Abstract
These studies focus on identification of vitamin D regulated pathways that impact development or progression of breast cancer. In mouse experiments, we assessed genomic profiles of glandular tissue and established tumors from MMTV-neu mice fed adequate (250 IU/kg) or high (5,000 IU/kg) vitamin D (cholecalciferol). Genomic profiles were also obtained in murine mammary cells that differentially express VDR that were cultured in vitro with 100nM 1,25-dihydroxyvitamin D (1,25D). Ten candidate genes were identified that were commonly regulated in murine cells treated with 1,25D in vitro and in mammary gland of mice fed high dietary vitamin D. In complementary studies, the vitamin D pathway was evaluated in human mammary epithelial cells as a function of transformation. Genes regulated by 1,25D in human mammary epithelial cells included those involved in innate immunity (CD14), differentiation (Bmp6), extracellular matrix remodeling (Plau) and cell survival (Birc3). Transformation reduced VDR content and blunted the induction of some, but not all, target genes by 1,25D in human mammary cells. Collectively, these in vivo and in vitro data demonstrate that vitamin D signaling impacts on common pathways that drive differentiation, alter metabolism, remodel the extracellular matrix and trigger innate immunity in mammary tissue.
Keywords: breast cancer, mammary gland, vitamin D, vitamin D receptor, genomics
1. Introduction
Vitamin D mediates its biological effects after conversion of the circulating metabolite 25-hydroxyvitamin D (25D) to 1,25-dihydroxyvitamin D (1,25D), the ligand for the vitamin D receptor (VDR). The majority of established breast cancer cell lines express VDR [1], and higher tumor VDR expression has been correlated with better prognosis in cancer patients [2]. This is likely because, even in breast cancer cells and tumors, active forms of vitamin D inhibit growth and induce apoptosis [3–5]. Furthermore, VDR agonists act synergistically with genotoxic drugs and radiation to kill breast cancer cells [6–10]. While synthetic vitamin D analogs that activate anti-proliferative signaling through VDR have shown efficacy in animal models of breast cancer [11–13], trials with human patients have been less successful due to dose-limiting calcemic toxicity and highly variable tumor response [14].
Recently, emphasis has been placed on the potential impact of vitamin D on breast cancer development. An early study found that intakes of dairy products, dairy calcium and vitamin D were inversely associated with breast cancer risk in premenopausal, but not postmenopausal, women [15]. John et al. [16] demonstrated that sunlight exposure and dietary vitamin D were associated with reduced risk of breast cancer, however, the association was dependent on region of residence. A prospective analysis of breast cancer incidence in relation to vitamin D intake for over 30,000 participants in the Women’s Health Study indicated that higher intake of vitamin D was moderately associated with a lower risk of pre- but not post- menopausal breast cancer [17]. These data are consistent with reports of inverse associations between vitamin D status and mammographic density in pre-menopausal women [18, 19]. A pooled analysis of studies that assessed serum 25-hydroxyvitamin D (25D) in relation to breast cancer demonstrated a clear dose-response relationship, with the highest quintile of serum 25D associated with a 50% reduction in breast cancer risk [20]. Consistent with these population studies, a four year, placebo-controlled pilot intervention trial demonstrated that vitamin D supplementation substantially reduced risk of cancer at all sites in postmenopausal women [21].
Laboratory studies with human and murine model systems support the concept that vitamin D status directly impacts on the mammary gland. VDR is expressed in epithelial, stromal and immune cells in the normal mammary gland, and is dynamically regulated in the epithelial compartment during puberty and pregnancy [22, 23]. Furthermore, mice lacking VDR are highly susceptible to hyperplasia and tumors in mammary gland in response to chemical carcinogens or oncogene activation [24, 25]. High dietary vitamin D also reduces mammary gland proliferation and tumor development in animals [26, 27]. Consistent with the population data, non-transformed human mammary epithelial (HME) cells grown in vitro express VDR and undergo growth arrest in response to 1,25D. Furthermore, HME cells express the 25D-1α-hydroxylase (CYP27B1) and synthesize 1,25D when incubated with physiological concentrations of 25D [28], providing a mechanistic basis for the observations that high serum 25D correlates with lower risk of breast cancer. Collectively, these data suggest that: a, 1,25D acts on normal mammary tissue to prevent cancer development; b, 1,25D can eliminate breast cancer cells that have already accumulated multiple oncogenic mutations; and c, increasing vitamin D status through diet or supplementation may inhibit breast cancer development.
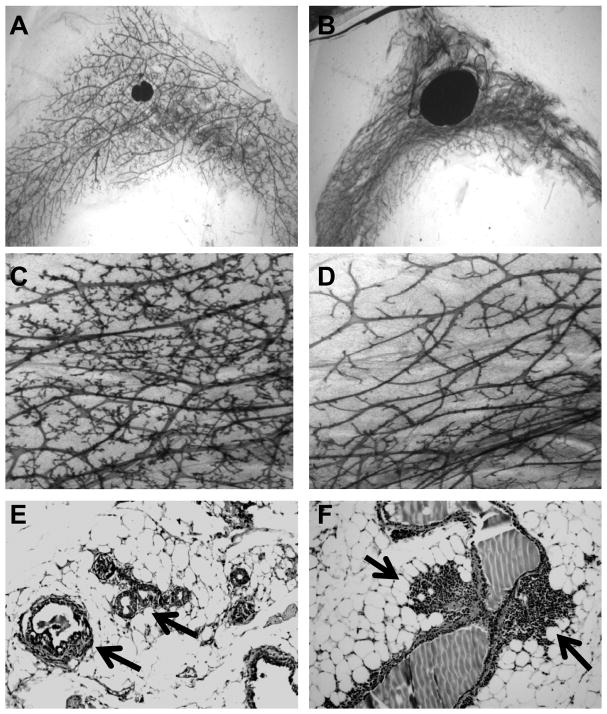
The function of VDR as a transcription factor suggests that any effects of dietary vitamin D to reduce risk of breast cancer would be mediated through changes in gene expression. Despite multiple lines of evidence that vitamin D signaling reduces the risk of breast cancer development and progression, little is known about the specific gene targets of VDR in mammary gland or breast tumors. During puberty and pregnancy, animals lacking VDR exhibit alterations in proliferation and apoptosis of the epithelial cells [22, 23], suggesting that vitamin D regulated pathways impact on glandular sensitivity to estrogen, progesterone and prolactin. Changes in the mammary gland in aging mice lacking the VDR include adipose tissue atrophy, reduced ductal branching and chronic inflammation (Figure 1). Thus, the phenotypic characterization of VDR null mice supports the concept that vitamin D signaling exerts stage-specific effects on both the stromal and epithelial compartments in the mammary gland. In our ongoing studies, we are testing the hypothesis that the anti-breast cancer effects of vitamin D are associated with global changes in gene expression mediated by the VDR present in mammary cells. This hypothesis predicts that common sets of genes will be modulated by dietary vitamin D in vivo or 1,25D treatment in vitro in a VDR-dependent manner.
Figure 1. Mammary gland pathology in aging VDR knockout mice.
Images of inguinal mammary gland whole mounts (A–D) and hematoxylin and eosin stained sections (E–F) from wild type (A,C) and VDR knockout (B,D,E,F) mice at 12 months of age. Note enlarged lymph nodes at center of mammary gland whole mounts in VDR knockout mice (B) compared to wild type controls (A). At higher magnification, glands from wild type animals display abundant secondary and tertiary branching off the main ducts (C), whereas glands from VDR knockout mice (D) exhibit markedly reduced branching. In addition, glands from VDR knockout mice exhibit hyperplastic lesions (arrows, E) and inflammation (arrows, F).
2. Genomic Studies on Vitamin D in Human and Animal Models of Breast Cancer
2.1. Effects of dietary vitamin D on gene expression profiles in a transgenic mouse model of breast cancer
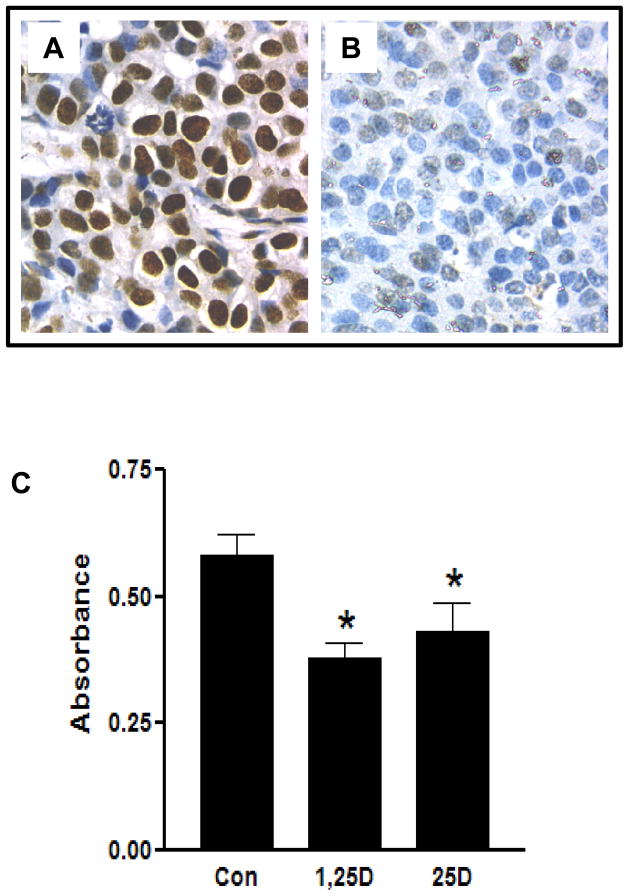
To gain insight into pathways regulated by dietary vitamin D that impact on mammary epithelial cell transformation, we conducted genomic profiling in the MMTV-neu transgenic mouse model of breast cancer. MMTV-neu mice express the oncogene neu, a member of the epidermal growth factor receptor family, in mammary gland and develop mammary tumors beginning at approximately four months of age. Mammary tumors that develop in MMTV-neu mice express high levels of VDR (Figure 2A), and tumor progression in this model is accelerated in mice heterozygous for VDR [24]. To test the sensitivity of neu expressing mammary cells to 1,25D, we performed growth assays with cells isolated from MMTV-neu tumors. As shown in Figure 2C, cell density was decreased in MMTV-neu cells treated with either 1,25D or 25D in vitro. In our experiments, we chronically fed female MMTV-neu mice diets containing either 250 IU cholecalciferol/kg diet (the standard amount present in rodent diets) or 5,000 IU cholecalciferol/kg diet (an amount that increases serum 25D approximately two fold). Glands (n=4 per dietary group) and tumors (n=7 per dietary group) were processed for global genomic profiling on mouse Affymetrix gene chips at NCI’s Laboratory of Molecular Technology through the Bioactive Nutrient Gene Omnibus (BANGEO) initiative. Genespring software was used to generate lists of differentially expressed genes as a function of dietary vitamin D.
Figure 2. Expression of VDR and sensitivity to vitamin D metabolites in mammary tumor cells from MMTV-neu mice.
A, Formalin fixed tumor sections from MMTV-neu wild-type mice were subjected to antigen retrieval and incubated with VDR antibody (clone 9A7) followed by anti-rat secondary antibody. VDR immunostaining was developed with DAB and appears brown against the blue hematoxylin counterstain.
B. Formalin fixed sections of MMTV-neu tumors that developed on the VDR knockout background were subjected to VDR immunostaining as described for A and used as negative control.
C. Cells were isolated by collagenase digestion from MMTV-neu tumors and plated in 24 well plates. After attachment, cells were treated with ethanol vehicle, 100nM 1,25D or 100nM 25D for 96 hours. Cell density was assessed by absorbance of crystal violet and expressed as mean±standard error of four replicates. *p<0.05, one way ANOVA.
Of 45K genes on the Affymetrix arrays, 592 genes were identified as differentially expressed (fold change >2) in MMTV-neu tumors as a function of dietary vitamin D. Genes that were altered more than 5-fold included ATP1A2 (Na+/K+ ATPase alpha 2), Clca2 (calcium activated chloride channel 2), Emb (embigin), Cpe (carboxypeptidase E), and Ltf (Lactoferrin), which were down regulated, and Enho (Energy homeostasis associated), Slc6a2 (solute carrier family 6A2 - neurotransmitter transporter), ApoC1 (Apolipoprotein C1), Dnajc6 (DnaJ homolog, subfamily C, member 6) and Rdh12 (retinol dehydrogenase 12), which were up regulated, in response to high dietary vitamin D. Interestingly, the profile of genes altered by dietary vitamin D in non-transformed mammary glands from these MMTV-neu mice was distinctly different from that observed in established MMTV-neu tumors. In non-transformed glands, candidate genes dose-dependently regulated by dietary vitamin D included Sncg (gamma synuclein), Lep (leptin), Fabp4 (fatty acid binding protein-4) and Tusc5 (tumor suppressor candidate-5), which were up-regulated, and Hey-1 (hairy/enhancer-of-split related with YRPW motif 1), Cldn10 (claudin 10) and Chm2 (chimaerin-2) which were down-regulated. While confirmation of these results is clearly necessary, the discordance between the effects of dietary vitamin D on gene expression profiles in tumors vs. mammary glands of MMTV-neu mice supports the concept that transformation significantly alters the tissue response to dietary vitamin D. Furthermore, these data suggest that the vitamin D regulated pathways that operate in normal mammary cells to suppress transformation are quite distinct from those triggered in transformed breast cells to exert anti-proliferative and pro-apoptotic effects.
2.2. Cellular Model for Studying Genomic Effects of VDR Signaling In Murine Cells
We have also developed a murine-based mammary tumor cell model for studying the role of the VDR in mediating the growth inhibitory effects of 1,25D. Cell lines were established from mammary tumors that were induced by carcinogen treatment of wild-type and VDR knockout mice [29]. WT145 cells, which represent a tumor cell line established from wild-type mice, express VDR and are growth inhibited by 1,25D (Figure 3A). As expected, KO240 cells - which were derived from a VDR knockout mouse - lack VDR and are not growth inhibited by 1,25D (Figure 3B). Recently we have engineered clones of KO240 cells that stably express human VDR (hVDR). Representative data from one of these clones (Figure 3C) demonstrates that introduction of hVDR into murine VDR null cells reconstitutes the growth inhibitory response to 1,25D that is present in the WT145 cells and absent in the parental KO240 cells. We have used this model to profile VDR dependent 1,25D mediated changes in gene expression in vitro. RNA isolated from WT145, KO240 and KO240hVDR cells that were treated with ethanol vehicle or 100nM 1,25D for 24h was used for Affymetrix microarray analysis. Data from the KO240 cells confirmed that gene expression profiles are unaffected by 1,25D in the absence of VDR. In contrast, significant changes in genomic profiles were observed in WT145 and KO240hVDR cells treated with 1,25D. In WT145 cells, 80 genes were altered more than 2-fold in response to 1,25D treatment, whereas in KOhVDR cells more than 200 genes were altered greater than 2-fold. Overall, there was about 50% concordance in the 1,25D target genes identified in WT145 cells and KOhVDR cells. Using a 1.5 fold change cut-off, we have compiled a list of 122 genes that were commonly altered in WT145 cells and KOhVDR cells. Of these, 31 (25%) were down-regulated. In both WT145 cells and KOhVDR cells the most highly induced gene in response to 1,25D was Cyp24A1, which was not induced by 1,25D in the parental KO240 cells lacking VDR. Through comparison of genomic profiles in WT145 and KOhVDR cells treated with 1,25D and in mammary glands of mice fed high dietary vitamin D, ten genes were identified that were commonly up-regulated in both model systems (Table 1). Validation of these candidate 1,25D regulated genes, pathway analysis, and mechanistic studies are ongoing to evaluate the significance of these potential VDR targets in relation to the anti-tumor effects of vitamin D.
Figure 3. Effects of 1,25D on growth of murine cells derived from wild-type and VDR knockout mice.
The effects of 1,25D on growth of murine cells that differentially express the VDR was assessed by crystal violet staining. A, WT145 cells; B, KO240 cells; and C, KO240 cells stably expressing human VDR (KOhVDR cells) were treated with 100nM 1,25D or ethanol vehicle for 24 hrs prior to assessment of cell density by crystal violet staining. Data are expressed in absorbance units, which is proportional to cell density under the conditions used. Bars represent mean±standard error of four measurements. *Significantly different (p<0.05; Students t test), control versus 1,25D treated.
Table 1. List of genes altered by vitamin D in two distinct murine model systems.
Vitamin D regulated genes were identified on the Affymetrix platform through comparison of expression profiles from VDR expressing murine cells treated with 1,25D in vitro and from mammary glands of mice fed different levels of dietary vitamin D. See text for details.
| Genes commonly altered by vitamin D signaling in murine model systems |
|---|
|
2.3. Effects of 1,25D on global gene expression in non-transformed human mammary epithelial cells
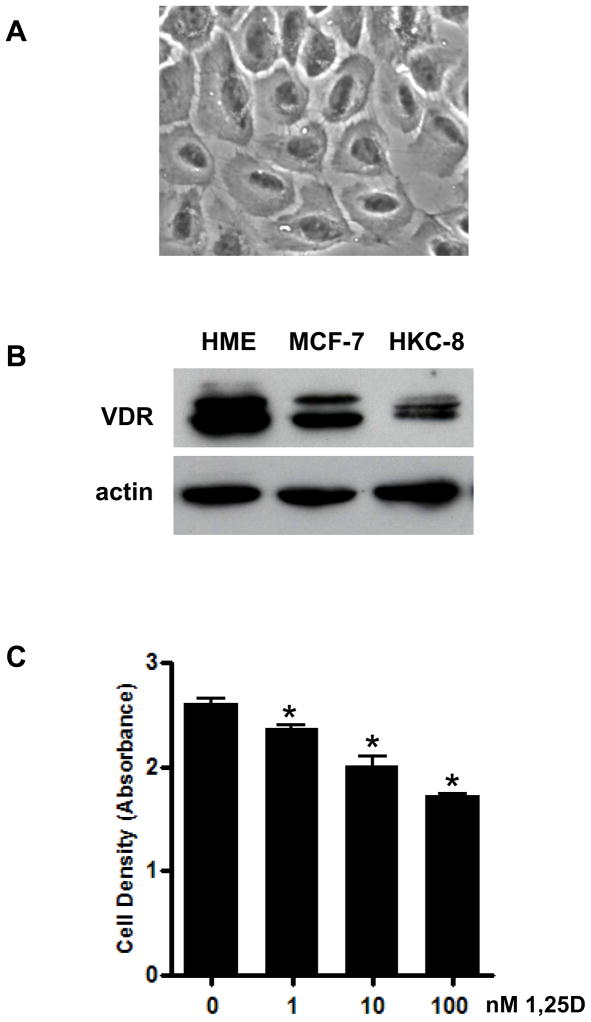
In complementary studies, we have examined 1,25D genomic profiles in human breast epithelial cells as a function of transformation. Non-transformed human mammary epithelial (HME) cells, which retain characteristic morphology of primary mammary epithelial cell cultures, express high levels of VDR and are dose dependently growth inhibited by 1,25D (Figure 4). Gene profiling was conducted on HME cells treated with 100nM 1,25D or ethanol vehicle in serum free mammary epithelial growth medium for 24h. Datasets were generated with Affymetrix human exon ST arrays and analyzed with Genespring software. Approximately 200 genes were identified as differentially expressed (fold change >2) in HME cells in response to 100nM 1,25D. As expected, Cyp24A1 was the most highly induced gene, with >200-fold induction by 1,25D. Other genes highly up-regulated by 1,25D in HME cells included CD14 (60x), Bone morphogenetic protein 6 (Bmp6, 15x) and Interleukin-1 receptor like 1 (Il1rl1, 10x). Down regulated genes included Kinase insert domain receptor (KDR, 8x), Regulator of G-protein signaling 2 (RGS2, 5x), Baculoviral IAP repeat-containing 3 (Birc3, 5x), glutamate synthetase (Glul, 4x) and FBJ murine osteosarcoma viral oncogene homolog B (Fosb, 3x).
Figure 4. Characteristics of non-transformed human mammary epithelial (HME) cells.
A. Phase contrast image of HME cells grown in serum free mammary epithelial growth medium. Cells display normal cuboidal epithelial morphology and express E-cadherin (not shown).
B. VDR expression in lysates from non-transformed HME cells, MCF-7 human breast cancer cells and HKC-8 immortalized renal cells was determined by western blotting.
C. HME cell density was quantitated by crystal violet staining after three day treatment with ethanol vehicle or 1–100nM 1,25D. Bars represent mean±standard error of four replicates. *p<0.05, one way ANOVA.
We have used real time PCR to confirm CD14, Bmp6 and Il1Rl1 as 1,25D target genes in HME cells, and to assess the possibility that transformation alters cellular responsiveness 1,25D. In previous studies [30], we demonstrated that VDR expression is down regulated in HME cells expressing SV-40 large T antigen (HMESV40) and HME cells expressing both SV-40 large T antigen and activated ras (HMESV40+Ras). Consistent with the reduced VDR content, the regulation of CD14 and Il1Rl1 by 1,25D was significantly blunted in HMESV40 and HMESV40+Ras cells relative to HME cells. Surprisingly, 1,25D induction of Bmp6 was comparable in HME, HMESV40 and HMESV40+Ras cells despite the reduced VDR content. These data suggest that transformation selectively alters the induction of a subset of VDR target genes in mammary cells.
3. Discussion
In these studies we have used both human and murine model systems to identify genomic patterns altered by vitamin D signaling in mammary cells. Since ours are the first studies to examine the genomic effects of dietary vitamin D on mammary gland in vivo, it is unclear to what extent the patterns of gene profiles we observed would be mimicked in other tissues. Li et al [31] assessed global gene expression in kidney as a function of 1,25D treatment in wild-type mice in comparison to VDR knockout mice, but there is little to no overlap in the candidate genes identified in their study with ours. This discrepancy likely reflects both tissue specificity as well as methodological differences (chronic dietary treatment with vitamin D in our study vs. acute injection of 1,25D in the study of Li et al [31]).
With respect to gene profiles of 1,25D treatment in vitro, published data from two array studies with human breast-derived cells are available for comparison. Swami et al [32] profiled MCF-7 and MDA-MB-231 breast cancer cell lines after treatment with 50nM 1,25D for 6 and 24hrs, while Lee et al [33] profiled the effects of a synthetic vitamin D analog (Ro3587, 1nM, 4 and 12 hrs) in premalignant (MCF10AT1) and fully malignant (MCF10CA1) human breast cells. We find very little overlap of our HME gene profiles with the list of 1,25D regulated genes reported by Swami et al [32], likely due to their use of a CMT Cancer Array format that contained 2000 cancer-related genes rather than a whole genome array. There is considerable overlap between our list of 1,25D regulated genes in HME cells and the list of Ro3587 regulated genes in the MCF10A cell series [33], with more than 20 genes appearing on both lists. Furthermore, many of the genes we have identified in HME cells contain consensus vitamin D response elements in their promoter regions [34], suggesting that they represent direct targets of the 1,25D/VDR complex. Validation of these candidate genes as VDR targets, and determination of their relevance, if any, to the anti-proliferative effects of the vitamin D pathway, is ongoing in our model systems.
4. Summary
In this series of studies, genomic profiling was used to gain insight into the anti-tumor mechanisms of vitamin D signaling. These studies were designed to test the hypothesis that vitamin D acts through the VDR, a ligand dependent transcription factor, to regulate patterns of gene expression that suppress the tumorigenic phenotype. Collectively, our in vivo and in vitro data indicate that, despite similar anti-proliferative effects of 1,25D in human and mouse mammary cells, the specific target genes regulated by vitamin D signaling vary considerably in different model systems. However, pathways that drive differentiation, alter metabolic flux, remodel the extracellular matrix and trigger innate immunity appear to be commonly regulated by vitamin D signaling in mammary tissue.
Acknowledgments
Supported by the National Institutes of Health [R01CA69700, RC1CA144963], A Sister’s Hope and the NIH Bio-Active Nutrient Gene Expression Omnibus Project (BANGEO).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frampton RJ, Suva LJ, Eisman JA, Findlay DM, Moore GE, Moseley JM, Martin TJ. Presence of 1,25-dihydroxyvitamin D3 receptors in established human cancer cell lines in culture. Cancer Res. 1982;42(3):1116–1119. [PubMed] [Google Scholar]
- 2.Berger U, McClelland RA, Wilson P, Greene GL, Haussler MR, Pike JW, Colston K, Easton D, Coombes RC. Immunocytochemical determination of estrogen receptor, progesterone receptor, and 1,25-dihydroxyvitamin D3 receptor in breast cancer and relationship to prognosis. Cancer Res. 1991;51:239–244. [PubMed] [Google Scholar]
- 3.Simboli-Campbell M, Narvaez CJ, VanWeelden K, Tenniswood M, Welsh J. Comparative effects of 1,25(OH)2D3 and EB1089 on cell cycle kinetics and apoptosis in MCF-7 breast cancer cells. Breast Cancer Res Treat. 1997;42(1):31–41. doi: 10.1023/a:1005772432465. [DOI] [PubMed] [Google Scholar]
- 4.Narvaez CJ, Welsh J. Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem. 2001;276(12):9101–9107. doi: 10.1074/jbc.M006876200. [DOI] [PubMed] [Google Scholar]
- 5.VanWeelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology. 1998;139(4):2102–2110. doi: 10.1210/endo.139.4.5892. [DOI] [PubMed] [Google Scholar]
- 6.Posner GH, Crawford KR, Peleg S, Welsh JE, Romu S, Gewirtz DA, Gupta MS, Dolan P, Kensler TW. A non-calcemic sulfone version of the vitamin D(3) analogue seocalcitol (EB 1089): chemical synthesis, biological evaluation and potency enhancement of the anticancer drug adriamycin. Bioorg Med Chem. 2001;9(9):2365–2371. doi: 10.1016/s0968-0896(01)00159-6. [DOI] [PubMed] [Google Scholar]
- 7.Demasters G, Di X, Newsham I, Shiu R, Gewirtz DA. Potentiation of radiation sensitivity in breast tumor cells by the vitamin D3 analogue, EB 1089, through promotion of autophagy and interference with proliferative recovery. Mol Cancer Ther. 2006;5(11):2786–2797. doi: 10.1158/1535-7163.MCT-06-0316. [DOI] [PubMed] [Google Scholar]
- 8.Sundaram S, Sea A, Feldman S, Strawbridge R, Hoopes PJ, Demidenko E, Binderup L, Gewirtz DA. The combination of a potent vitamin D3 analog, EB 1089, with ionizing radiation reduces tumor growth and induces apoptosis of MCF-7 breast tumor xenografts in nude mice. Clin Cancer Res. 2003;9(6):2350–2356. [PubMed] [Google Scholar]
- 9.Chaudhry M, Sundaram S, Gennings C, Carter H, Gewirtz DA. The vitamin D3 analog, ILX-23-7553, enhances the response to adriamycin and irradiation in MCF-7 breast tumor cells. Cancer Chemother Pharmacol. 2001;47(5):429–436. doi: 10.1007/s002800000251. [DOI] [PubMed] [Google Scholar]
- 10.Sundaram S, Gewirtz DA. The vitamin D3 analog EB 1089 enhances the response of human breast tumor cells to radiation. Radiat Res. 1999;152(5):479–486. [PubMed] [Google Scholar]
- 11.Flanagan L, Packman K, Juba B, O_Neill S, Tenniswood M, Welsh J. Efficacy of Vitamin D compounds to modulate estrogen receptor negative breast cancer growth and invasion. J Steroid Biochem Mol Biol. 2003;84(2–3):181–192. doi: 10.1016/s0960-0760(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 12.Vanweelden K, Flanagan L, Binderup L, Tenniswood M, Welsh J. Apoptotic regression of MCF-7 xenografts in nude mice treated with the vitamin D3 analog, EB1089. Endocrinology. 1998;139(4):2102–2110. doi: 10.1210/endo.139.4.5892. [DOI] [PubMed] [Google Scholar]
- 13.James SY, Mercer E, Brady M, Binderup L, Colston KW. EB1089, a synthetic analogue of vitamin D, induces apoptosis in breast cancer cells in vivo and in vitro. Br J Pharmacol. 1998;125(5):953–962. doi: 10.1038/sj.bjp.0702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulliford T, English J, Colston KW, Menday P, Moller S, Coombes RC. A phase I study of the vitamin D analogue EB1089 in patients with advanced breast and colorectal cancer. Br J Cancer. 1998;78:6–13. doi: 10.1038/bjc.1998.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knekt P, Jarvinen R, Seppanen R, Pukkala E, Aromaa A. Intake of dairy products and the risk of breast cancer. Br J Cancer. 1996;73(5):687–691. doi: 10.1038/bjc.1996.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971–1975 to 1992, National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8(5):399–406. [PubMed] [Google Scholar]
- 17.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167(10):1050–1059. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- 18.Brisson J, Berube S, Diorio C, Sinotte M, Pollak M, Masse B. Synchronized seasonal variations of mammographic breast density and plasma 25-hydroxyvitamin D. Cancer Epidemiol Biomarkers Prev. 2007;16(5):929–933. doi: 10.1158/1055-9965.EPI-06-0746. [DOI] [PubMed] [Google Scholar]
- 19.Berube S, Diorio C, Verhoek-Oftedah W, Brisson lJ. Vitamin D, calcium and mammographic breast densities. Cancer Epidemiology Biomarkers Prevention. 2004;13(9):1466–1472. [PubMed] [Google Scholar]
- 20.Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, Newmark H, Holick MF, Garland FC. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103(3–5):708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 22.Zinser GM, Packman K, Welsh JE. Mammary gland development in vitamin D3 receptor knockout mice. Development. 2002;129:3067–3076. doi: 10.1242/dev.129.13.3067. [DOI] [PubMed] [Google Scholar]
- 23.Zinser GM, Welsh JE. Accelerated mammary gland development during pregnancy and delayed post-lactational involution in vitamin D3 receptor null mice. Mol Endocrinol. 2004;18:2208–2223. doi: 10.1210/me.2003-0469. [DOI] [PubMed] [Google Scholar]
- 24.Zinser GM, Welsh JE. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis. 2004;25(12):2361–2372. doi: 10.1093/carcin/bgh271. [DOI] [PubMed] [Google Scholar]
- 25.Zinser GM, Suckow M, Welsh JE. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97(1–2):153–164. doi: 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Kurihara N, Fan K, Thaler HT, Yang K, Lipkin M. Effect of a Western-style diet fortified with increased calcium and vitamin D on mammary gland of C57BL/6 mice. J Med Food. 2008;11(2):201–206. doi: 10.1089/jmf.2007.619. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson EA, James KA, Newmark HL, Carroll KK. Effects of dietary fat, calcium, and vitamin D on growth and mammary tumorigenesis induced by 7,12-dimethylbenz(a)anthracene in female Sprague-Dawley rats. Cancer Res. 1989;49(22):6300–6303. [PubMed] [Google Scholar]
- 28.Kemmis CM, Salvador S, Smith K, Welsh JE. Human mammary cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D3. J Nutrition. 2006;136:887–92. doi: 10.1093/jn/136.4.887. [DOI] [PubMed] [Google Scholar]
- 29.Zinser GM, McEleney K, Welsh JE. Characterization of mammary tumor cell lines from wild type and vitamin D receptor knockout mice. Mol Cell Endocrinol. 2003;200:67–80. doi: 10.1016/s0303-7207(02)00416-1. [DOI] [PubMed] [Google Scholar]
- 30.Kemmis CM, Welsh JE. Mammary epithelial cell transformation is associated with deregulation of the vitamin D pathway. J Cell Biochem. 2008;105:980–988. doi: 10.1002/jcb.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Zheng W, Li YC. Altered gene expression profile in the kidney of vitamin D receptor knockout mice. J Cell Biochem. 2003;89(4):709–719. doi: 10.1002/jcb.10547. [DOI] [PubMed] [Google Scholar]
- 32.Swami S, Raghavachari N, Muller UR, Bao YP, Feldman D. Vitamin D growth inhibition of breast cancer cells: gene expression patterns assessed by cDNA microarray. Breast Cancer Res Treat. 2003;80(1):49–62. doi: 10.1023/A:1024487118457. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, Notterman D, Reiss M, Suh N. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72(3):332–343. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Wang TT, Tavera-Mendoza LE, Laperriere D, Libby E, MacLeod NB, Nagai Y, Bourdeau V, Konstorum A, Lallemant B, Zhang R, Mader S, White JH. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Mol Endocrinol. 2005;19(11):2685–2695. doi: 10.1210/me.2005-0106. [DOI] [PubMed] [Google Scholar]