Abstract
The aim of this study was to investigate modifiable predictors of vitamin D status in healthy individuals, aged 55-74, and living across the USA. Vitamin D status [serum 25-hydroxyvitamin D (25(OH)D)] was measured along with age and season at blood collection, demographics, anthropometry, physical activity (PA), diet, and other lifestyle factors in 1357 male and 1264 female controls selected from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) cohort. Multivariate linear and logistic regression analyses were used to identify associations with vitamin D status. Three, 29 and 79% of the population had serum 25(OH)D levels <25, <50 and <80 nmol/L, respectively. The major modifiable predictors of low vitamin D status were low vitamin D dietary and supplement intake, body mass index (BMI) >30 kg/m2, physical inactivity (PA) and low milk and calcium supplement intake. In men, 25(OH)D was determined more by milk intake on cereal and in women, by vitamin D and calcium supplement and menopausal hormone therapy (MHT) use. Thus targeting an increase in vigorous activity and vitamin D and calcium intake and decreasing obesity could be public health interventions independent of sun exposure to improve vitamin D status in middle-aged Americans.
Keywords: Vitamin D status, 25(OH)D, vitamin D deficiency, exercise, physical activity, obesity, body mass index(BMI), vitamin D dietary and supplement intake, calcium supplement intake, menopausal hormone therapy (MHT), milk intake
1. INTRODUCTION
Established determinants of vitamin D status, as measured by serum 25-hydroxyvitamin D (25(OH)D) are exposure to sunlight and intake of vitamin D, either from foods or vitamin supplements [1-3]. Decreased physical activity (PA) and obesity and low social status have also been associated with low vitamin D levels in Europe and the USA [4-6]. The aim of the present study was to investigate predictors of vitamin D status within a large non-elderly population of healthy men and women living across the USA.
2. MATERIALS AND METHODS
The subjects in this study were healthy controls, age-matched to case distributions, selected for five case-control studies of serum vitamin D and cancer nested within the original Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) cohort. The PLCO Cancer Screening Trial is a large randomized controlled multicenter trial in the United States of approximately 155 000 men and women at sites in Birmingham, AL; Denver, CO; Detroit, MI; Honolulu, HI; Marshfield, WI; Minneapolis, MN; Pittsburgh, PA; Salt Lake City, UT; St Louis, MO; and Washington, DC, that was designed to evaluate selected methods for the early detection of these four cancers as well as non-Hodgkins lymphoma (NHL), breast and pancreatic cancer: enrollment began November 1, 1993, and ended June 30, 2001 [7]. Details of these studies of colorectal adenoma, non-Hodgkins lymphoma (NHL) and prostate, breast, and pancreatic cancer are described elsewhere [8-12]. Briefly, we included, 399 controls used for a study of colorectal adenoma (matched to cases by gender and race) [10], 286 controls used for a study of non-Hodgkin lymphoma [11], 713 controls used for a study of prostate cancer (matched to cases by age, time since screening, and year of follow-up) [8], 932 controls used for a study of breast cancer (matched to cases by age and year of blood draw) [9], and 350 used for a study of pancreatic cancer (matched to cases by age, gender, race and date of blood draw) [12]. Of these controls, 59 were included in more than one study; thus, in total 2621 control subjects from PLCO were included in this present data analysis. At the initial screening, all participants were asked to complete a questionnaire including demography, anthropometry, lifestyle factors (including smoking history and vigorous physical activity (PA) during the last year), and usual dietary intake over the 12 months before enrolment (137-item food frequency questionnaire and 14 questions about intake of vitamin and mineral supplements [13]). Daily nutrient intake from foods was calculated by multiplying the reported frequency of consumption of each food item by the nutrient composition of the imputed gender-specific portion size using the nutrient database from the U.S. Department of Agriculture [14]. Calcium and vitamin D intake were measured both from food and supplemental sources. Serum samples were collected during the baseline visit, and stored at −70 °C. Levels of the serum 25(OH)D for subjects were determined using a radio-iodinated tracer assay in the laboratories of Hollis and Horst [8-12, 15]. Replicate blinded quality control samples from 2 to 4 different individuals were included in all 25(OH)D batches. The overall coefficients of variation were 16.3% for the colorectal adenoma, 11.4 % for NHL, 5.9% for prostate, 8.2% for the breast study, and 4.7% for the pancreas study. Separate variables, representing each of the ten study centers across the USA and each of the five nested cancer case-control studies were entered as confounders in all models. Age at blood draw and pack-years of smoking, current smoking and educational level were entered as a priori confounders in all models. Total dietary energy was also added as a confounder in order to adjust for individual variation in total energy intake. As the aim of this study was to identify predictors of 25(OH)D levels, initial data screening was performed in order to identify statistically significant and biologically meaningful variables associated with continuous and categorical vitamin D status. T-tests for continuous variables and chi-square tests for categorical variables were used to determine statistical significance with two-sided p-values less than 0.01. Those unadjusted factors found to be significant were then included in a forward stepwise multiple linear regression analysis in order to ascertain the independent predictors of serum 25(OH)D either continuously or < 50 nmol/L 25(OH)D, respectively. Linear trends of ordered categorical variables were assessed using ordinal values consisting of the mid-range values for each category and applying a likelihood ratio test [16].
3. RESULTS
3.1 Descriptive characteristics of the population by 25(OH)D level are presented in Table 1
Table 1.
Characteristics of seasonal, demographic, anthropometric, lifestyle and dietary factors by serum 25(OH)D in US men and women living across the USA
| Predictor Variables | Total n=2621 | 25(OH)D <37 nmol/L n=305 |
25(OH)D ≥37 to<50nmol/L n=466 |
25(OH)D ≥50 to<80nmol/L n=1306 |
25(OH)D ≥80 to<100nmol/L n=403 |
25(OH)D ≥100nmol/L n=141 |
|---|---|---|---|---|---|---|
| Percentage | ||||||
| Season: Winter (December-May) *** % | 47 | 72 | 62 | 43 | 31 | 23 |
| Demography Race: White *** % | 94 | 88 | 95 | 95 | 95 | 94 |
| Current Menopausal Hormone Therapy (MHT) *** % yes | 53 | 43 | 44 | 52 | 60 | 74 |
| Current smoking * % yes | 8 | 11 | 8 | 8 | 6 | 4 |
| Education college or above % yes | 36 | 35 | 35 | 36 | 37 | 37 |
| Current Vigorous Physical Activity (PA) ( ≥ 3 hours/week) *** % | 40 | 29 | 32 | 41 | 49 | 55 |
| Mean (SD) | ||||||
| Height (inches) *** | 67 (4) | 67(4) | 68(4) | 67(4) | 66(4) | 66(4) |
| Current Body Mass Index (BMI kg/m2)*** | 27(5) | 29(6) | 28(5) | 27(5) | 26(4) | 25(4) |
| Red meat group (g/day)*** | 77(67) | 82(76) | 88(78) | 76(66) | 66(54) | 69(61) |
| Milk, on cereal (g/day)*** | 92(90) | 64(76) | 84(81) | 99(94) | 99(91) | 105(94) |
| Energy from diet (kcal/day)* | 2059 (856) | 2054( 882) | 2137(905) | 2073(884) | 1964(714) | 1974(757) |
| Dietary Calcium (mg/day)* | 966(518) | 865(453) | 961(499) | 989(564) | 981(450) | 940(410) |
| Dietary Vitamin D (μg/day)** | 5.3(3.6) | 4.3(3.0) | 5.3(3.3) | 5.6(4.0) | 5.4(3.1) | 5.2(2.6) |
| Supplemental calcium (mg/day)** | 281(385) | 146(279) | 222(354) | 281(379) | 406(421) | 426(479) |
| Supplemental vitamin D( (μg/day)** | 6.4(8.0) | 3.1(6.1) | 4.9(7.4) | 6.6(7.9) | 8.6(8.6) | 9.7(9.9) |
| Supplemental vitamin E((IU/day)** | 168(254) | 106(218) | 160(259) | 168(251) | 212(267) | 213(279) |
p<0.05
p < 0.01
p < 0.001
There was very little overt clinical vitamin D deficiency in this study population, with only 3% of the population having serum 25(OH)D < 25 nmol/L; 12%, 29% , 79% and 95% had levels of serum 25(OH)D < 37 nmol/L, <50 nmol/L , <80 nmol/L, <100 nmol/L, respectively. The average age was 63±5 years (males (M): 64±5.years; females (F): 63±5years), 6% were of non-Caucasian origin, 8% were current smokers and 36% had education above college level. Forty % (M: 41%; F: 39%) had engaged in vigorous activity ≥3 hours/wk during the last year and average body mass index (BMI) was 27.± 5 kg/m2 (M: 27±4 kg/m2; F: 27±6kg/m2).
3.2 Serum 25(OH)D levels varied by season of blood collection with the highest levels during the summer and autumn and the lowest levels during winter and spring
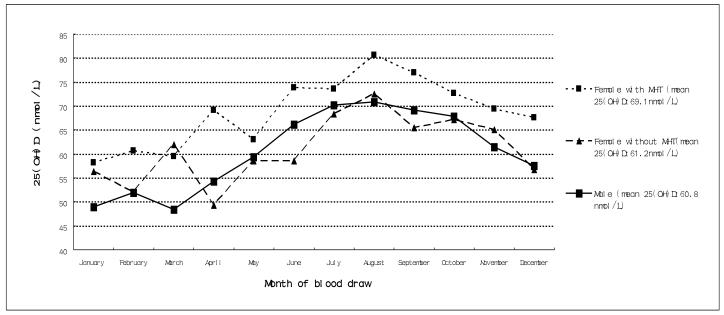
Mean 25(OH)D levels were significantly higher in females than males (p <0.001)(Figure 1). However, when mean 25(OH)D levels of females not taking MHT were compared to those of males, there were no significant differences between females and males ( p=0.5). Similarly there were significant differences between 25(OH)D levels by vitamin D supplement use between females and males over the year p<0.001)(Figure 2). However, when mean 25(OH)D levels of females not taking vitamin D supplement were compared to the mean of males, there were no significant differences.
Figure 1.
Seasonal variation of serum 25(OH)D concentrations in US men and women: including with and without use of menopausal hormone therapy (MHT) in women
Figure 2.
Seasonal variation of serum 25(OH)D concentrations in US men and women showing intaake or not of vitamin D supplement use
3.3 In this sample, twelve factors were found to be significantly (p < 0.01) associated with vitamin D status (Table 1)
Table 2 presents the categorical analyses for those factors that remained significant (p < 0.01) on step-down multivariate linear regression analyses of 25(OH)D (Appendix). The factors associated with low vitamin D status (adjusted for confounders and mutually) were: donating blood in winter, being of non-Caucasian background, being female, being obese (BMI≥30 kg/m2), not being physically active, having low dietary vitamin D intake and vitamin D and Ca supplement use. It is interesting that, independent of dietary vitamin D and calcium intake, consuming less milk on cereal remained a significant predictor as did current MHT use in women. There were gender differences in these data: women had a higher 25(OH)D levels when they either took MHT or vitamin D or calcium supplements (p *interaction for vitamin D supplement intake * gender = 0.005). Vitamin D supplement and MHT use were found to be significantly associated in women: those who took vitamin D supplements were 1.5(1.2-1.9) more likely to take MHT as were those who took Ca supplements OR= 1.9(1.5-2.4). It is interesting that the association between MHT and serum 25(OH)D strengthens when women took supplements (the odds for low vitamin D status ( < 50 nmol/L 25(OH)D) for those using both MHT and vitamin D supplements =0.5; 95% CI=0.3-0.8 versus those who didn’t take supplements =0.7(0.5-1.1). Similar risk factor associations were seen with 25(OH)D <80 nmol/L and linear regression analyses of continuous serum vitamin D (Appendix).
Table 2.
Categorical analysis of those predictors of vitamin D status, as measured by serum 25(OH)D (less than 50nmol/L) that remained significant after stepwise multiple logistic regression analysis in men and women living in the USA
| Total = 2621 | Men=1357 | Women=1264 | ||
|---|---|---|---|---|
| OR1 (95%CI) | OR1 (95%CI) | OR1 (95%CI) | ||
| Season | Summer | 1.0 | 1.0 | 1.0 |
| Winter | 3.4 (2.8-4.1) | 2.7(1.9-3.7) | 3.4 (2.3-5.0) | |
| Race | Other | 1.0 | 1.0 | 1.0 |
| Caucasian | 0.2 (0.1-0.4) | 0.2 (0.1-0.6) | 0.2 (0.1-0.4) | |
| MHT (current use) | No | 1.0 | ||
| Yes | 0.8 (0.5-1.0) | |||
| Vigorous PA (hours/week) | ||||
| none | 1.0 | 1.0 | 1.0 | |
| < 3 hours | 0.7 (0.5-0.9) | 0.7 (0.4-1.0) | 0.7 (0.5-1.1) | |
| ≥3 hours | 0.5 (0.4-0.7) | 0.5 (0.3-0.7) | 0.5 (0.3-0.8) | |
| p trend | *** | *** | *** | |
| BMI(kg/m2) | ||||
| <25 | 1.0 | 1.0 | 1.0 | |
| ≥25-<30 | 1.4 (1.1-1.8) | 1.2 (0.9-1.7) | 1.6 (1.1-2.3) | |
| ≥30 | 2.7 (2.0-3.5) | 2.0 (1.4-3.0) | 3.6 (2.4-5.4) | |
| p trend | *** | ** | *** | |
| Milk(gms/day) | ||||
| <13 | 1.0 | 1.0 | 1.0 | |
| ≥ 13-80 | 0.8 (0.6-1.1) | 0.8 (0.5-1.2) | 0.7 (0.5-1.1) | |
| ≥ 80-159 | 0.8 (0.6-1.0) | 0.6 (0.4-0.9) | 1.0 (0.7-1.6) | |
| ≥ 159 | 0.6 (0.5-0.9) | 0.6 (0.4-1.0) | 0.6 (0.3-0.9) | |
| p trend | ** | * | ns | |
| Dietary Vitamin D(μg/day) | ||||
| <3.0 | 1.0 | 1.0 | 1.0 | |
| ≥ 3.0-4.5 | 0.9 (0.6-1.2) | 0.9 (0.6-1.5) | 0.8 (0.5-1.3) | |
| ≥ 4.5-6.7 | 0.6 (0.4-0.9) | 0.7 (0.4-1.2) | 0.6 (0.3-1.1) | |
| ≥ 6.7 | 0.4 (0.3-0.7) | 0.5 (0.2-0.9) | 0.5 (0.2-1.0) | |
| p trend | *** | * | * | |
| Supplemental calcium (mg/day) | ||||
| None | 1.0 | 1.0 | 1.0 | |
| ≥ 0-162 | 1.1 (0.7-1.9) | 1.2 (0.6-2.3) | 1.2 (0.5-2.6) | |
| ≥ 162-500 | 0.8 (0.6-1.2) | 0.8 (0.5-1.3) | 0.8 (0.5-1.3) | |
| ≥ 500 | 0.7 (0.5-1.0) | 0.8 (0.4-1.6) | 0.7 (0.4-1.1) | |
| p trend | * | ns | * | |
| Supplemental Vitamin D (μg/day) | ||||
| 0-10 | 1.0 | 1.0 | 1.0 | |
| ≥ 10-20 | 0.5 (0.4-0.7) | 0.6 (0.4-1.0) | 0.5 (0.3-0.7) | |
| ≥ 20-30 | 0.4 (0.3-0.7) | 0.7 (0.3-1.5) | 0.3 (0.1-0.5) | |
| ≥ 30 | 0.5 (0.2-1.2) | 0.3 (0.1-1.4) | 0.7 (0.2-2.0) | |
| p trend | *** | * | *** | |
OR: odds ratio; BMI: Body Mass Index; MHT: Menopausal Hormone Therapy; PA: Vigorous Physical Activity
p<0.05
p < 0.01
p < 0.001
adjusted for age at blood draw, gender, smoking pack-years, vitamin D laboratory analysis, PLCO center, total energy intake, educational level and mutually for listed predictor variables
4. DISCUSSION
4.1 Our observation that 25(OH)D serum levels are higher in summer and autumn than in winter and spring is consistent with studies where date of blood draw has been ascertained [17-18]
Mean serum 25(OH)D levels in our study of middle-aged men and women (men=60.8 nmol/L; women=65.4 nmol/L) were similar to the overall mean data from the latest US National Health and Nutrition Examination Survey (men = 62.9 nmol/L; women = 61.5 nmol/L) [19] and higher than most European studies [20-24] ( British men: 53.8; women: 51.5) [5]; except in Northern Europe, where there is a high consumption of fatty fish (e.g. Norway :74.1 nmol/L [22]). We found on initial analysis that women had higher serum25(OH)D levels than men, a finding that was not found in other studies, where women tended to have lower values [17]. In our sample, the gender effect disappeared when the effect of MHT and vitamin D supplement use was taken into account , the MHT effect as has previously been reported [25-26].
4.2 Dietary vitamin D has consistently been reported as a determinant of vitamin D status in all reported predictor studies, especially in Nordic and northern European countries [5, 17, 20-24, 27] with high intake of fatty fish and low sunlight exposure
Milk intake is a significant source of vitamin D in the diet in the US where supplementation of milk with vitamin D has been standard for many decades [6, 17, 19]. As with our findings, in all other studies where vitamin D supplement intake was high there was a strong association with serum 25(OH)D levels [17]. To our knowledge the interesting and divergent associations of serum 25(OH)D with intake of vitamin D and calcium supplements in women and milk on cereal in men has not been previously reported.
4.3 We also found vigorous physical activity to be a strong and modifiable contributor to vitamin D status, consistent with other studies [11, 17, 20-24, 28-32]
This association has often been attributed to physical activity being a surrogate for sun exposure; however, in the few studies in which both exposures were measured simultaneously [28, 32], the vigorous activity-vitamin D relationship persisted. Some aspect of exercise might be contributing to the maintenance of vitamin D status, other than by increasing exposure of skin to sunlight; indeed, this is supported by small clinical studies [33]. Further investigation into the independent role of PA in vitamin D bioavailability and metabolism would appear warranted.
4.4 Our findings of high BMI being associated with low vitamin D levels is consistent with other studies in the US [6, 11, 17, 30] and in other countries [17, 21, 27]
The inverse 25(OH)D and obesity relationship has been explained by “trapping” of the vitamin D parent compound, cholecalciferol, in adipose tissue [34]. Our study is limited, by not having a direct measure of sun exposure or indoor or outdoor exercise patterns, although our measures are adjusted for date of blood draw.
4.6 Strengths of the present investigation are its relatively large sample size and the fact that the vitamin D analyses were all performed with the same assay
Thus targeting an increase in vigorous activity and vitamin D and Ca intake and decreasing obesity could be public health interventions independent of sun exposure to improve vitamin D status in middle-aged Americans.
Appendix
Appendix 1.
Linear Regression Predictors of Vitamin D status as measured by serum 25(OH)D that remained significant after stepwise multiple logistic regression analysis in men and women living in the USA
| Total = 2621 | Men=1357 | Women=1264 | ||||
|---|---|---|---|---|---|---|
| Linear regression1 | Linear regression2 | Linear regression2 | ||||
| Beta Coefficient |
p | Beta Coefficient |
p | Beta Coefficient |
p | |
| Season (Winter versus Summer) | −0.3 | *** | −0.3 | *** | −0.2 | *** |
| Race (Caucasian versus Other) | 0.2 | *** | 0.1 | *** | 0.2 | *** |
| MHT (current use yes/no) | 0.1 | ** | ||||
| Vigorous PA (hours/week) | 0.1 | *** | 0.1 | *** | 0.1 | ** |
| BMI(kg/m2) | -0.2 | *** | -0.1 | *** | -0.2 | *** |
| Milk(gms/day) | 0.1 | ** | 0.1 | ** | 0.03 | |
| Dietary Vitamin D(μg/day) | 0.1 | ** | 0.1 | 0.2 | ** | |
| Supplemental calcium (mg/day) | 0.1 | *** | 0.04 | 0.1 | ** | |
| Supplemental Vitamin D (μg/day) | 0.1 | *** | 0.1 | ** | 0.2 | * |
| r2 | 0.255 | 0.263 | 0.254 | |||
BMI= Body Mass Index, MHT= Menopausal Hormone Therapy, PA=Vigorous Physical Activity
p<0.05
p < 0.01
p < 0.001
adjusted for age at blood draw, smoking pack-years, gender, vitamin D laboratory analysis, PLCO center, total energy intake, educational level and mutually for listed predictor variables
adjusted for age at blood draw, smoking pack-years, vitamin D laboratory analysis, PLCO center, total energy intake, educational level and mutually for listed predictor variables
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fraser DR. Vitamin D. Lancet. 1995;345(8942):104–7. doi: 10.1016/s0140-6736(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. NEJM. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Lips P. Vitamin D physiology. Prog Biophys Mol Biol. 2006;92(1):4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Hintzpeter B, Mensink GB, Thierfelder W, et al. Vitamin D status among German adults. Eur J Nutr. 2007;62(9):1079. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 5.Hirani V, MosdØl A, Mishra G. Predictors of 25-(OH)D status among adults in two British national surveys. Br J Nutr. 2008;17:1–5. doi: 10.1017/S0007114508023416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status. J Natl Cancer Inst. 2006;98(7):451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 7.Hayes RB, Reding D, Kopp W, et al. Etiologic and early marker studies in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21(6 Suppl):349S–355S. doi: 10.1016/s0197-2456(00)00101-x. [DOI] [PubMed] [Google Scholar]
- 8.Peters U, Hayes RB, Chatterjee N, et al. Circulating vitamin D metabolites, polymorphism in vitamin D receptor, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2004;13(4):546–52. [PubMed] [Google Scholar]
- 9.Purdue MP, Freedman DM, Gapstur SM, et al. Circulating 25-hydroxyvitamin D and Risk of non-Hodgkin Lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. American Journal of Epidemiology. 2010 doi: 10.1093/aje/kwq117. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn J, Peters U, Albanes D, et al. Serum vitamin D concentration and prostate cancer risk. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman DM, Chang SC, Falk RT, et al. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008;17(4):889–94. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolzenberg-Solomon RZ, Hayes RB, Horst RL, et al. Serum vitamin D and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian screening trial. Cancer Res. 2009;69(4):1439–47. doi: 10.1158/0008-5472.CAN-08-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subar AF, Midthune D, Kulldorff M, et al. Evaluation of alternative approaches to assign nutrient values to food groups in food frequency questionnaires. Am J Epidemiol. 2000;3:279–86. doi: 10.1093/aje/152.3.279. [DOI] [PubMed] [Google Scholar]
- 14.Tippett KS, Cypel YS. US Department of Agriculture, Agricultural Research Service; Washington, DC: Design and operation: the continuing survey of food intakes by individuals and diet and health knowledge survey, 1994-96. 1997
- 15.Wagner D, Hanwell HE, Vieth R. An evaluation of methods for measurement of serum 25(OH)D. Clin Biochem. 2009;42(15):1549–56. doi: 10.1016/j.clinbiochem.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Breslow NE, Day NE. The analysis of case-control studies. Vol. 32. IARC Scientific Publications; 1980. Statistical methods in cancer; pp. 5–338. [PubMed] [Google Scholar]
- 17.IARC . Vitamin D and Cancer IARC Working Group Reports 5 International Agency for Research on Cancer. Lyon, France: 2008. [Google Scholar]
- 18.Hagenau T, Vest R, Gissel TN, Poulsen CS. Global vitamin D levels in relation to age, gender,skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int. 2009;20(1):133–40. doi: 10.1007/s00198-008-0626-y. [DOI] [PubMed] [Google Scholar]
- 19.Looker AC, Pfeiffer CM, Lacher DA, L. R, et al. Serum 25-OHD status of the US population: 1988-1994 cf 2000-2004. Am J Clin Nutr. 2008;88(6):1519–27. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Brock K, Graubard B, Fraser DR, et al. Predictors of vitamin D biochemical status in a large sample of middle-aged male smokers from Finland. Eur J Clin Nutr. 2010;64(3):280–8. doi: 10.1038/ejcn.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgaz A, Akesson A, Oster A, et al. Associations of diet, supplement use, and ultraviolet B radiation exposure with vitamin D status in Swedish women during winter. Am J Clin Nutr. 2007;86(5):1399–404. doi: 10.1093/ajcn/86.5.1399. [DOI] [PubMed] [Google Scholar]
- 22.Lips P, Hosking D, Lippuner K, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260(3):245–54. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 23.van Dam RM, Snijder MB, Dekker JM, D C, et al. Potentially modifiable determinants of vitamin D status in an older population in the Netherlands: the Hoorn Study. Am J Clin Nutr. 2007;85(3):755–61. doi: 10.1093/ajcn/85.3.755. [DOI] [PubMed] [Google Scholar]
- 24.Holvik K, Meyer HE, Søgaard AJ, et al. Biochemical markers of bone turnover and their relation to forearm bone mineral density in persons of Pakistani and Norwegian background living in Oslo, Norway. Eur J Endocrinol. 2006;155(5):693–9. doi: 10.1530/eje.1.02282. [DOI] [PubMed] [Google Scholar]
- 25.Looker AC. Do body fat and exercise modulate vitamin D status? Nutr Rev. 2007;65(8 Pt 2):S124–6. doi: 10.1301/nr.2007.aug.s124-s126. [DOI] [PubMed] [Google Scholar]
- 26.Sowers MF, Hollis BW, Shapiro B, et al. Elevated pth-related peptide associated with lactation and bone density loss. JAMA. 1996;276(7):549–554. [PubMed] [Google Scholar]
- 27.Abbas S, Linseisen J, Slanger T, et al. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer–results of a large case–control study. Carcinogenesis. 2008;29(1):93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- 28.Brock K, Cant R, Clemson L, Mason RS, Fraser DR. Effects of diet and exercise on plasma vitamin D (25(OH)D) levels in Vietnamese immigrant elderly in Sydney, Australia. J Steroid Biochem Mol Biol. 2007;103(3-5):786–92. doi: 10.1016/j.jsbmb.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 29.Foo LH, Zhang O, Zhu K, et al. Relationship between Vitamin D Status, Body Composition and Physical Exercise of Adolescent Girls in Beijing. Accepted Osteoporosis International. 2008;20:417–425. doi: 10.1007/s00198-008-0667-2. [DOI] [PubMed] [Google Scholar]
- 30.Scragg R, Camargo CA., Jr Frequency of Leisure-Time Physical Activity and Serum 25(OH)D Levels in the US Population: Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168(6):577–586. doi: 10.1093/aje/kwn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scragg R, Holdaway I, Jackson R, et al. 25(OH) D3 and its relation to physical activity. Ann Epidemiol. 1992;2(5):697–703. doi: 10.1016/1047-2797(92)90014-h. [DOI] [PubMed] [Google Scholar]
- 32.Bell N, Godsen RN, Henry DP, et al. The effects of muscle-building exercise on vitamin D. J Bone Miner Res. 1988;3(4):369–73. doi: 10.1002/jbmr.5650030402. [DOI] [PubMed] [Google Scholar]
- 33.Wortsman J, Matsuoka L, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–3. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]