lines on cells infected with either wild-type parasite; instead, gating behavior was highly reproducible and distinct between the five cloned parasites we examined (Fig. 5), suggesting that both toxins select for changes in a single channel type. Second, although there were significant differences in open channel dwell distributions, the closed channel distributions were essentially identical on our parasite lines and quite different from other well-characterized ion channels. Conservation of closed durations along with similar single channel conductances on these isolates also suggests relatively modest changes in a single channel type. Finally, two distinct PSAC inhibitors have parallel effects on parasite killing by these two toxins (Fig. 1E; [16]), suggesting a single shared uptake mechanism. The similar properties of the channels detected (closed channel durations, 1/f power spectra, single channel conductance, and pharmacology) combined with the failure to detect mutant channel behavior on wild-type parasite isolates despite intensive efforts implicate alterations of a single ion channel type in the selection of resistance to one or both antimalarial toxins. Nevertheless, we cannot exclude contributions of unrelated channels that might have escaped detection by our study.
Solutes that share a single ion channel may compete for binding sites within the pore and reduce each other's flux. We examined this possibility and found no detectable slowing of sorbitol uptake in osmotic lysis experiments by either 5 μM blasticidin S or 50 μM leupeptin. We also performed single channel patch-clamp in the presence of 100 μM leupeptin and did not see significant changes in PSAC conductance or dwell time distributions (data not shown). This lack of effect is consistent with the poor saturability of this channel [6, 8]. Indeed, competition between permeating solutes is a complex function of channel and solute geometries, the strength of solute interactions within the pore, and solute diffusion coefficients, which may vary along the length of the channel pore [47]. Thus, competition between solutes in a pore frequently escapes detection.
Only certain laboratory parasite isolates appear capable of accruing the changes required for resistance to leupeptin or blasticidin S (Fig. 3), suggesting DNA-level polymorphisms in one or more parasite genes that are permissive for selection of resistance. Despite multiple attempts with other parasite isolates, we successfully generated channel-mediated blasticidin S resistance in only the FCB laboratory isolate. This may account for the successful use of this toxin in parasite DNA transfection experiments that select for plasmid-encoded blasticidin S deaminase (BSD): most of those studies utilized other parasite isolates [17, 19, 20]. Continued, cautious use of this reagent seems appropriate, especially because there are only a few selectable markers available in this system. Acquired leupeptin resistance via reduced channel-mediated permeability also required use of permissive parasite isolates in our hands (HB3 and FCB-br1, but not FCB). Identification of the channel's gene(s) may help reveal the molecular basis of these isolate-specific differences.
We noticed that the concentrations of leupeptin and blasticidin S required to kill parasite cultures are significantly higher than those required to inhibit their intracellular targets. For example, leupeptin inhibits falcipains, a family of proteases in the parasite's digestive vacuole, with low nanomolar affinity [48], but parasite growth inhibition is not seen until ~ 104-fold higher extracellular concentrations are applied. Blasticidin S also appears to inhibit protein synthesis by cellular extracts with ~10-fold greater affinity than one might predict from in vitro parasite killing [49]. These discrepancies are consistent with our studies that suggest toxin uptake at the erythrocyte membrane is rate-limiting. It is also consistent with pore sieving considerations that predict relatively low permeability for these large toxin molecules. Modest reductions in toxin permeability, such as we have selected, should be sufficient to confer in vitro resistance to killing.
While our single channel recordings reveal a correlation between gating and toxin resistance phenotypes, it is important to recognize that altered gating behavior does not directly contribute to acquired resistance. Instead, changes in channel solute selectivity that reduce toxin uptake (Fig. 4) are more likely to be directly responsible for toxin resistance; altered gating appears to be a byproduct and is fortuitous for our patch-clamp studies. This model is consistent with channel mutants in other systems, where relatively modest changes in sequence or structure can often have multiple effects on single channel properties [50, 51]. We speculate that residues on the PSAC protein critical for determining the channel's solute selectivity overlap or interact with those involved in gating.
Our study highlights PSAC's remarkable ability to distinguish between structurally similar solutes despite a broad spectrum of solutes that can be transported. Leupeptin and blasticidin S are both linear hydrocarbon compounds, carry a charged guanidinium moiety, and have almost identical molecular weights; nevertheless, they can still be distinguished within the PSAC pore, as evidenced by their abilities to select for distinct in vitro resistance phenotypes and channel activities. Other studies have also pointed to unusual selectivity mechanisms in this channel. For example, the small Na+ ion is stringently excluded from permeation in spite of the broad permeability of larger organic cations [13]. Additional examples include the channel's ability to pass some amino acids and sugars while excluding others [52] and to transport proline and hydroxyproline by distinct mechanisms [53]. While several other channels can also distinguish similar solutes [54, 55], PSAC appears to be relatively unique in its ability to pass a diverse collection of solutes that cannot be precisely defined based on net charge, molecular weight, hydrophobicity, or other simple criteria. Our study extends the understanding of PSAC's selectivity filter by identifying mutants capable of fine-tuning the list of permeant solutes. How permeating solutes interact with the channel pore is presently unknown, but we propose that there may be multiple contact points that can be individually tweaked to produce changes in relative permeabilities of structurally similar solutes.
Our study also has important implications for antimalarial drug discovery programs targeting intraerythrocytic parasite activities. Water-soluble drug leads that are bulky or charged may require PSAC-mediated uptake to enter the erythrocyte and reach their targets. This requirement can be confirmed through straightforward examination of the lead compound's in vitro growth inhibitory efficacy in the presence of a known PSAC inhibitor (Fig. 1E); we suggest that such testing be incorporated into all antimalarial drug discovery and development programs. If decreased growth inhibitory effectiveness of an antimalarial drug lead is seen when PSAC inhibitors are added, there is a real concern that parasites may acquire resistance via the drug resistance mechanism described here.
Acknowledgements
We thank Ian Bathurst, the Medicines for Malaria Venture Project Director for these studies, and Michael Fay for help with statistical analysis. This research was funded by the Intramural Research Programs of the National Institutes of Health, NIAID and NICHD, and by the Medicines for Malaria Venture (MMV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat. Rev. Microbiol. 2009;7:341–354. doi: 10.1038/nrmicro2110. [DOI] [PubMed] [Google Scholar]
- 2.van Ooij C, Tamez P, Bhattacharjee S, Hiller NL, Harrison T, Liolios K, Kooij T, Ramesar J, Balu B, Adams J, Waters A, Janse C, Haldar K. The malaria secretome: from algorithms to essential function in blood stage infection. PLoS. Pathog. 2008;4:e1000084. doi: 10.1371/journal.ppat.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilley L, Hanssen E. A 3D view of the host cell compartment in P. falciparum-infected erythrocytes. Transfus. Clin. Biol. 2008;15:72–81. doi: 10.1016/j.tracli.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Ginsburg H, Krugliak M, Eidelman O, Cabantchik ZI. New permeability pathways induced in membranes of Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 1983;8:177–190. doi: 10.1016/0166-6851(83)90008-7. [DOI] [PubMed] [Google Scholar]
- 5.Kirk K, Horner HA, Elford BC, Ellory JC, Newbold CI. Transport of diverse substrates into malaria-infected erythrocytes via a pathway showing functional characteristics of a chloride channel. J. Biol. Chem. 1994;269:3339–3347. [PubMed] [Google Scholar]
- 6.Desai SA, Bezrukov SM, Zimmerberg J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406:1001–1005. doi: 10.1038/35023000. [DOI] [PubMed] [Google Scholar]
- 7.Staines HM, Alkhalil A, Allen RJ, De Jonge HR, Derbyshire E, Egee S, Ginsburg H, Hill DA, Huber SM, Kirk K, Lang F, Lisk G, Oteng E, Pillai AD, Rayavara K, Rouhani S, Saliba KJ, Shen C, Solomon T, Thomas SL, Verloo P, Desai SA. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int. J Parasitol. 2007;37:475–482. doi: 10.1016/j.ijpara.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhalil A, Cohn JV, Wagner MA, Cabrera JS, Rajapandi T, Desai SA. Plasmodium falciparum likely encodes the principal anion channel on infected human erythrocytes. Blood. 2004;104:4279–4286. doi: 10.1182/blood-2004-05-2047. [DOI] [PubMed] [Google Scholar]
- 9.Hill DA, Pillai AD, Nawaz F, Hayton K, Doan L, Lisk G, Desai SA. A blasticidin S-resistant Plasmodium falciparum mutant with a defective plasmodial surface anion channel. Proc Natl Acad Sci U S A. 2007;104:1063–1068. doi: 10.1073/pnas.0610353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkhalil A, Pillai AD, Bokhari AA, Vaidya AB, Desai SA. Complex inheritance of the plasmodial surface anion channel in a Plasmodium falciparum genetic cross. Mol Microbiol. 2009;72:459–469. doi: 10.1111/j.1365-2958.2009.06661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisk G, Desai SA. The plasmodial surface anion channel is functionally conserved in divergent malaria parasites. Eukaryot. Cell. 2005;4:2153–2159. doi: 10.1128/EC.4.12.2153-2159.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alkhalil A, Hill DA, Desai SA. Babesia and plasmodia increase host erythrocyte permeability through distinct mechanisms. Cell Microbiol. 2007;9:851–860. doi: 10.1111/j.1462-5822.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 13.Cohn JV, Alkhalil A, Wagner MA, Rajapandi T, Desai SA. Extracellular lysines on the plasmodial surface anion channel involved in Na+ exclusion. Mol. Biochem. Parasitol. 2003;132:27–34. doi: 10.1016/j.molbiopara.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Desai SA. Open and closed states of the plasmodial surface anion channel. Nanomedicine. 2005;1:58–66. doi: 10.1016/j.nano.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Lisk G, Scott S, Solomon T, Pillai AD, Desai SA. Solute-inhibitor interactions in the plasmodial surface anion channel reveal complexities in the transport process. Mol Pharmacol. 2007;71:1241–1250. doi: 10.1124/mol.106.030734. [DOI] [PubMed] [Google Scholar]
- 16.Lisk G, Pain M, Gluzman IY, Kambhampati S, Furuya T, Su XZ, Fay MP, Goldberg DE, Desai SA. Changes in the plasmodial surface anion channel reduce leupeptin uptake and can confer drug resistance in P. falciparum-infected erythrocytes. Antimicrob. Agents Chemother. 2008;52:2346–2354. doi: 10.1128/AAC.00057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mamoun CB, Gluzman IY, Goyard S, Beverley SM, Goldberg DE. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8716–8720. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crabb BS, Rug M, Gilberger TW, Thompson JK, Triglia T, Maier AG, Cowman AF. Transfection of the human malaria parasite Plasmodium falciparum. Methods Mol. Biol. 2004;270:263–276. doi: 10.1385/1-59259-793-9:263. [DOI] [PubMed] [Google Scholar]
- 19.Epp C, Raskolnikov D, Deitsch KW. A regulatable transgene expression system for cultured Plasmodium falciparum parasites. Malar. J. 2008;7:86. doi: 10.1186/1475-2875-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonilla JA, Moura PA, Bonilla TD, Yowell CA, Fidock DA, Dame JB. Effects on growth, hemoglobin metabolism and paralogous gene expression resulting from disruption of genes encoding the digestive vacuole plasmepsins of Plasmodium falciparum. Int. J Parasitol. 2007;37:317–327. doi: 10.1016/j.ijpara.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal PJ, McKerrow JH, Aikawa M, Nagasawa H, Leech JH. A malarial cysteine proteinase is necessary for hemoglobin degradation by Plasmodium falciparum. J. Clin. Invest. 1988;82:1560–1566. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debrabant A, Delplace P. Leupeptin alters the proteolytic processing of P126, the major parasitophorous vacuole antigen of Plasmodium falciparum. Mol. Biochem. Parasitol. 1989;33:151–158. doi: 10.1016/0166-6851(89)90029-7. [DOI] [PubMed] [Google Scholar]
- 23.Moura PA, Dame JB, Fidock DA. Evaluation of the role of Plasmodium falciparum digestive vacuole plasmepsins in the specificity and antimalarial mode of action of cysteine and aspartic protease inhibitors. Antimicrob. Agents Chemother. 2009;53:4968–4978. doi: 10.1128/AAC.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner MA, Andemariam B, Desai SA. A two-compartment model of osmotic lysis in Plasmodium falciparum-infected erythrocytes. Biophys. J. 2003;84:116–123. doi: 10.1016/S0006-3495(03)74836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigworth FJ, Sine SM. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys. J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bezrukov SM, Winterhalter M. Examining noise sources at the single-molecule level: 1/f noise of an open maltoporin channel. Phys. Rev. Lett. 2000;85:202–205. doi: 10.1103/PhysRevLett.85.202. [DOI] [PubMed] [Google Scholar]
- 27.Contreras A, Carrasco L. Selective inhibition of protein synthesis in virus-infected mammalian cells. J. Virol. 1979;29:114–122. doi: 10.1128/jvi.29.1.114-122.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iordanov MS, Pribnow D, Magun JL, Dinh TH, Pearson JA, Chen SL, Magun BE. Ribotoxic stress response: activation of the stress-activated protein kinase JNK1 by inhibitors of the peptidyl transferase reaction and by sequence-specific RNA damage to the alpha-sarcin/ricin loop in the 28S rRNA. Mol. Cell Biol. 1997;17:3373–3381. doi: 10.1128/mcb.17.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehdi S. Cell-penetrating inhibitors of calpain. Trends Biochem. Sci. 1991;16:150–153. doi: 10.1016/0968-0004(91)90058-4. [DOI] [PubMed] [Google Scholar]
- 30.Ishiguro J, Miyazaki M. Characterization of blasticidin S-resistant mutants of Saccharomyces cerevisiae. Curr. Genet. 1985;9:179–181. doi: 10.1007/BF00436968. [DOI] [PubMed] [Google Scholar]
- 31.Shenai BR, Semenov AV, Rosenthal PJ. Stage-specific antimalarial activity of cysteine protease inhibitors. Biol Chem. 2002;383:843–847. doi: 10.1515/BC.2002.089. [DOI] [PubMed] [Google Scholar]
- 32.Pillai AD, Pain M, Solomon T, Bokhari AA, Desai SA. A cell-based high-throughput screen validates the plasmodial surface anion channel as an antimalarial target. Mol Pharmacol. 2010 doi: 10.1124/mol.109.062711. doi:10.1124/mol.109.062711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang M, Lisk G, Hollingworth S, Baylor SM, Desai SA. Malaria parasites are rapidly killed by dantrolene derivatives specific for the plasmodial surface anion channel. Mol. Pharmacol. 2005;68:34–40. doi: 10.1124/mol.104.010553. [DOI] [PubMed] [Google Scholar]
- 34.Liebovitch LS, Sullivan JM. Fractal analysis of a voltage-dependent potassium channel from cultured mouse hippocampal neurons. Biophys. J. 1987;52:979–988. doi: 10.1016/S0006-3495(87)83290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fulinski A, Grzywna Z, Mellor I, Siwy Z, Usherwood PNR. Non-Markovian character of ionic current fluctuations in membrane channels. Phys. Rev. E. 1998;58:919–924. [Google Scholar]
- 36.Siwy Z, Fulinski A. Origin of 1/f noise in membrane channel currents. Phys. Rev. Lett. 2002;89:158101. doi: 10.1103/PhysRevLett.89.158101. [DOI] [PubMed] [Google Scholar]
- 37.Smeets RM, Keyser UF, Dekker NH, Dekker C. Noise in solid-state nanopores. Proc. Natl. Acad. Sci. U. S. A. 2008;105:417–421. doi: 10.1073/pnas.0705349105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staines HM, Rae C, Kirk K. Increased permeability of the malaria-infected erythrocyte to organic cations. Biochim. Biophys. Acta. 2000;1463:88–98. doi: 10.1016/s0005-2736(99)00187-x. [DOI] [PubMed] [Google Scholar]
- 39.Mortlock RP. Metabolic acquisitions through laboratory selection. Annu. Rev. Microbiol. 1982;36:259–84. doi: 10.1146/annurev.mi.36.100182.001355. 259-284. [DOI] [PubMed] [Google Scholar]
- 40.Spencer JF, Spencer DM. Mutagenesis in yeast. Methods Mol Biol. 1996;53:17–38. doi: 10.1385/0-89603-319-8:17. 17-38. [DOI] [PubMed] [Google Scholar]
- 41.Inselburg J. Isolation of temperature-sensitive mutants of Plasmodium falciparum. Mol Biochem. Parasitol. 1985;16:75–83. doi: 10.1016/0166-6851(85)90050-7. [DOI] [PubMed] [Google Scholar]
- 42.Dolan SA, Miller LH, Wellems TE. Selection of genetic variants from Plasmodium clones. Acta Leiden. 1991;60:93–99. [PubMed] [Google Scholar]
- 43.Singh A, Rosenthal PJ. Selection of cysteine protease inhibitor-resistant malaria parasites is accompanied by amplification of falcipain genes and alteration in inhibitor transport. J. Biol. Chem. 2004;279:35236–35241. doi: 10.1074/jbc.M404235200. [DOI] [PubMed] [Google Scholar]
- 44.Verloo P, Kocken CH, van der WA, Tilly BC, Hogema BM, Sinaasappel M, Thomas AW, De Jonge HR. Plasmodium falciparum-activated chloride channels are defective in erythrocytes from cystic fibrosis patients. J. Biol. Chem. 2004;279:10316–10322. doi: 10.1074/jbc.M311540200. [DOI] [PubMed] [Google Scholar]
- 45.Merckx A, Nivez MP, Bouyer G, Alano P, Langsley G, Deitsch K, Thomas S, Doerig C, Egee S. Plasmodium falciparum regulatory subunit of cAMP-dependent PKA and anion channel conductance. PLoS. Pathog. 2008;4:e19. doi: 10.1371/journal.ppat.0040019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanneur V, Duranton C, Brand VB, Sandu CD, Akkaya C, Kasinathan RS, Gachet C, Sluyter R, Barden JA, Wiley JS, Lang F, Huber SM. Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J. 2006;20:133–135. doi: 10.1096/fj.04-3371fje. [DOI] [PubMed] [Google Scholar]
- 47.Berezhkovskii AM, Bezrukov SM. Optimizing transport of metabolites through large channels: molecular sieves with and without binding. Biophys. J. 2005;88:L17–L19. doi: 10.1529/biophysj.104.057588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerr ID, Lee JH, Pandey KC, Harrison A, Sajid M, Rosenthal PJ, Brinen LS. Structures of falcipain-2 and falcipain-3 bound to small molecule inhibitors: implications for substrate specificity. J Med. Chem. 2009;52:852–857. doi: 10.1021/jm8013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi H, Yamamoto C, Tanaka N. Inhibition of protein synthesis by blasticidin S. I. Studies with cell-free systems from bacterial and mammalian cells. J. Biochem. (Tokyo) 1965;57:667–677. [PubMed] [Google Scholar]
- 50.Shen XM, Fukuda T, Ohno K, Sine SM, Engel AG. Congenital myasthenia-related AChR delta subunit mutation interferes with intersubunit communication essential for channel gating. J Clin. Invest. 2008;118:1867–1876. doi: 10.1172/JCI34527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ginsburg H, Kutner S, Krugliak M, Cabantchik ZI. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol. Biochem. Parasitol. 1985;14:313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 53.Bokhari AA, Solomon T, Desai SA. Two distinct mechanisms of transport through the plasmodial surface anion channel. J. Membr. Biol. 2008;226:27–34. doi: 10.1007/s00232-008-9136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J Physiol. 1993;264:C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 55.Rostovtseva TK, Komarov A, Bezrukov SM, Colombini M. VDAC channels differentiate between natural metabolites and synthetic molecules. J Membr. Biol. 2002;187:147–156. doi: 10.1007/s00232-001-0159-1. [DOI] [PubMed] [Google Scholar]