Abstract
Trastuzumab (Herceptin) is a recombinant humanized monoclonal antibody directed against an extracellular region of the HER2 protein. We hypothesized that a single adeno-associated virus mediated genetic delivery of an anti-HER2 antibody should be effective in mediating long term production of anti-HER2 and in suppressing the growth of human tumors in a xenograft model in nude mice. The adeno-associated virus gene transfer vector AAVrh.10αHER2 was constructed based on non-human primate AAV serotype rh.10 to express the cDNAs for the heavy and light chains of monoclonal antibody 4D5, the murine precursor to trastuzumab. The data demonstrates that genetically transferred anti-HER2 selectively bound human HER2 protein and suppressed proliferation of HER2 positive tumor cell lines. A single administration of AAVrh.10αHER2 provided long term therapeutic levels of anti-HER2 antibody expression without inducing anti-idiotype response, suppressed the growth of HER2 positive tumors and increased survival of the tumor-bearing mice. In the context that trastuzumab therapy requires frequent, repeated administration, this strategy might be developed as an alternate platform for delivery anti-HER2 therapy.
Introduction
Human epidermal growth factor receptor type 2 (HER2, also referred to as HER2/neu or ErbB-2) is a 185 kDa tyrosine kinase cell surface receptor1. HER2 was originally identified as a proto-oncogene and is known to be overexpressed and/or amplified in many types of human malignancies such as such as breast, ovarian, stomach, salivary gland and kidney tumor2. Overexpression of HER2, with subsequent constitutive kinase activation, is observed in approximately 20 to 30% of human breast cancers, and is associated with reduced disease-free and overall survival3,4.
A variety of anti-HER2 therapies effectively suppress the growth of HER2 dependent tumors in experimental models5–7. Trastuzumab (Herceptin), a recombinant humanized monoclonal antibody (mAb) directed against an extracellular region of the HER2 protein, was the first HER2-targeted therapy approved by the United States Food and Drug Administration for the treatment of HER2-overexpressing metastatic breast cancer8. Clinical trials have established that addition of trastuzumab to adjuvant chemotherapy (either in sequence or in combination) resulted in significant improvements in disease-free and overall survival rates in patients with early-stage HER2-overexpressing breast cancer8. Trastuzumab is administered either once a week or once every three weeks intravenously for 30 to 90 min to achieve a minimum trough serum level of >10 μg/ml9. Since follow up treatment is indefinite, this places a substantial burden on patients and the health care system in terms of time and cost.
With the goal of developing an alternative platform for delivering trastuzumab, we hypothesized that genetic delivery of an anti-HER2 antibody with the trastuzumab antigen binding site should be effective in suppressing the growth of human tumors. An adeno-associated virus (AAV) gene transfer vector (AAVrh.10αHER2) was constructed based on the non-human primate AAV serotype rh.10 expressing the cDNA for the heavy and light chains of monoclonal antibody 4D5, the murine precursor to trastuzumab. The data demonstrates that following a single administration to mice, the AAVrh.10αHER2 vector expressed persistent levels of an antibody that selectively bound human HER2 protein and suppressed proliferation of HER2 positive tumor cell line and the tumor in vivo, and increased survival of the HER2+ human tumor-bearing nude mice.
Methods
Anti-HER2 Expression Cassette
Molecular clones of HER2 antibody heavy and light chains were amplified from mouse anti-human HER2 hybridoma 4D5 (ATCC, Manassas, VA). The antigen binding regions of 4D5 correspond to those in trastuzumab10. pAAVαHER2 and pAAVαHER2-myc are plasmids used to express full length HER2 antibody in a single open reading frame in an AAV vector. The anti-HER2 expression cassettes contains (5′ to 3′) the cytomegalovirus immediate early promoter/enhancer followed by the cDNA encoding the anti-HER2 heavy chain, a 13 amino acid myc tag (only for αHER2-myc), a 4 amino acid furin cleavage site, the 24 amino acid self-cleaving 2A peptide from foot-and-mouth disease virus, the anti-HER2 light chain, and the bovine growth hormone polyadenylation signal11,12. The AAVαHER2 and AAVαHER2-myc expression cassettes are both flanked by the inverted terminal repeats (ITR) of AAV2.
Adeno-associated Virus Vectors
AAVrh.10αHER2 and AAVrh.10αHER2-myc are AAV gene transfer vectors expressing full length anti-HER2 antibody11. AAVrh.10αPA-myc expresses an unrelated antibody against anthrax protective antigen and was used as a negative control vector13. AAVrh.10 vectors expressing GFP and LacZ expression cassettes were used as further controls11,13. All AAV vectors were based on rh.10, a non-human primate derived AAV which provides high efficiency of gene transfer11,13. The vectors were produced using three plasmids: (1) an expression plasmid described above containing (5′ to 3′) the AAV2 ITR including the packaging signal, the expression cassette, and the 3′ AAV2ITR; (2) pAAV44.2, a plasmid that provides rep proteins derived from AAV2 and cap proteins derived from AAVrh.1011,13; and (3) pAdDeltaF6, an adenovirus helper plasmid that provides adenovirus helper functions of E2, E4, and VA RNA. To create the vectors, the expression plasmid (600 μg), pAAV44.2 (800 μg), and pAdDeltaF6 (1200 μg) were co-transfected into human embryonic kidney 293 cells with Polyfect (Qiagen, Valencia, CA). At 72 hr post-transfection, cells were harvested, a crude viral lysate was prepared by three cycles of freeze/thaw, and clarified by centrifugation. The AAV vectors were purified by iodixinol gradient and QHP anion exchange chromatography. The purified AAV vectors were concentrated using a BioMax 100 membrane concentrator (Millipore, Billerica, MA) and stored in phosphate buffered saline, pH 7.4 (PBS) at −80°C11,13. Vector genome titers were determined by TaqMan real-time PCR using a CMV promoter-specific primer-probe set, designed using Primer Express software (Applied Biosystems, Foster City, CA). An AAVα1AT plasmid DNA standard of known copy number was used to generate a standard curve11. The control AAV6.2 vectors expressing lacZ transgene was made by a similar method but with the pAAV6.2 AAV helper plasmid with the AAV6.2 capsid sequence instead of the p44.2 AAV helper plasmid14,15.
Cell Lines
BT474 breast cancer, SK-BR-3 breast cancer and Calu-3 lung cancer cell lines are HER2 positive human tumor cell lines16–18. A-673 rhabdomyosarcoma and HeLa cervical cancer are HER2 negative human tumor cell lines and BEAS-2b is an immortalized bronchial epithelial HER2 negative cell line19–21. All of these cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA) and maintained in medium recommended by the ATCC.
In Vitro Assessment of anti-HER2 Expression
To assess the function of expressing cassettes, expression of the anti-HER2 antibody from pAAVαHER2 and pAAVαHER2-myc was examined following transfection of cells in vitro using Western analysis. 293 cells were transfected with pAAVαHER2 or pAAVαHER2-myc (8 μg plasmids/10 cm plate) and transfected cell medium were harvested at 72 hr post-transfection. Medium from mock and pGFP-transfected cells served as negative controls. Medium from pAAVαPA-myc transfected cells and purified mouse IgG1 served as additional controls. All media were evaluated for the expression of anti-HER2 antibody by Western analysis under non-reducing and reducing conditions using a sheep anti-mouse IgG heavy chain and light chain secondary antibody (SIGMA, Saint Louis, MO) and ECL reagent (Amersham, Piscataway, NJ). Peroxidase-conjugated mouse anti-myc antibody (GeneTex, San Antonio, TX) was used to detect the myc tagged antibody heavy chain under non-reducing and reducing conditions.
The specificity of the AAV plasmids-expressing anti-HER2 antibodies for human HER2 was evaluated by immunohistochemistry. HER2 positive SK-BR-3 and HER2 negative BEAS-2b cells were fixed by 4% paraformaldehyde and blocked with 5% goat serum. After washing, the cells were incubated with medium from pAAVαHER2-, pAAVαHER2-myc- or pAAVαPA-myc-transfected 293 cells for 60 min. Cells were then washed and incubated with Alexa 488 labeled goat anti-mouse antibody (Jackson, West Grove, PA) at a dilution of 1:100 for 60 min. DAPI (Invitrogen, Carlsbad, CA) was used to stain nuclei. Labeled cells were assessed by fluorescent microscopy (Olympus IX 70, Olympus America, Center Valley, PA).
Cell Proliferation
The anti-proliferation effect of the AAV plasmids-expressing anti-HER2 antibody was assayed using the HER2 positive BT474 breast tumor cell line and the HER2 negative HeLa cervical cancer cell line. Cells (104 per well) were plated in 96 wells plates. Following cell adherence, medium collected from 293 cells transfected with mock, pGFP, pAAVαPA-myc, pAAVαHER2 or pAAVαHER2-myc were added (1:2 diluted, 8 wells per group). After incubation at 37°C for 3 days, 20 μl/well of CellTiter 96 AQueous One Solution Reagent (Promega, Madison, WI) was added. After 1 hr incubation, the absorbance at 490 nm was recorded using an ELISA plate reader. The data was expressed as the ratio of A490 with the experimental group relative to A490 for the untransfected control group. For assessment of dose-dependent inhibition, medium collected from 293 cells transfected with mock, pAAVαPA-myc, AAVαHER2 or pAAVαHER2-myc were 2-fold serial diluted from 50% to 6.25% and then added to BT474 adhered cells. After 5 days, cell proliferation was assessed as described above.
Flow Cytometry
Female C57Bl/6 mice, 6 to 8 weeks of age, were obtained from Taconic (Germantown, NY) and were housed under specific pathogen-free conditions. AAVrh.10αHER2-myc or, as controls, AAVrh.10LacZ or PBS (1011 genome copies for the vectors) were administrated by the intravenous route. To assess the expression of the anti-HER2 antibody in serum from AAVrh.10αHER2-myc treated mice, HER2 positive BT474 and HER2 negative A673 cell lines were chosen for binding specificity determination. BT474 or A673 cells were trypsinized and resuspended in PBS containing 1% fetal bovine serum and then blocked with 5% goat serum in PBS for 20 min. After 3 times wash, BT474 or A673 cells were incubated with 1:40 diluted serum (collected at day 30 post-administration) from AAVrh.10αHER2-myc treated mice for 60 min. Serum from PBS and AAVrh.α10aPA-myc treated mice served as negative controls. Cells were then washed and incubated with FITC-labeled goat anti-mouse antibody (R&D Systems, Minneapolis, MN). After 30 min, cells were washed and assessed with a BD FACSCalibur cytometer (BD Biosciences, San Jose, CA) with CellQuest (BD Biosciences) and FlowJo (Tree Star, Ashland, OR) software.
Organ Distribution of AAVrh.10 Vector-mediated Transgene Expression Following Intravenous Administration
To evaluate organ distribution of the AAVrh.10 vector following intravenous administration, AAVrh.10-Luc (1011 genome copies) in 100 μl PBS was administered intravenously into female C57BL/6 mice (n=6). As control, the same volume of PBS was administered intravenously to 3 mice. Three wk after administration, the animals were sacrificed and tissues samples were collected from each animal, including: brain, heart, lung, diaphragm, liver, spleen, kidney, uterus, and quadriceps muscle. The total weight of the organs was determined, and then the organs were analyzed for luciferase transgene expression. The luciferase enzyme activity in homogenates of the individual organs was assessed, and activities were represented as relative light units quantified in a luminometer (Promega, Madison, WI), and standardized by total protein concentration, using a bicinchoninic acid (BCA) assay (BioRad, Hercules, CA). The relative light units were also multiplied by total organ weight11.
Anti-HER2 Antibody Levels in Mice Following AAVrh.10aHER2-myc Administration
Anti-human HER2 antibody levels in serum were assessed as a function of time in C57Bl/6 mice receiving AAVrh.10αHER2-myc (1011 genome copies, intravenously) by Western analysis and by a human HER2-specific ELISA. The Western analysis was carried out under non-reducing conditions with myc-specific antibody as described above. The HER2-specific ELISA was carried out using flat bottomed 96-well EIA/RIA plates (Corning, New York, NY) coated with 0.3 μg extracelluar domain of human HER2 per well in a total volume of 100 μl PBS overnight at 4°C. The extracellular domain of HER2 was expressed as a His-tagged protein in 293 cells and purified by nickle affinity chromatography (GE Healthcare, Piscataway, NJ). The coated ELISA plates were blocked with 5% dry milk, washed, and 1:100 dilutions of mouse serum in PBS containing 1% dry milk were added to each well and incubated at 25 °C for 60 min. The plates were washed three times with PBS-Tween and 100 μl/well of 1:10,000 diluted horseradish peroxidase (HRP) conjugated goat anti-mouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in PBS containing 1% dry milk was added and incubated for 60 min. The plates were washed four times and peroxidase substrate (100 μl/well; Bio-Rad, Hercules, CA) was added; after 17 min, the reaction was stopped by addition of 2% oxalic acid (100 μl/well) and absorbance at 415 nm was measured. The standards were prepared by protein G column purification of anti-HER2 antibody from supernatant of pAAVαHER2-myc transfected 293 cells. Antibody levels to human HER2 were estimated by calculating antibody concentrations of a serially diluted standard of known concentration.
Anti-AAV and Anti-idiotypic Antibody Responses
Anti-AAVrh.10 antibodies in serum from AAVrh.10αHER2-myc treated C57Bl/6 mice were assessed by ELISA. Serum from naive mice were used as controls. Flat bottomed 96-well EIA/RIA plates (Corning, New York, NY) were coated with 109 genome copies AAVrh.10GFP per well, or AAV6.2LacZ as control in a total volume of 100 μl PBS overnight at 4°C. Plates were then blocked with 5% dry milk and washed. Serum dilutions (1:100) in PBS containing 1% dry milk were added to each well and incubated for 60 min. Anti-AAVrh.10 antibodies were detected with HRP conjugated goat anti-mouse antibody.
For analysis of a possible anti-idiotypic antibody response against the anti-HER2 anti-body, the Fab fragment of anti-HER2-myc antibody was prepared from protein G column purified full length anti-HER2-myc protein using mouse IgG1 Fab and F(ab)2 Preparation Kit (Pierce, Rockford, IL). Flat bottomed 96-well EIA/RIA plates (Corning, New York, NY) were coated with 0.5 μg Fab fragment of anti-HER2-myc per well, or as control, full length anti-HER2-myc, in a total volume of 100 μl PBS overnight at 4°C. The purity of the Fab fragment was assessed by ELISA using 1:8000 diluted HRP-conjugated sheep anti-mouse IgG heavy and light chain antibody, mouse anti-myc antibody (GeneTex, San Antonio, TX) and goat anti-mouse Fc antibody (SIGMA, Saint Louis, MO). The specific binding to the HER2 protein by Fab fragment was also assessed by HER2-specific ELISA as described above. After purity determination, Fab fragment of anti-HER2-myc was used to coat the plates and then blocked and washed as described above. Serum from AAVrh.10αHER2-myc treated C57Bl/6 mice or naive mice were diluted 1:100 and then added to each well and incubated at 25°C for 60 min. Anti-idiotypic antibodies were detected by HRP conjugated goat anti-mouse Fc antibody.
Human Tumor Model
Female nude mice, 6 to 8 wk of age, were obtained from Taconic (Germantown, NY) and housed under specific pathogen-free conditions. On day 0, mice were treated by intravenous administration of 1011 genome copies/mouse AAVrh.10αHER2-myc or, as controls, AAVrh.10αPA-myc or PBS. Tumor cells (107 Calu-3) were injected subcutaneously in the right flank of mice on day 21. The size of each tumor was measured using calipers 2 times weekly, and tumor volume was calculated as length×width2×0.52. When the mice became morbid, the tumor ulcerated, tumor diameter reached 15 mm, or tumor volume reached 1000 mm3, the mice were sacrificed, and this was recorded as the date of death.
Statistical Analysis
All data are shown as mean ± standard error. Statistical comparison was made using the two-tailed Students t-test and a value of p<0.05 indicating significance. Survival evaluation was carried out using Kaplan-Meier analysis.
Results
Characterization of the anti-HER2 Antibody Expression Cassettes
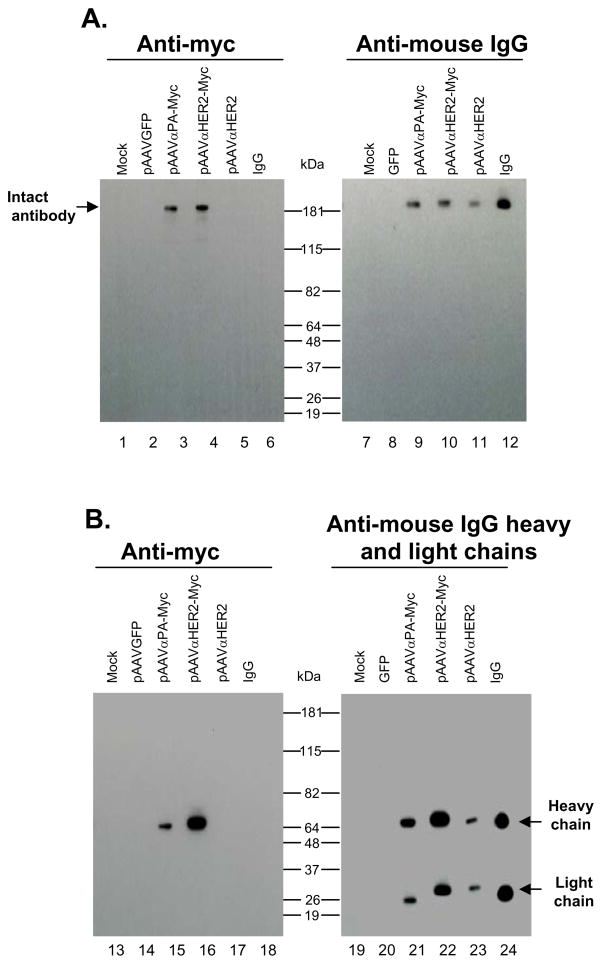
To examine expression of the anti-HER2 antibody by the expression cassettes, 293 cells were transfected with pAAVαHER2 and pAAVαHER2-myc, or as controls, pAAVαPA-myc, pGFP and mock. At 72 hr, cell media were collected and antibody expression was examined by Western analysis under non-reducing (Figure 1A) and reducing condition (Figure 1B). Medium collected from pAAVαHER2 and pAAVαHER2-myc transfected cells demonstrated the presence of full length antibody under non-reducing condition (lanes 10, 11) and separated antibody heavy and light chains under reducing condition (lanes 22, 23). The stoichiometry of the heavy and light chains was similar for the IgG control and the antibody expressed by the pAAVαHER2 vector (0.92 versus 1.02 by densitometry). Medium collected from pAAVαHER2-myc transfected cells also demonstrated the presence of myc tag-labeled heavy chain under reducing conditions (lane 4) and non-reducing condition (lane 16). No antibody was detected in medium from mock transfected or pGFP transfected cells.
Figure 1.
Expression of anti-HER2 monoclonal antibody in 293 cells transfected with expression plasmids. The plasmids were those used to construct AAV vectors. After 72 hr, cell medium were collected and assessed for anti-HER2 antibody expression by Western analysis with an anti-myc or sheep anti-mouse IgG heavy and light chain antibody. A. Western analysis under non-reducing conditions. Lanes 1–6, assessed with mouse anti-myc antibody. Lanes 7–12, assessed with sheep anti-mouse IgG antibody. The expected migration position of expressed IgG antibody is shown by the arrow. Lane 1 - mock; lane 2-pGFP negative control; lane 3 -pAAVαPA-myc; lane 4 - pAAVαHER2-myc; lane 5 - pAAVαHER2; lane 6 - purified mouse IgG1; lane 7 - mock; lane 8; pGFP negative control; lane 9 - pAAVαPA-myc; lane 10 -pAAVαHER2-myc; lane 11 - pAAVαHER2; and lane 12 - purified mouse IgG1. B. Western analysis under reducing conditions. Lanes 13–18, assessed with mouse anti-myc antibody. Lanes 19–24, assessed with sheep anti-mouse IgG antibody. Expected migration position of the expressed heavy and light chains of IgG antibody are shown by the arrows. Lane 13 - mock; lane 14 - pGFP negative control; lane 15 - pAAVαPA-myc; lane 16 - pAAVαHER2-myc; lane 17 -pAAVαHER2; lane 18 - purified mouse IgG1; lane 19 - mock; lane 20 - pGFP negative control; lane 21 - pAAVαPA-myc; lane 22 - pAAVαHER2-myc; lane 23 - pAAVαHER2; and lane 24 -purified mouse IgG1.
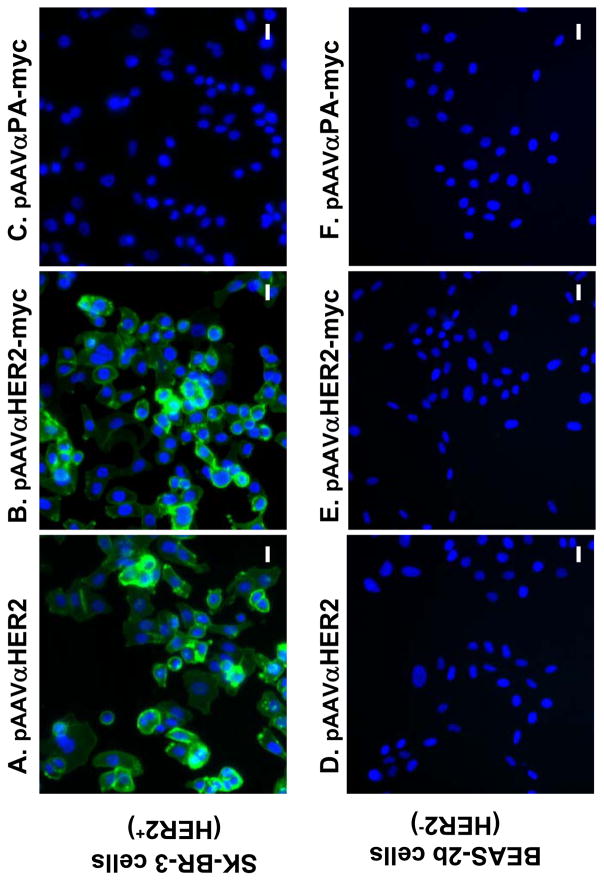
The specificity of the anti-HER2 antibody expressed from the plasmids for human HER2 was first assessed by immunofluorescent staining. Medium collected from pAAVαHER2 and pAAVαHER2-myc transfected cells recognized HER2 positive SK-BR-3 cells (Figure 2A, B) but not HER2 negative BEAS-2b cells (Figure 2D, E). In contrast, medium collected from pAAVαPA-myc transfected cells did not recognize either SK-BR-3 or BEAS-2b cells (Figure 2C, F).
Figure 2.
Specific binding of expressed anti-HER2 antibody to HER2 positive cells. Medium were collected from 293 cells transfected with pAAVαHER2, pAAVαHER2-myc and pAAVαPA-myc, respectively and added to fixed HER2 positive SK-BR-3 cells or fixed HER2 negative BEAS-2b cells. HER2 specific binding was assessed by immunofluorescent microscopy with Alexa 488 labeled goat anti-mouse second antibody. A. SK-BR-3 cells incubated with αHER2; B. SK-BR-3 cells, αHER2-myc; C. SK-BR-3 cells, αPA-myc; D. BEAS-2b, αHER2; E. BEAS-2b cells, αHER2-myc; and F. BEAS-2b cells, αPA-myc. All panels, bar = 20 μm.
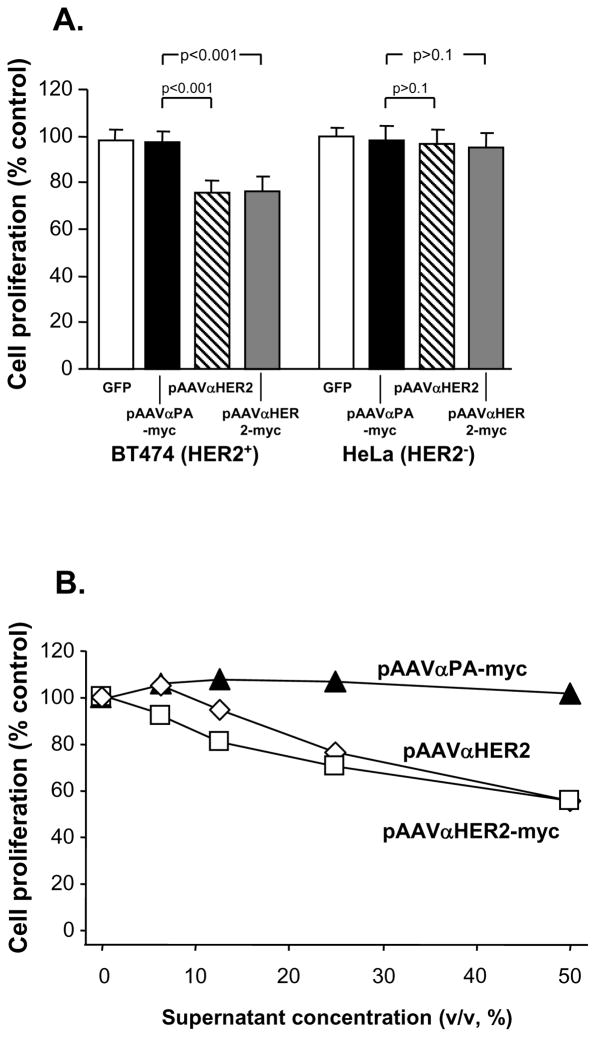
To assess whether the anti-HER2 antibody directed by the expression cassettes was functional, inhibition of cell growth was demonstrated in vitro with HER2 positive (BT474) and HER2 negative (HeLa) cell lines. Cells growth was assessed after exposure to medium from pAAVαHER2, pAAVαHER2-myc, pAAVαPA-myc or pGFP transfected 293 cells, using medium from mock transfected cells as a control. When HeLa cells were used, there was no impact of any conditioned medium on the growth rate (p>0.1 comparing all groups to the HeLa cells exposed to conditioned medium of mock transfected cells, Figure 3A). After 3 days, treatment of BT474 cells with medium from pAAVαPA or pGFP transfected cells had no impact on growth rate compared to that of BT474 cells treated with mock transfected cell media (p>0.2). In contrast, BT474 cells treated with medium from pAAVαHER2 or pAAVαHER2-myc transfected cells showed suppression of growth relative to the controls (p<0.0001).
Figure 3.
Inhibition of HER2 positive cell proliferation by anti-HER2 antibody expressed by pAAVαHER2 and pAAVαHER2-myc plasmids. Media were collected from 293 cells transfected with mock, pGFP, pAAVαPA-myc, pAAVαHER2, pAAVαHER2-myc and added to HER2 positive BT474 cells and HER2 negative HeLa cells. After various time periods, cell proliferation was assessed. The data is expressed as the relative cell number compared to mock medium treated cells. Each point represents the mean of 8 replicates. A. Specific suppression of HER2+ cells proliferation by anti HER2 antibody expressed by the 293 cells. Medium from pGFP, pAAVαHER2, pAAVαHER2-myc or pAAVαPA-myc or transfected cells were added to BT474 (HER2+) or HeLa cells (HER2-) and cell proliferation assessed after 3 days. B. Dose-dependent suppression of HER2+ cells proliferation by anti HER2 antibody expressed by the 293 cells. Medium from pAAVαPA-myc, pAAVαHER2 or pAAVαHER2-myc transfected cells was 2-fold serial diluted and then added to BT474 cells. After 5 days, cells proliferation was assessed.
To determine the dose-dependent anti-tumor growth effect of anti-HER2 antibody, medium from pAAVαHER2, pAAVαHER2-myc and pAAVαPA-myc transfected 293 cells was serially diluted from 1:2 to 1:16 and the impact on proliferation of BT474 HER2 positive cells assessed (Figure 3B). The anti-HER2 antibody showed a dose dependent anti-proliferation effect with more than 40% growth inhibition effect achieved when 1:2 dilutions of medium containing anti-HER2 antibody were used (p<0.0001 compared to growth rate of cells treated with medium from pAAVαPA transfected cells).
Distribution of Transgene Following Intravenous Administration of AAVrh.10 Vectors
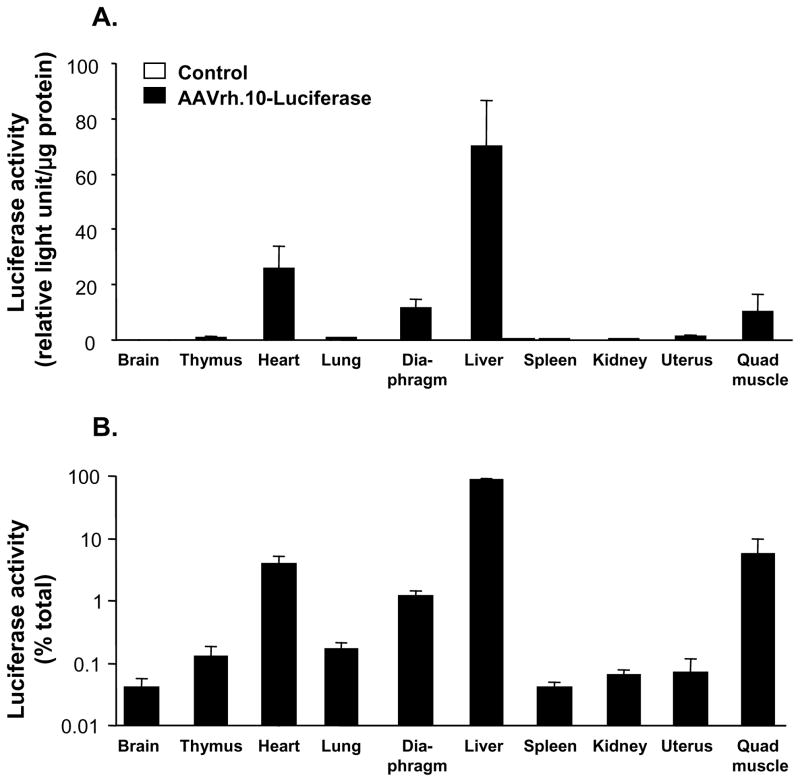
To determine the pattern of organ distribution of transgene expression directed by the AAVrh.10 serotype following intravenous administration, expression of luciferase following injection of AAVrh.10-Luc was assessed. Luciferase specific activity was highest in the liver with much smaller amounts of activity in the heart, diaphragm and quadriceps (Figure 4A) and negligible expression level in spleen and thymus. When expressed as the total amount of luciferase expressed in each organ the liver expressed 88 ± 4% of the total body luciferase activity (Figure 4B) implying that the liver is the primary tissue expressing the anti-HER2 antibody.
Figure 4.
Organ distribution of transgene expression following intravenous administration of AAVrh.10 vector expressing the luciferase reporter transgene. On day 0, Female C57B1/6 mice (n=6) received intravenous administration of 1011 genome copies AAVrh.10-luciferase vector, or as controls, PBS (n=3). After 3 wk, the mice were sacrificed, the organs were collected and the weight of each organ was determined. Samples of each organ were homogenized and the organ distribution of luciferase activity was determined and normalized to protein concentration. A. Luciferase activity in relative light units per μg of protein (mean ± standard error). B. Percent luciferase activity per organ relative to total body luciferase activity. The total luciferase activity was determined as the sum for all organs derived by multiplying the weight of each organ by the luciferase activity in relative light units/mg protein.
Specificity of anti-HER2 Antibody Expressed In Vivo
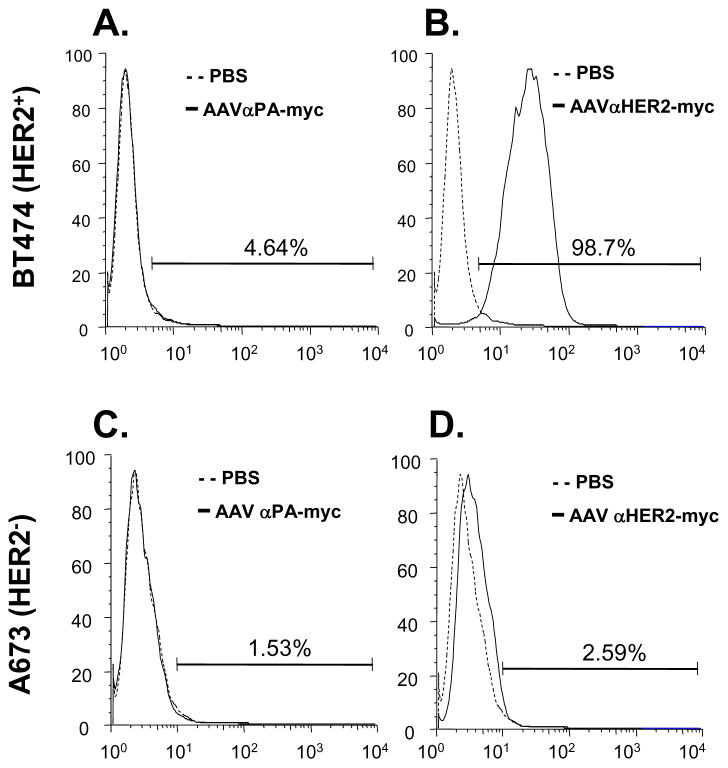
The expression cassettes for anti-HER2 and anti-HER2-myc were packaged into the AAVrh.10 capsid, and administrated intravenously into C57Bl/6 mice. The specificity of anti-HER2 antibody expressed by AAVrh.10 in vivo to human HER2 was assessed by flow cytometry. Serum from AAVrh.10αHER2-myc-treated mice specifically recognized HER2 positive BT474 cells but not HER2 negative A673 cells (Figure 5A, B). As controls, serum from PBS and AAVrh.10αPA-myc-treated mice did not recognize either of these cells (Figure 5C, D).
Figure 5.
HER2-specific cell binding from αHER2 antibody expressed in vivo following administration of AAVrh.10αHER2. PBS, AAVrh.10αHER2-myc or AAVrh.10αPA-myc (1011 ge-nome copies each) were administrated intravenously into C57Bl/6 mice and after 30 days serum was collected and pooled and used as the primary antibody. HER2 positive BT474 cells (panels A, B) and HER2 negative A673 cells (panels C, D) were exposed to the sera using FITC-labeled goat anti-mouse antibody and flow cytometry for detection. Solid lines - AAVrh.10αPA-myc sera or AAVrh.10αHER2-myc sera. Dashed lines - PBS control sera. A. BT474 cells exposed to sera from mice receiving the control vector AAVrh.10αPA-myc or PBS. B. BT474 cells exposed to sera from mice receiving AAVrh.10αHER2-myc or PBS. C. A673 cells exposed to sera from mice receiving the control vector AAVrh.10αPA-myc or PBS. D. A673 cells exposed to sera from mice receiving AAVrh.10αHER2-myc or PBS.
Persistent AAVrh.10αHER2-mediated Anti-HER2 Antibody Expression
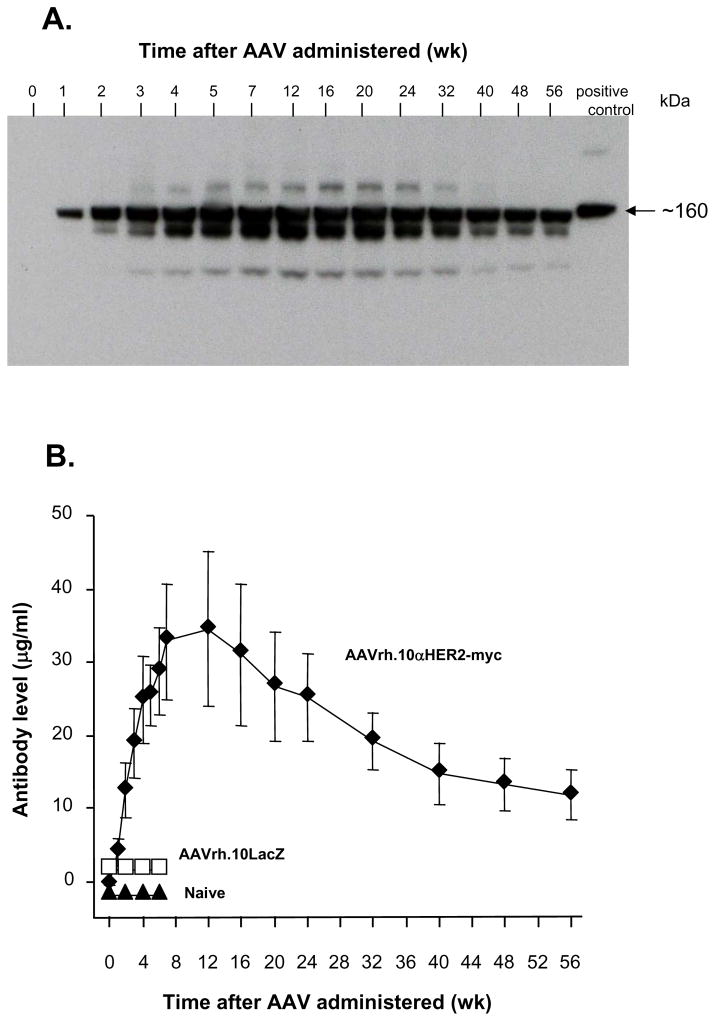
To evaluate the persistence of in vivo expression of anti-HER2 antibodies in serum of C57Bl/6 mice, electrophoresis under non-reducing condition followed by Western analysis was employed to detect the myc tag attached to the heavy chain. Pooled serum samples from AAVrh.10αHER2-treated mice taken at various time points were assessed. The Western analysis showed the myc tagged anti-HER2 antibody was detectable at 1 wk, peaked at about wk 5 to 6 months, and was sustained for more than 1 yr (Figure 6A).
Figure 6.
Time course of AAVrh.10 mediated expression of anti-HER2 antibody in vivo. On day 0, C57Bl/6 mice (n=5/group) received intravenous administration of 1011 genome copies AAVrh.10αHER2-myc or AAVrh.10LacZ. Naive mice were used as an additional control. Serum was collected at different time points. A. Anti-HER2 antibody levels assessed by myc-specific Western analysis under non-reducing conditions. Serum from AAVrh.10αHER2-myc mice was pooled. Purified anti-HER2-myc was used as positive control. B. Serum anti-HER2 antibody levels were measured by human HER2-specific ELISA. Purified anti-HER2 antibody served as standard. Data was obtained from n=5 mice per group and is plotted as mean ± standard error.
The time course of expression of anti-HER2 antibodies in the serum following intravenous adminstration of AAVrh.10αHER2-myc in C57Bl/6 mice was quantitated using a human HER2-specific ELISA. The antibody was detectable by week 1, peaked at week 12 and slowly declined over for 1 yr (Figure 6B). At 56 wk, the last time point tested, the anti-HER2 antibody levels were still 12.2 ± 1.5 μg/ml. No anti-HER2 antibody was detected in the sera from the control mice receiving AAVrh.10LacZ or naive mice.
AAVrh.10αHER2-induced Humoral Anti-AAV and Anti-idiotypic Responses
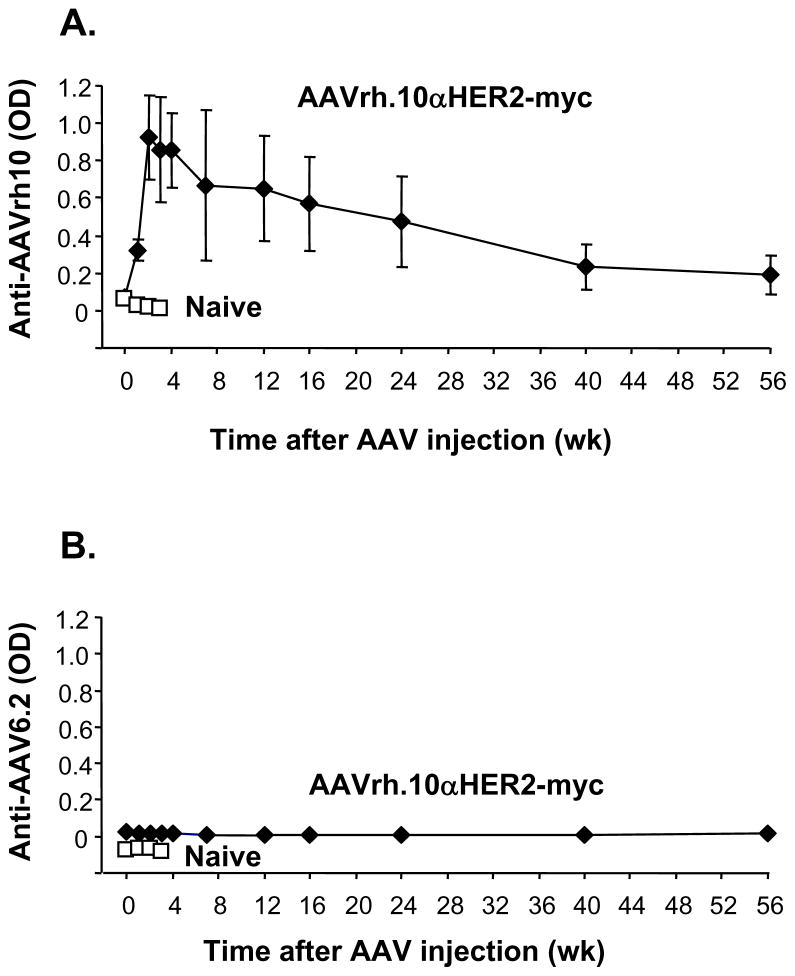
For analysis of the humoral anti-adeno-associated virus response, serum samples taken at various time points were assessed by ELISA against AAV capsid proteins. Anti-AAVrh.10 antibodies could be detected as early as 1 wk after AAV administration, peaked at 2 to 3 wk, and then slowly declined over 1 yr (Figure 7A). There were no detectable antibody responses against the capsid of a different AAV serotype (AAV6.2 LacZ; Figure 7B), demonstrating the specificity of the anti-AAV humoral immune response.
Figure 7.
Humoral anti-AAV immune response. Sera from C57Bl/6 mice treated with AAVrh10αHER2-myc or naive mice were assayed for anti-AAV antibodies by ELISA. A. Anti-AAVrh.10 ELISA. The ELISA plate was coated with AAVrh.10GFP. B. Anti-AAV6.2 ELISA. The ELISA plate was coated with AAV6.2LacZ. Data (mean ± standard error) was obtained from n=5 animals per group.
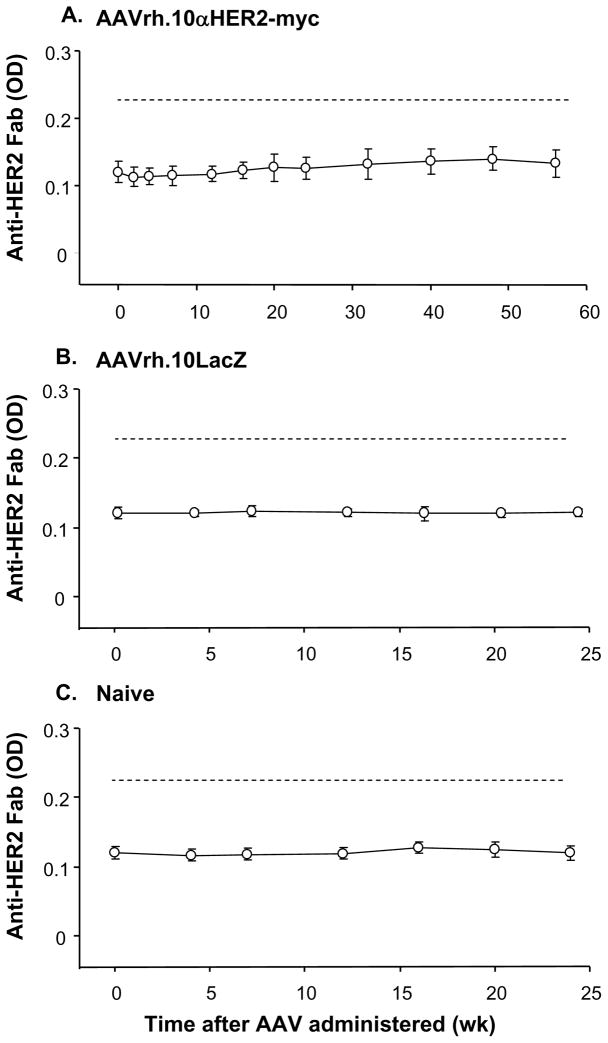
The possibility of an anti-idiotype response against genetically delivered anti-HER2 antibody was investigated by direct ELISA, in which microtiter plates were coated with purified anti-HER2-myc Fab fragments. The purity of the anti-HER2-myc Fab fragment was first verified by ELISA showing that the purified anti-HER2 Fab could be recognized by anti-mouse heavy and light chain antibody but not by anti-myc antibody or anti-mouse Fc antibody (i.e., there was no residual Fc domain in the coating antigen). The integrity and anti-HER2 functionality of the Fab fragment was further demonstrated by ELISA showing binding to the HER2 extracellular domain. Using ELISA with this verified anti-HER2 Fab, no anti-idiotypic antibodies were detectable in the serum from all AAVrh.10αHER2-myc treated mice assessed over 1 yr (Figure 8).
Figure 8.
Detection of anti-idiotype immune response in mice administrated AAVrh.10αHER2-myc. The anti-idiotypic response against anti-HER2 antibody was analyzed by a direct ELISA using purified Fab of anti-HER2 antibody as coating antigen and anti-Fc as detection. Controls showed that the Fab coating antigen retained recognition of HER2 and was not recognized by the anti-Fc detection antibody. A. Sera from AAVrh.10αHER2-myc treated mice (n=5). B. Sera from AAVrh.10LacZ treated mice (n=5). C. Sera from naive mice (n=5). The dashed line shows the detection limit calculated as two times the mean background of 3 wells with no serum added.
Effects of AAVrh.10αHER2 on HER2+ Human Tumor Growth
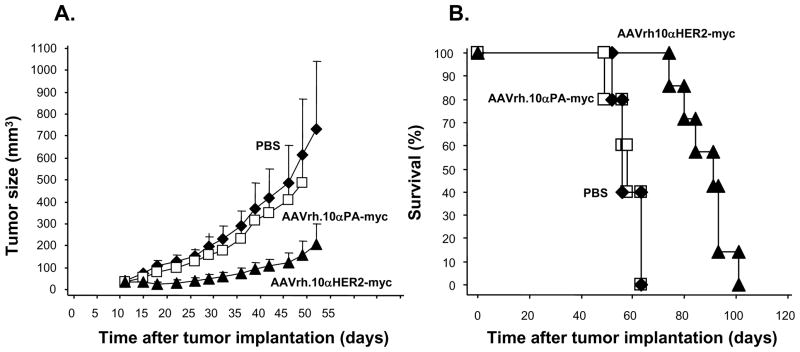
To determine the effects of AAVrh.10 expressed anti-HER2 antibody on tumor growth in vivo, Balb/c nu/nu mice were treated with AAVrh.10αHER2-myc, or as controls, AAVrh.10αPA-myc and PBS. After 21 days, tumors were generated by implanting HER2 overexpressing Calu-3 cells subcutaneously onto the right flank of the mice. Tumor growth was determined by measurement tumor volume every 3 to 4 days post-vector administration. Intravenous administration of AAVrh.10αHER2-myc to nude mice induced a significant suppression of Calu-3 tumor growth relative to animals that received AAVrh.10αPA-myc or PBS (p<0.01 compared with all other groups, Figure 9A). The survival of Calu-3 tumor-bearing mice was also monitored (Figure 9B). In contrast to mice that received AAVrh.10αPA-myc or PBS, mice treated with AAVrh.10αHER2-myc exhibited a significant survival advantage (p<0.01 compared with all other groups).
Figure 9.
Anti-tumor efficacy mediated by AAVrh.10αHER2. On day 0, Balb/c nu/nu mice received intravenous administration of 1011 genome copies AAVrh.10αHER2-myc (n=7) or, as controls, AAVrh.10αPA-myc (n=5) or PBS (n=5). After 21 days, Calu-3 tumor cells (107) were implanted subcutaneously in the right flank of the mice. A. Tumor volume was assessed 2 times weekly and is reported as the average tumor volume (mm3) ± standard error. B. Survival of AAVrh.10αHER2-myc treated Calu-3 tumor bearing mice. Survival is presented as the percentage of surviving mice in each group.
Discussion
There are now over 20 US Food and Drug Administration approved therapeutic monoclonal antibodies for the treatment of cancer and of autoimmune, inflammatory and infectious diseases, with many more in preclinical and clinical development22. Chronic diseases such as cancer require long-term and repeated administration of the therapeutic monoclonal antibody over time. Trastuzumab, a humanized monoclonal anti-HER2 antibody, is effective in the treatment of HER2 overexpression breast cancer8. In this study, we demonstrate that a single administration of an AAVrh.10 vector encoding the murine equivalent of Trastuzumab specific for human HER2, expressed anti-HER2 primarily in the liver and persisted for at least 1 yr without inducing an anti-idiotype response, and resulted in slower growth of xenografted human tumors in mice.
Trastuzumab (Herceptin)
Trastuzumab was derived from the murine anti-HER2 monoclonal antibody 4D52,10. Humanization of 4D5 to reduce immunogenicity and to increase half-life in humans resulted in an antibody molecule that is 95% human and 5% murine but retaining the same biochemical and pharmacologic properties of the parental antibody10,23. In preclinical studies with immunodeficient mice, trastuzumab effectively inhibited the growth of various implanted human tumors overexpressing HER224,25. The mechanism of action of trastuzumab includes initiation of G1 arrest and induction of p27, inhibition of HER2 shedding, inhibition of the PI3K pathway, inhibition of angiogenesis, and induction of immune mechanism26.
The initial clinical trials of trastuzumab tested it as a single agent in HER2-overexpressing metastatic breast cancer, with resulting response rates of 12 to 34% for a median duration of 9 months. Phase III trials combining trastuzumab with paclitaxel or docetaxel demonstrated increased response rates, time to disease progression and overall survival compared with trastuzumab monotherapy8. The recommended duration of trastuzumab treatment is currently one yr (adjuvant) or until the progression of the disease (e.g., metastatic disease). In spite of the encouraging clinical results, the high cost of trastuzumab has evoked a heated debate.
Genetic Antibody-based Immunotherapy
Because of the costs and time involved in producing and manufacturing monoclonal antibodies, genetic transfer of therapeutic antibodies became an attractive strategy as both short-term and long-term therapy27,28. Initially gene therapy was used to deliver single chain antibodies, but this strategy has some inherent limitations. Compared to full length antibodies, single chain antibodies have a short half-life, fast clearance, low affinity, the lack of Fc functional domain, and inability to induce antibody-dependent cellular cytotoxicity and complement dependent cytotoxicity, all contributing to reduce their efficacy compared to full length antibodies29.
The challenge for successful genetic delivery of full-length antibodies is to find ways of generating stoichiometric amounts of both the heavy and light chains in the antibody producing cells28. Various expression methods have been developed utilizing mono-and bicistronic expression cassettes encoded on one or two vectors30. The use of a single vector with two identical promoters or two different promoters for stable expression introduces the risk of recombination events or may result in unbalanced expression of the two different chains respectively30. In contrast, internal ribosome entry sites allow the coexpression of multiple polypeptide chains from the same mRNA, and has been used to express full length antibodies successfully both in vitro and in vivo31–33. However, this method is both cell and gene dependent and can often result in lower expression of the gene encoded by the second cistron34. An antibody expression system that uses the foot-and-mouth-disease virus (FMDV)-derived 2A self-processing sequence to express full-length antibodies from a single open reading frame was first described by Fang et al.12 In the current study, the FMDV-2A-based cassette was employed, yielding apparent equimolar antibody expression of heavy chain and light chain, with functional full length anti-HER2 antibody generated in both immune competent and immune compromised mice.
Although antibodies have been expressed in vivo using plasmids or cells transduced with retroviral vectors, these approaches achieved only marginal levels of antibody production35,36. In contrast, high therapeutic antibody level have been achieved with adenovirus and adeno-associated virus vectors. With adenovirus as a platform, detectable serum CD20 antibody level was obtained in nude mice from day 3 through at least 8 wk post-administration, levels high enough for complete elimination of preestablished B-cell lymphoma cells in nude mice37. A single administration of an adenovirus encoding anti-VEGF antibody suppresses the proliferation and vascularity of pre-existing human tumors in immunodeficient mice, with concomitant suppression of the growth of the tumors and increased survival of the tumor-bearing mice 33. With adeno-associated virus as a platform, high level and long term antibody expression has been achieved by using various serotypes, including AAV812, AAVrh.1011,13 and AAV138.
Relevant to expressing HER2 antibodies, delivery of a gene encoding an anti-HER2 intracellular single-chain antibody resulted in down-regulation of cell surface HER2 levels and induction of apoptosis in HER2 overexpressing cancer cells39,40. A single chain anti-HER2 antibody linked to caspase 6, a pro-apoptotic molecule, generated a chimeric HER2-targeted toxic protein41. More recently, an adenovirus was used to express full-length HER2 antibody, resulting in high transient anti-HER2 antibody levels with concomitant tumor suppression in nude mice42.
In the present study, a single AAVrh.10 mediated genetic delivery of an anti-HER2 antibody suppressed but did not reverse the growth of HER2-positive tumors, possibly due to the sensitivity of Calu-3 cells to HER2 antibody. Expression of anti-HER2 might be enhanced if the vector was further optimized. Instead of CMV promoter, CMV enhancer/chicken β-actin/rabbit β-globin hybrid promoter (CAG), elongation factor-1 alpha (EF1alpha), phosphoglycerokinase (PGK) or other promoters might be used to provide enhanced antibody gene expression in vivo43. To enhance gene expression post-transcriptionally, the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) can also be used44. Codon and GC content optimization of expression cassette might also help.
Immune Responses
One therapeutical concern using genetic delivery of antibodies is that the viral vector will induce correspondent anti-virus antibodies45. In the current study, we found that AAVrh.10 coding for anti-HER2 induces an anti-AAVrh.10 response in immune competent mice, but long term expression will still observed for at least 1 yr. Compared to liver and other major target organs of AAVrh.10, the immune organ spleen and thymus only have negligible transgene expression after AAVrh.10 intravenously administration.
Another therapeutical concern is in the development of anti-idiotype antibodies, in that high dose and repeated administration of monoclonal antibodies can result in anti-idiotype responses46. Additionally, the ability of virus vectors to stimulate an immune response against ectopic proteins expressed from the transgene they carry make the immune responses to genetic delivered antibodies more likely47. With adenovirus as a platform, high levels of circulating ectopic monoclonal antibodies was obtained following in vivo gene transfer without inducing an anti-idiotype response sufficiently robust to exert a neutralizing effect32. Consistent with this observation, following AAVrh.10αaHER2 administration in immune competent mice in the present study, no anti-idiotype antibodies were observed.
Acknowledgments
We thank B. Ferris, B.P, De and A. Bajak for technical assistance, and T. Bryan and N. Mohamed for help in preparing this manuscript. These studies were supported, in part, by U01 HL66952 and the Will Rogers Memorial Fund, Los Angeles, CA.
Footnotes
These studies were supported, in part, by U01 HL66952
References
- 1.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 2.Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990;50:1550–1558. [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 5.Chan T, Sami A, El-Gayed A, Guo X, Xiang J. HER-2/neu-gene engineered dendritic cell vaccine stimulates stronger HER-2/neu-specific immune responses compared to DNA vaccination. Gene Ther. 2006;13:1391–1402. doi: 10.1038/sj.gt.3302797. [DOI] [PubMed] [Google Scholar]
- 6.Drebin JA, Link VC, Greene MI. Monoclonal antibodies specific for the neu oncogene product directly mediate anti-tumor effects in vivo. Oncogene. 1988;2:387–394. [PubMed] [Google Scholar]
- 7.Garrett JT, Rawale S, Allen SD, Phillips G, Forni G, Morris JC, et al. Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2/neu. J Immunol. 2007;178:7120–7131. doi: 10.4049/jimmunol.178.11.7120. [DOI] [PubMed] [Google Scholar]
- 8.Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26:3637–3643. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 9.Leyland-Jones B, Arnold A, Gelmon K, Verma S, Ayoub JP, Seidman A, et al. Pharmacologic insights into the future of trastuzumab. Ann Oncol. 2001;12 (Suppl 1):S43–S47. doi: 10.1093/annonc/12.suppl_1.s43. [DOI] [PubMed] [Google Scholar]
- 10.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J, et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther. 2006;13:67–76. doi: 10.1016/j.ymthe.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M, et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol. 2005;23:584–590. doi: 10.1038/nbt1087. [DOI] [PubMed] [Google Scholar]
- 13.De BP, Hackett NR, Crystal RG, Boyer JL. Rapid/sustained anti-anthrax passive immunity mediated by co-administration of Ad/AAV. Mol Ther. 2008;16:203–209. doi: 10.1038/sj.mt.6300344. [DOI] [PubMed] [Google Scholar]
- 14.Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ, Wilson JM. Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther. 2009;17:294–301. doi: 10.1038/mt.2008.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song Y, Lou H, Boyer JL, Limberis MP, Vandenberghe LH, Hackett NR, Leopold PL, Wilson JM, Crystal RG. Functional CFTR expression in cystic fibrosis airway epithelial cells by AAV6.2-mediated segmental trans-splicing. Hum Gene Ther. 2009;20:267–81. doi: 10.1089/hum.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunn PA, Jr, Helfrich B, Soriano AF, Franklin WA, Varella-Garcia M, Hirsch FR, et al. Expression of Her-2/neu in human lung cancer cell lines by immunohistochemistry and fluorescence in situ hybridization and its relationship to in vitro cytotoxicity by trastuzumab and chemotherapeutic agents. Clin Cancer Res. 2001;7:3239–3250. [PubMed] [Google Scholar]
- 17.Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M, et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene. 1999;18:2241–2251. doi: 10.1038/sj.onc.1202526. [DOI] [PubMed] [Google Scholar]
- 18.Spiridon CI, Ghetie MA, Uhr J, Marches R, Li JL, Shen GL, et al. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res. 2002;8:1720–1730. [PubMed] [Google Scholar]
- 19.Jia LT, Zhang LH, Yu CJ, Zhao J, Xu YM, Gui JH, et al. Specific tumoricidal activity of a secreted proapoptotic protein consisting of HER2 antibody and constitutively active caspase-3. Cancer Res. 2003;63:3257–3262. [PubMed] [Google Scholar]
- 20.Kern JA, Torney L, Weiner D, Gazdar A, Shepard HM, Fendly B. Inhibition of human lung cancer cell line growth by an anti-p185HER2 antibody. Am J Respir Cell Mol Biol. 1993;9:448–454. doi: 10.1165/ajrcmb/9.4.448. [DOI] [PubMed] [Google Scholar]
- 21.Martinez-Ramirez A, Rodriguez-Perales S, Melendez B, Martinez-Delgado B, Urioste M, Cigudosa JC, et al. Characterization of the A673 cell line (Ewing tumor) by molecular cytogenetic techniques. Cancer Genet Cytogenet. 2003;141:138–142. doi: 10.1016/s0165-4608(02)00670-2. [DOI] [PubMed] [Google Scholar]
- 22.Dubel S. Recombinant therapeutic antibodies. Appl Microbiol Biotechnol. 2007;74:723–729. doi: 10.1007/s00253-006-0810-y. [DOI] [PubMed] [Google Scholar]
- 23.Harries M, Smith I. The development and clinical use of trastuzumab (Herceptin) Endocr Relat Cancer. 2002;9:75–85. doi: 10.1677/erc.0.0090075. [DOI] [PubMed] [Google Scholar]
- 24.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–2831. [PubMed] [Google Scholar]
- 25.Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17:2235–2249. doi: 10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]
- 26.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 27.Bakker JM, Bleeker WK, Parren PW. Therapeutic antibody gene transfer: an active approach to passive immunity. Mol Ther. 2004;10:411–416. doi: 10.1016/j.ymthe.2004.06.865. [DOI] [PubMed] [Google Scholar]
- 28.Marasco WA. Therapeutic antibody gene transfer. Nat Biotechnol. 2005;23:551–552. doi: 10.1038/nbt0505-551. [DOI] [PubMed] [Google Scholar]
- 29.Liu XY, Pop LM, Vitetta ES. Engineering therapeutic monoclonal antibodies. Immunol Rev. 2008;222:9–27. doi: 10.1111/j.1600-065X.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Menzel C, Meier D, Zhang C, Dubel S, Jostock T. A comparative study of different vector designs for the mammalian expression of recombinant IgG antibodies. J Immunol Methods. 2007;318:113–124. doi: 10.1016/j.jim.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Hotta A, Kamihira M, Itoh K, Morshed M, Kawabe Y, Ono K, et al. Production of anti-CD2 chimeric antibody by recombinant animal cells. J Biosci Bioeng. 2004;98:298–303. doi: 10.1016/S1389-1723(04)00285-3. [DOI] [PubMed] [Google Scholar]
- 32.Noel D, Pelegrin M, Kramer S, Jacquet C, Skander N, Piechaczyk M. High in vivo production of a model monoclonal antibody on adenoviral gene transfer. Hum Gene Ther. 2002;13:1483–1493. doi: 10.1089/10430340260185111. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe M, Boyer JL, Hackett NR, Qiu J, Crystal RG. Genetic delivery of the murine equivalent of bevacizumab (avastin), an anti-vascular endothelial growth factor monoclonal antibody, to suppress growth of human tumors in immunodeficient mice. Hum Gene Ther. 2008;19:300–310. doi: 10.1089/hum.2007.109. [DOI] [PubMed] [Google Scholar]
- 34.Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther. 2000;1:376–382. doi: 10.1006/mthe.2000.0050. [DOI] [PubMed] [Google Scholar]
- 35.Noel D, Pelegrin M, Marin M, Biard-Piechaczyk M, Ourlin JC, Mani JC, et al. In vitro and in vivo secretion of cloned antibodies by genetically modified myogenic cells. Hum Gene Ther. 1997;8:1219–1229. doi: 10.1089/hum.1997.8.10-1219. [DOI] [PubMed] [Google Scholar]
- 36.Tjelle TE, Corthay A, Lunde E, Sandlie I, Michaelsen TE, Mathiesen I, et al. Monoclonal antibodies produced by muscle after plasmid injection and electroporation. Mol Ther. 2004;9:328–336. doi: 10.1016/j.ymthe.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Su C, Lu Q, Shi W, Zhang Q, Wang X, et al. Generation of adenovirus-mediated anti-CD20 antibody and its effect on B-cell deletion in mice and nonhuman primate cynomolgus monkey. Mol Cancer Ther. 2008;7:1562–1568. doi: 10.1158/1535-7163.MCT-08-0297. [DOI] [PubMed] [Google Scholar]
- 38.Ho DT, Wykoff-Clary S, Gross CS, Schneider D, Jin F, Kretschmer PJ, et al. Growth inhibition of an established A431 xenograft tumor by a full-length anti-EGFR antibody following gene delivery by AAV. Cancer Gene Ther. 2008 doi: 10.1038/cgt.2008.68. [DOI] [PubMed] [Google Scholar]
- 39.Deshane J, Loechel F, Conry RM, Siegal GP, King CR, Curiel DT. Intracellular single-chain antibody directed against erbB2 down-regulates cell surface erbB2 and exhibits a selective anti-proliferative effect in erbB2 overexpressing cancer cell lines. Gene Ther. 1994;1:332–337. [PubMed] [Google Scholar]
- 40.Deshane J, Siegal GP, Alvarez RD, Wang MH, Feng M, Cabrera G, et al. Targeted tumor killing via an intracellular antibody against erbB-2. J Clin Invest. 1995;96:2980–2989. doi: 10.1172/JCI118370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu YM, Wang LF, Jia LT, Qiu XC, Zhao J, Yu CJ, et al. A caspase-6 and anti-human epidermal growth factor receptor-2 (HER2) antibody chimeric molecule suppresses the growth of HER2-overexpressing tumors. J Immunol. 2004;173:61–67. doi: 10.4049/jimmunol.173.1.61. [DOI] [PubMed] [Google Scholar]
- 42.Jiang M, Shi W, Zhang Q, Wang X, Guo M, Cui Z, et al. Gene therapy using adenovirus-mediated full-length anti-HER-2 antibody for HER-2 overexpression cancers. Clin Cancer Res. 2006;12:6179–6185. doi: 10.1158/1078-0432.CCR-06-0746. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen AT, Dow AC, Kupiec-Weglinski J, Busuttil RW, Lipshutz GS. Evaluation of gene promoters for liver expression by hydrodynamic gene transfer. J Surg Res. 2008;148:60–66. doi: 10.1016/j.jss.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ. Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther. 1999;10:2295–2305. doi: 10.1089/10430349950016942. [DOI] [PubMed] [Google Scholar]
- 45.Hackett NR, Kaminsky SM, Sondhi D, Crystal RG. Antivector and antitransgene host responses in gene therapy. Curr Opin Mol Ther. 2000;2:376–382. [PubMed] [Google Scholar]
- 46.Pelegrin M, Marin M, Noel D, Piechaczyk M. Genetically engineered antibodies in gene transfer and gene therapy. Hum Gene Ther. 1998;9:2165–2175. doi: 10.1089/hum.1998.9.15-2165. [DOI] [PubMed] [Google Scholar]
- 47.Cesco-Gaspere M, Zentilin L, Giacca M, Burrone OR. Boosting anti-idiotype immune response with recombinant AAV enhances tumour protection induced by gene gun vaccination. Scand J Immunol. 2008;68:58–66. doi: 10.1111/j.1365-3083.2008.02119.x. [DOI] [PubMed] [Google Scholar]