Introduction
Calcifying epithelial odontogenic tumors (CEOT; Pindborg tumor) are rare benign epithelial tumors of odontogenic apparatus comprising approximately 1% of all odontogenic tumors [1-4]. Although benign, CEOT are locally infiltrative tumors with a tendency to invade adjacent bone, displace the teeth and cause root resorption. CEOT have two clinical variants, namely intraosseous (central) CEOT representing 94% of all tumors and extraosseous (peripheral) CEOT accounting for less than 5% of cases[2, 5]. Clinically, intraosseous CEOT often present as slow-growing painless bony expansions which radiographically appear as irregular unior multilocular radiolucencies with scattered flecks of calcification[1, 2, 5]. CEOT have a well-recognized propensity to recur (10-15%) after surgical excision and exhibit even greater recurrence rate if they are treated by curettage or if the resection is incomplete [1, 6]. However, there have been a few reported cases of locally aggressive CEOT that either invaded into neighboring structures such as the maxillary sinus, caused cortical bone perforation or involved the surrounding soft tissue [7-12]. Both locally aggressive and non-aggressive CEOT are cytologically benign and cannot be distinguished by their histopathologic presentation [7-12]. On the other hand, malignant transformation of CEOT is extremely rare with only seven cases have been reported so far in the English literature (Table 1) [13-19]. Here, we report a unique case of malignant CEOT of the mandible that showed locally aggressive growth with multiple local recurrences, eventually metastasizing to the base of the skull leading to intracranial extension and death.
Table 1. Clinicopathologic features of previously reported cases of CEOT malignant transformation.
| Author | Age/Gender | Location | Clinical features | Histology | Follow-up |
|---|---|---|---|---|---|
| Basu et al. [13] | 75/M | Right mandible | Perforation of mandibular cortical plate and soft tissue invasion Regional lymph node metastasis |
Primary Tumor: Cellular and nuclear pleomorphism, increased mitotic activity | ANED at 4 year |
| Bouckaert et al. [14] | 54/M | Left maxilla | Invasion of orbital floor with intracranial extension | Primary tumor: Cellular and nuclear pleomorphism | N/A |
| Veness et al. [19] | 64/M | Left mandible | Perforation of the mandibular cortical plate and soft tissue invasion Regional lymph node metastasis |
Recurrent tumor: Increased mitotic activity, vascular invasion | ANED at 12 months |
| Cheng et al. [15] | 83/F | Anterior mandible | Perforation of mandibular cortical plates and pathologic fracture | Primary tumor: Clear cells, increased and atypical mitotic activity, pleomorphism and vascular invasion | ANED at 10 months |
| Kumar et al. [18] | 43/F | Left posterior mandible | Lingual cortical plate expansion and perforation Distant metastasis to lumbar vertebrae and pelvic bone |
Primary tumor: CEOT Recurrent tumor: Clear cell odontogenic carcinoma |
AWD at 3 years |
| Goldenberg et al. [16] | 40/M | Mandible | Rapidly growing mandibular mass | Primary tumor: N/A | N/A |
| Kawano et al. [17] | 54/M | Left mandible | Mandibular cortical plate perforation and soft tissue invasion Distant metastasis to lung |
Recurrent tumor: Cellular and nuclear pleomorphism, vascular invasion | AWD at 6 years |
| Current case | 45/F | Right mandible | Cortical plate perforation with soft tissue extension Distant metastasis to base of the skull and orbit |
Primary tumor: Nuclear and cellular pleomorphism, increased mitotic activity, necrosis | DOD within two years |
M: male; F: female; N/A: not available; ANED: alive with no evidence of disease; AWD: alive with disease; DOD: dead of disease
Report of a Case
A 45-year-old female presented to her dentist for evaluation and treatment of right mandibular swelling. The exact duration of this swelling is not known but the patient remembered having had it for approximately one year. This swelling was apparently asymptomatic until she heard a small “cracking” sound, which prompted her to seek treatment from her dentist. This mandibular swelling was not associated with local trauma or inflammation. Subsequently, the patient was referred to an oral and maxillofacial surgeon who performed an incisional biopsy of this lesion, which was diagnosed as calcifying epithelial odontogenic tumor (CEOT). The patient was then referred to the oral and maxillofacial surgery department at the authors' institution for further evaluation and treatment.
The patient's medical history was non-contributory and she was taking no medication at presentation. Her surgical history was significant for a complete abdominal hysterectomy. Patient reported smoking a pack of cigarettes per day for more than 25-years. Extraoral examination revealed a hard, immobile mass involving the right angle of the mandible and preauricular region (Figure 1A). Intraoral examination showed expansion of the buccal and lingual surface of the right posterior mandible extending from tooth #28 to the retro-molar region (Figure 1B). There were no pulsations, bruits, or thrills associated with this swelling. No submandibular or cervical lymphadenopathy was present. Cranial nerve examination was unremarkable.
Figure 1.
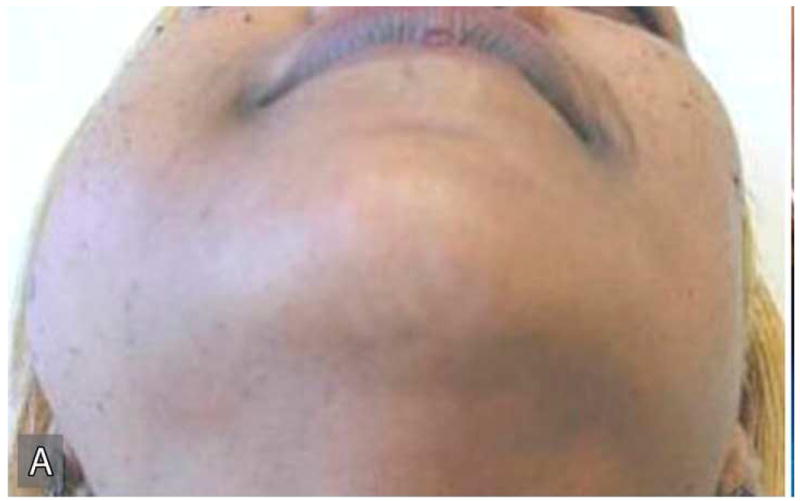
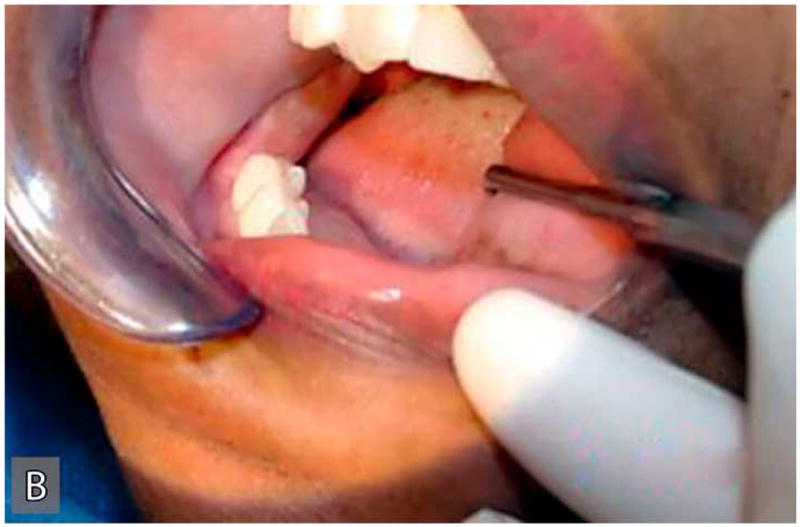
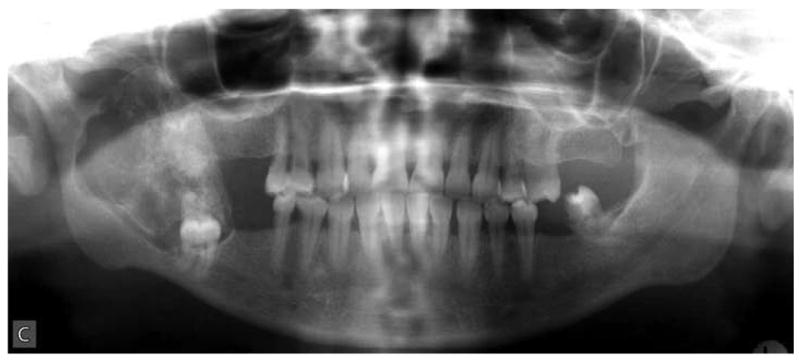
Extraoral (A), intraoral (B) clinical and radiographic (C) findings at the initial presentation of the patient. Extraoral view (A) reveals an expansile mass involving the right angle of the mandible and preauricular region leading to notable facial asymmetry. Intraoral view demonstrates the expansion of lingual and buccal aspects of the right posterior mandible. The panoramic radiograph (C) shows an impacted tooth #32 which is associated with a large, multilocular, expansile and destructive radiolucent lesion with foci of calcification.
Panoramic radiographic examination revealed a large ill-defined, expansile, mixed radiolucent-radiopaque lesion involving the right posterior mandible and ascending ramus which was associated with an unerupted 3rd molar (Figure 1C). Axial computer tomographic (CT) scans of the head and face filmed at bone and soft tissue window settings demonstrated a large expansile osteolytic lesion involving the posterior body, angle and ramus of the mandible (Figure 2: A,B,C,D).
Figure 2.
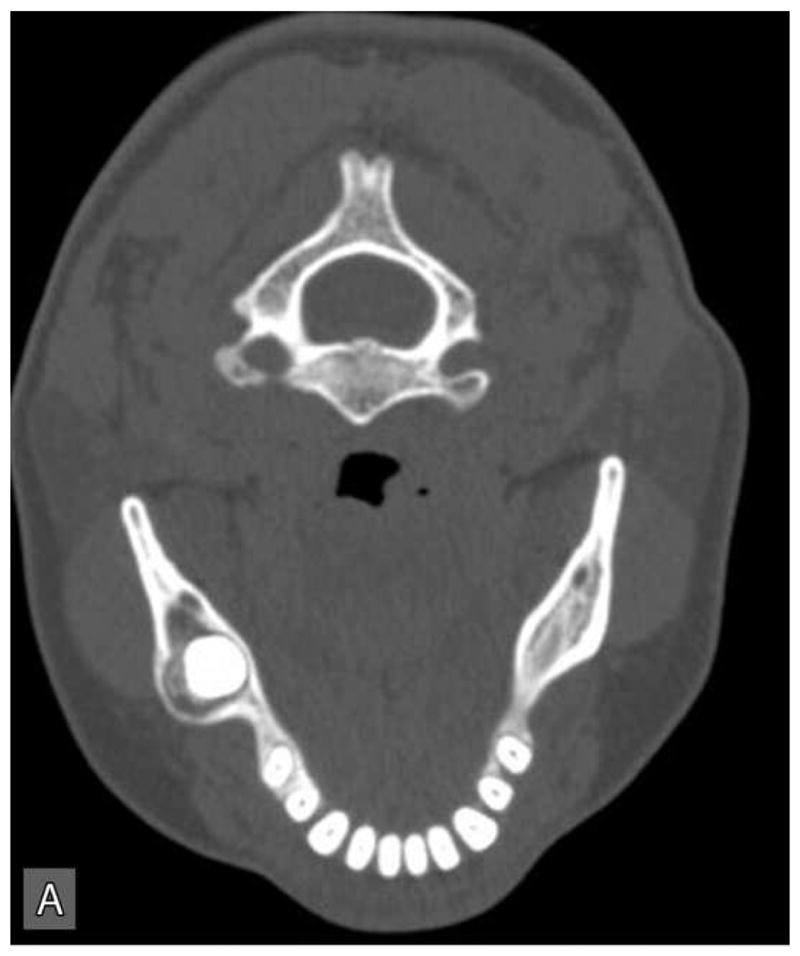
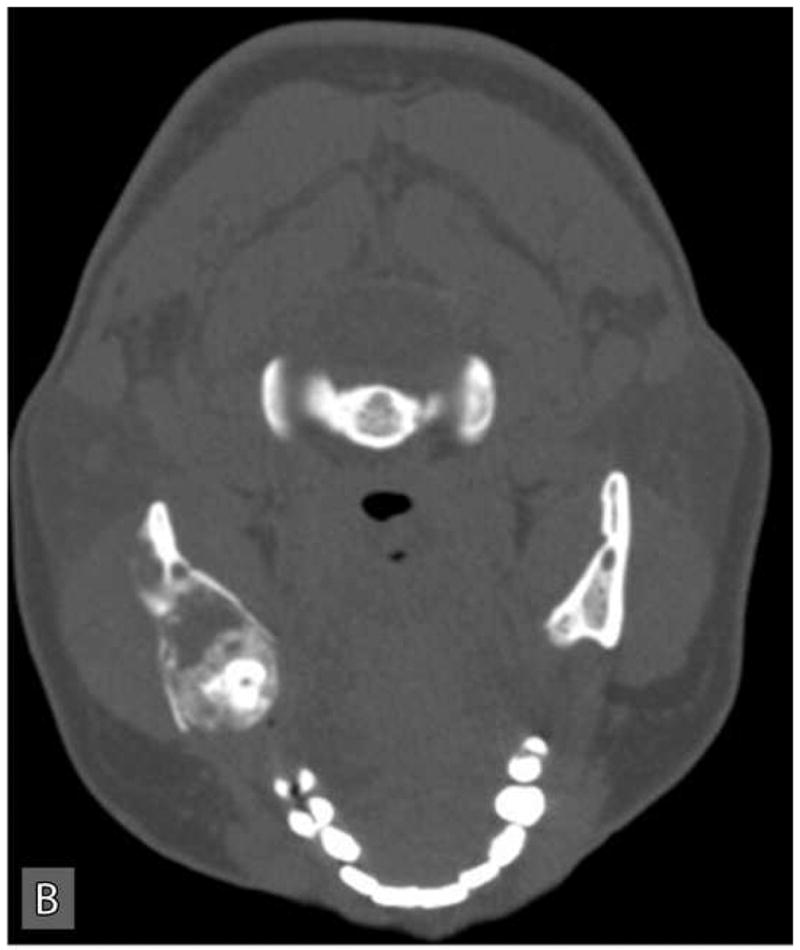
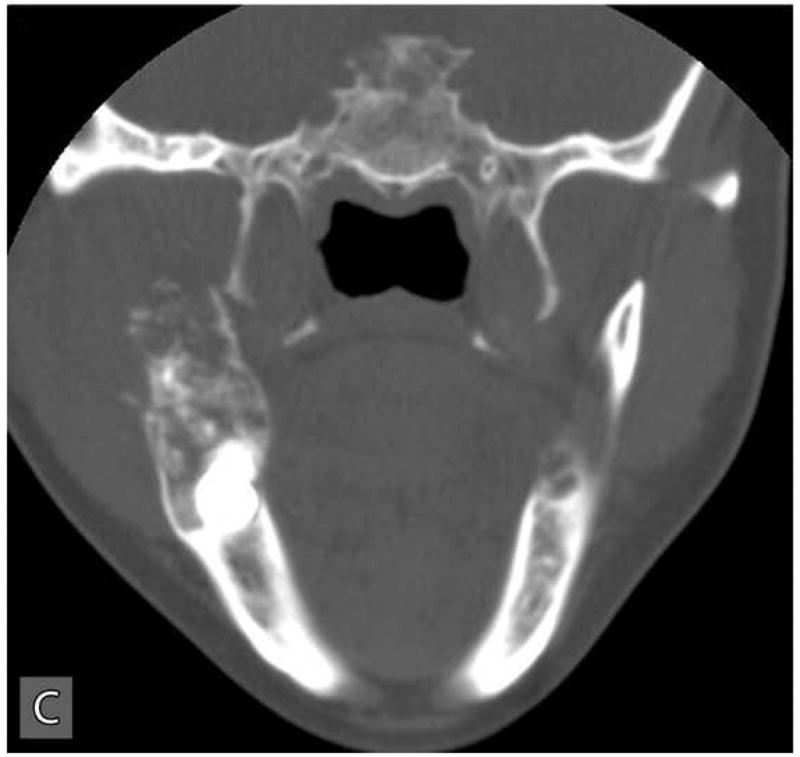
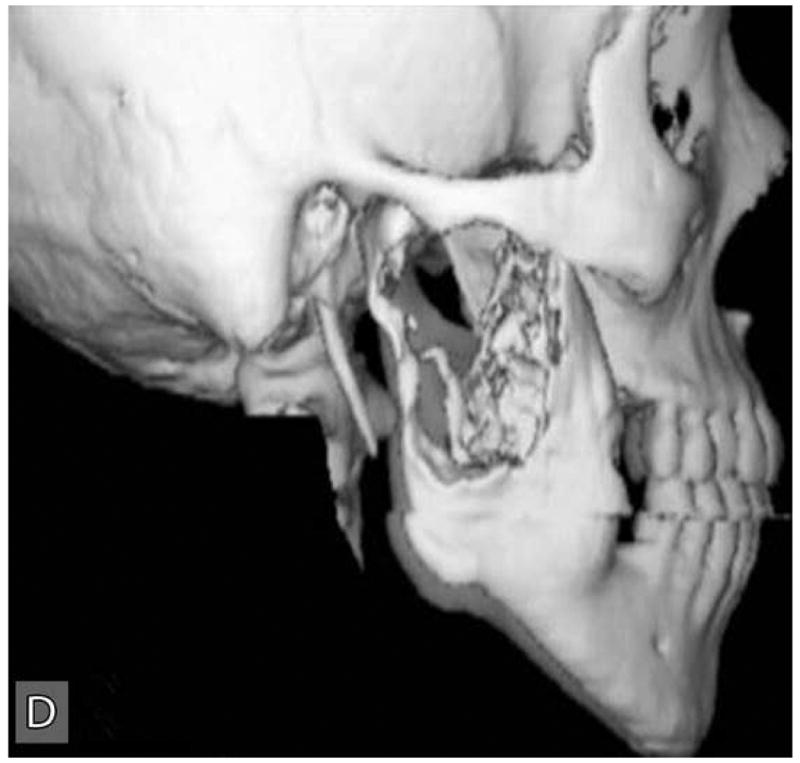
Computer tomographic (CT; soft tissue window) scans showing an expansile and destructive mass involving the right posterior mandible. Three-dimensional reconstruction of the CT scan exhibits an expansile lytic lesion involving the right mandibular ramus with perforation of the medial and lateral cortical plates.
Right hemimandibulectomy using a transfascial approach was done in March 2004. Areas of the tumor where lateral perforations were suspected were dissected supra-periosteal with surrounding soft tissue (Figure 3A). The mandibular defect was immediately reconstructed using costochondral rib graft and titanium plate (Figure 3B). The patient developed postoperative infection approximately one month after surgery requiring surgical exploration, drainage and removal of the costochondral graft and the reconstruction plate.
Figure 3.
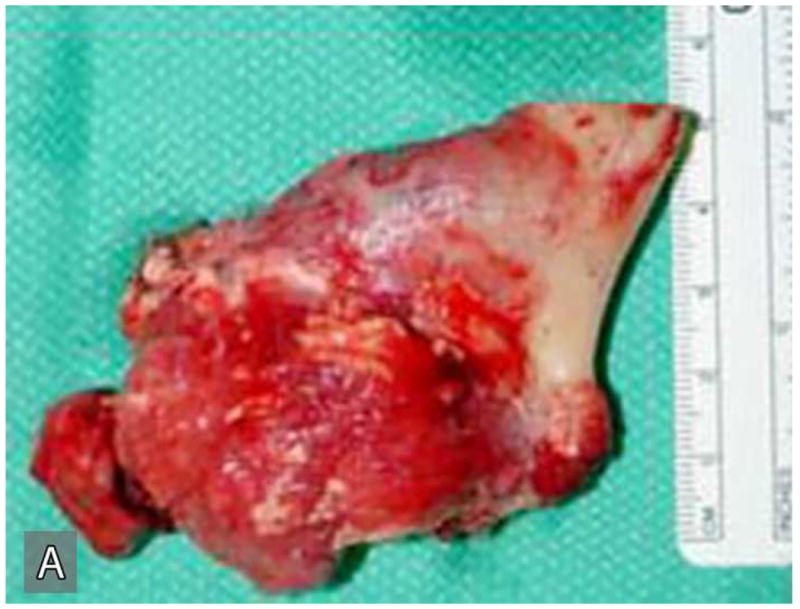
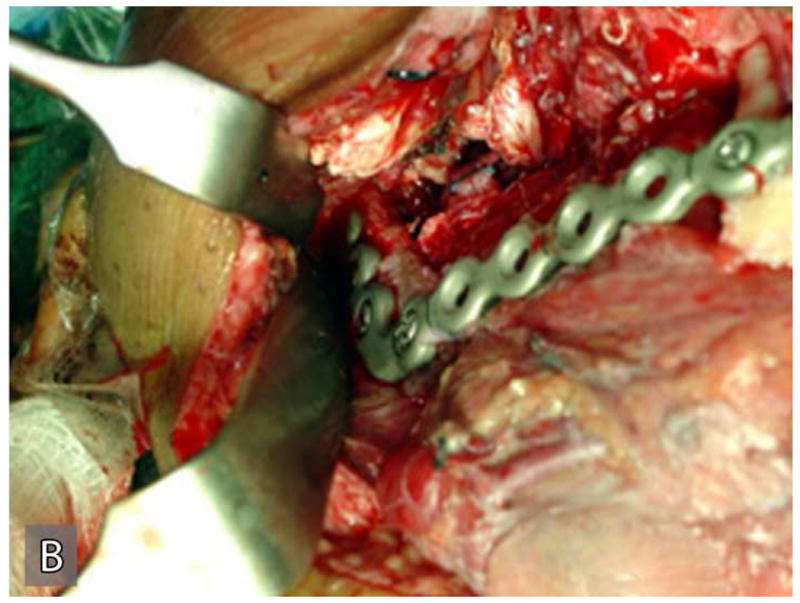
Gross specimen of the initial resection (A) and postoperative reconstruction of the mandibular defect (B). Gross specimen shows the resected portion of the posterior mandible with tumor perforating through the cortex into the surrounding soft tissue.
This tumor recurred within the right infratemporal fossa four months after surgery. In August 2004, this recurrent tumor was re-excised with total parotidectomy, infratemporal fossa resection, neck dissection, and free flap reconstruction. She underwent postoperative radiation treatment with 60 Gy over 30 fractions. Follow-up CT scans obtained in January 2005, revealed local tumor recurrence and metastatic spread to right orbital roof and lateral orbital wall with extraosseous extension into the right orbit. A second metastasis was noted within the left middle cranial fossa floor, the greater sphenoid wing, with intracranial extension into the parasellar region and extradural space of the left middle cranial fossa. This metastatic tumor also extended inferiorly into the left masticator space resulting in destruction of the pterygoid plates. Subsequently, she was treated with palliative radiation and chemotherapy consisting of three cycles of paclitaxel and carboplatin, and a course of capecitabine. However, the tumor was non-responsive to this treatment and grew rapidly. The CT and MRI scans performed in October, 2005 exhibited massive metastatic disease involving the entire skull base and invaded into the middle cranial fossa, frontal lobe and orbits causing loss of vision (Figure 4: A,B,C). The patient was palliatively managed and died of the tumor within a few months.
Figure 4.
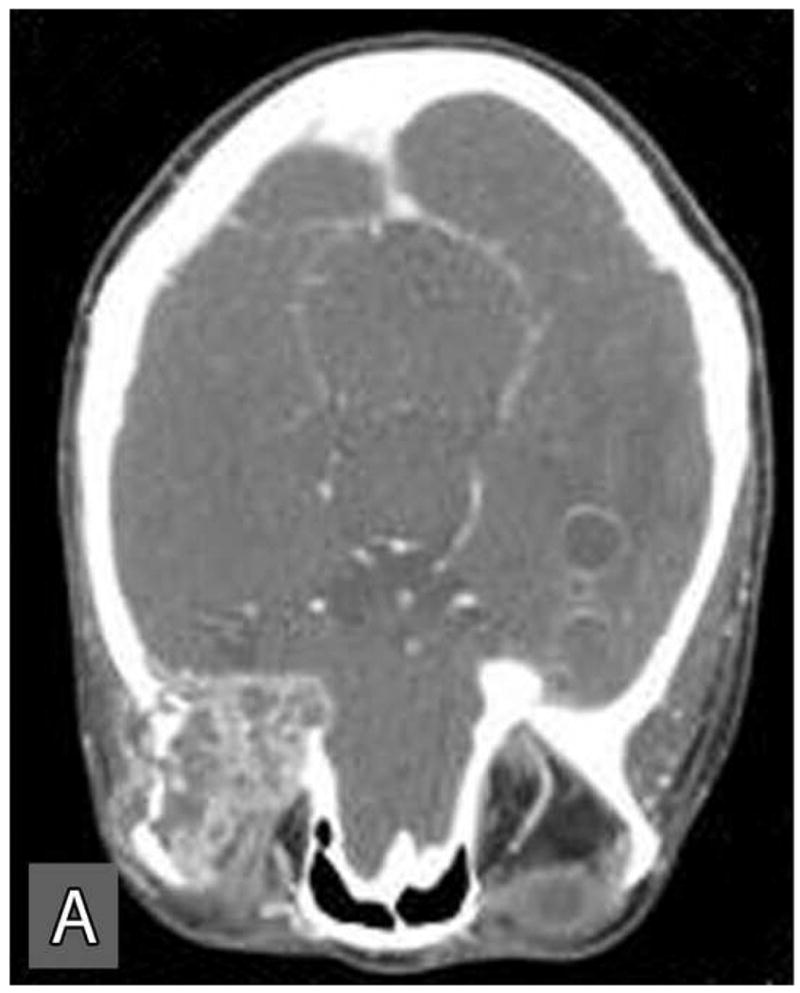
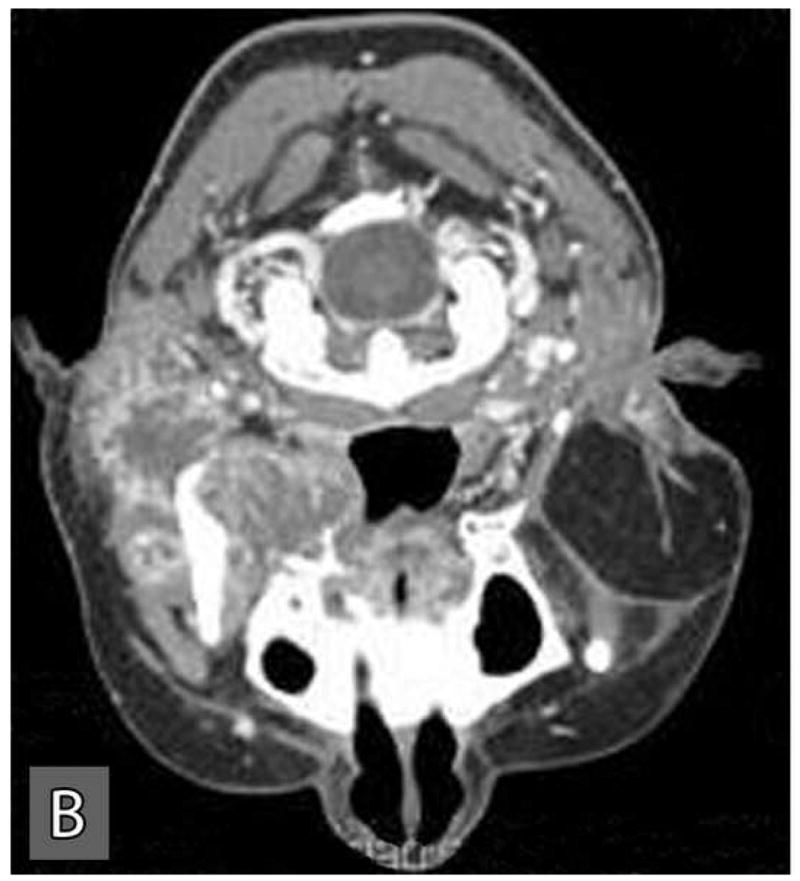
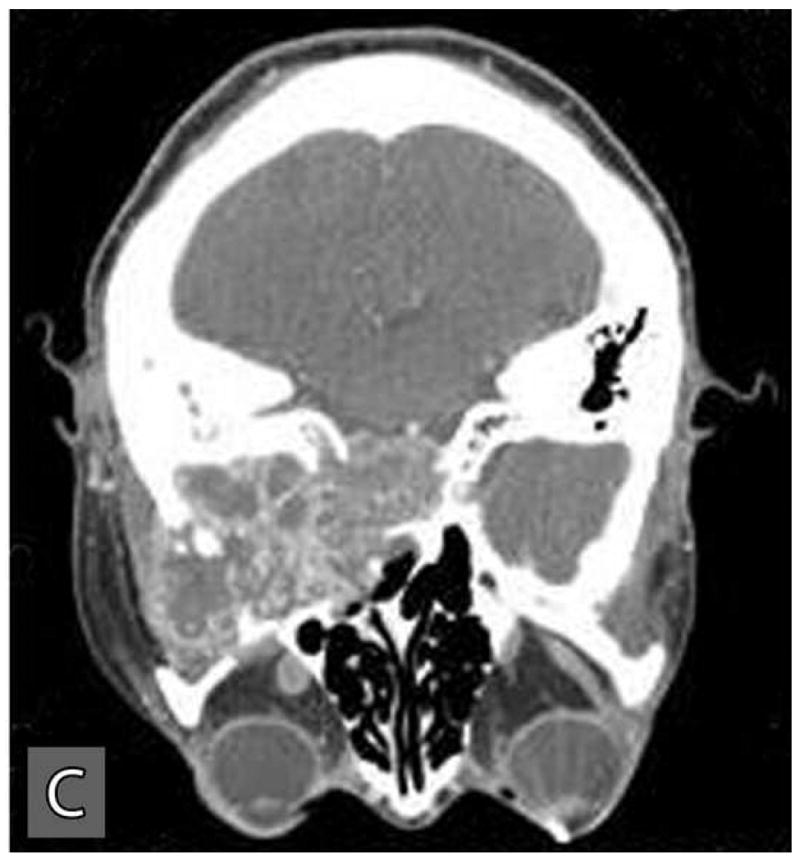
Computer tomographic scans at a later stage of the disease revealed extensive local and distance spread of the tumor. Distant spread of tumor involved the orbits (A), skull base (B), and the middle cranial fossa (C).
Pathology
Gross findings
Grossly, the original tumor specimen resected in March 2004, contained the posterior body and ramus of the mandible with a tumor mass (Figure 3A). This specimen measured 7.8 cm from anterior to posterior, 4.4 cm from superior to inferior, and 2.4 cm from lateral to medial. The posterior 2/3 of the mandible is replaced by partially calcified tumor mass measuring 4.5 × 4.0 × 2.2 cm. This tumor mass was partially covered by a thin shell of cortical bone on its lateral and superior aspect. Sectioning of the specimen prior to decalcification demonstrated a calcified spongy consistency in the anterior portion and a soft fleshy consistency with focal hemorrhage and necrosis in the posterior and superior portion of the tumor mass. An impacted tooth was noted within the tumor. The tumor grossly involved the posterior resection margin.
Microscopic findings
Tissue sections submitted from the incisional biopsy performed in December of 1983 and the subsequent resection in March of 1984 were reviewed. The original incisional biopsy revealed histopathologic features characteristic of a classic CEOT (Figure 5). This tumor was composed of islands and sheets of uniform polyhedral cells with well-defined cell borders and eosinophilic cytoplasm (Figure 5: A, B). These tumor cells contained prominent centrally placed ovoid nuclei that showed minor variation in size and shape. However, the tumor cells were relatively uniform in size and shape and there were no atypical cytologic features characteristic of malignancy (Figure 5: A, B). The mitotic count was less than 1 per 10 high-power fields. These tumor islands were surrounded by dense fibrous connective tissue stroma that revealed focal deposits of pink, amorphous amyloid-like material (Figure 5B). Concentric basophilic Liesegang ring-like calcifications were noted within these amyloid-like deposits (Figure 5B). This amyloid-like material when stained with Congo red, exhibited an apple-green birefringence when viewed with polarized light.
Figure 5.
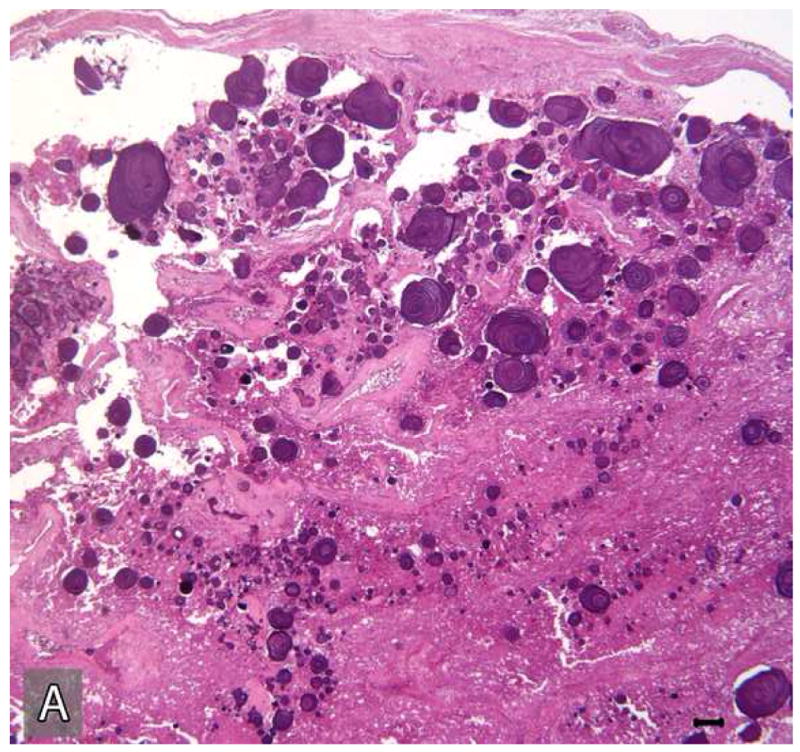
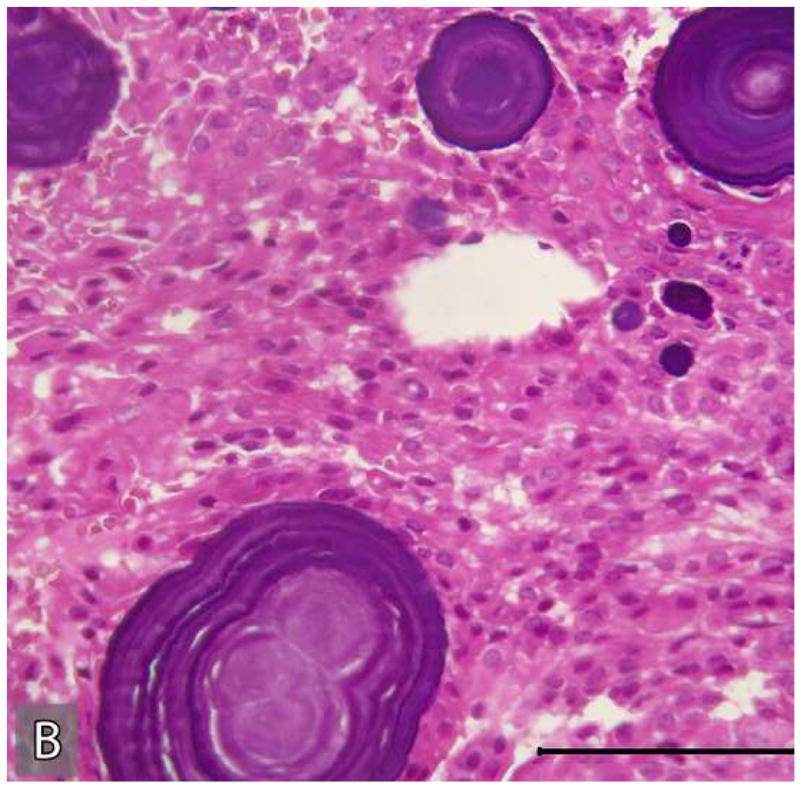
Microscopic appearance of the tumor in the initial incisional biopsy. The tumor consists of sheets and islands polygonal epithelial cells with eosinophilic cytoplasm and large ovoid nuclei. These tumor islands are surrounded by fibro-collagenous stroma which reveals multifocal concentric “Liesegang ring”-like calcifications in association with amyloid-like material. (Hematoxylin-eosin stain, Original magnifications × 40 (A) and × 200 (B); Bar: 100 μM)
Multiple H&E stained serial sections of the tumor resected in 2004 were reviewed. These tissue sections exhibited a tumor consisting of two parts, both embedded in a dense fibrous stroma, one part with histopathologic features of a benign CEOT (Figure 6 A,B) the other part, an odontogenic carcinoma displaying the histologic features of a poorly differentiated squamous cell carcinoma (Figure 6 C,D).
Figure 6.
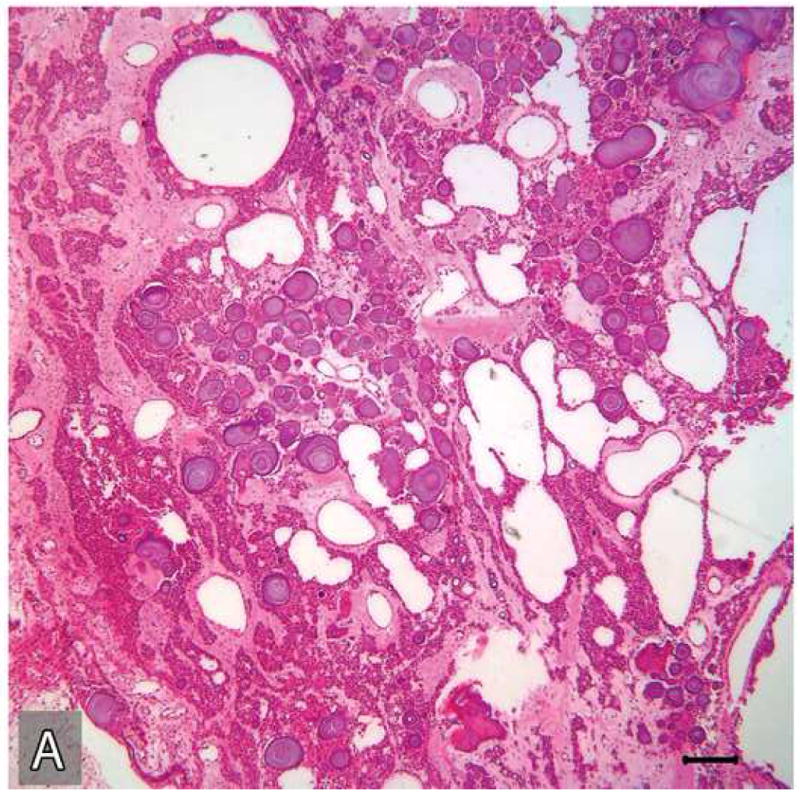
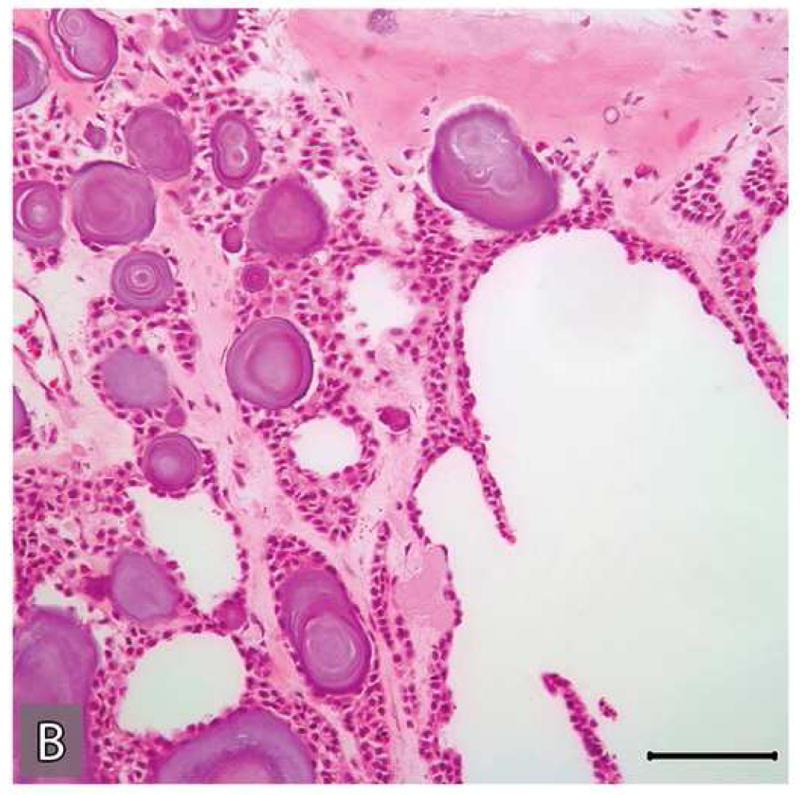
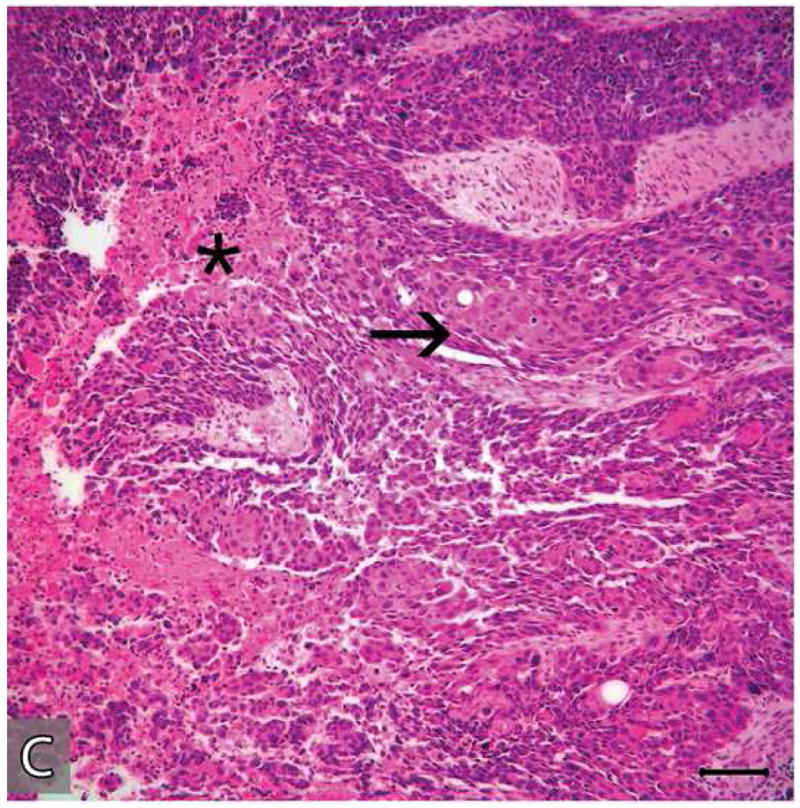
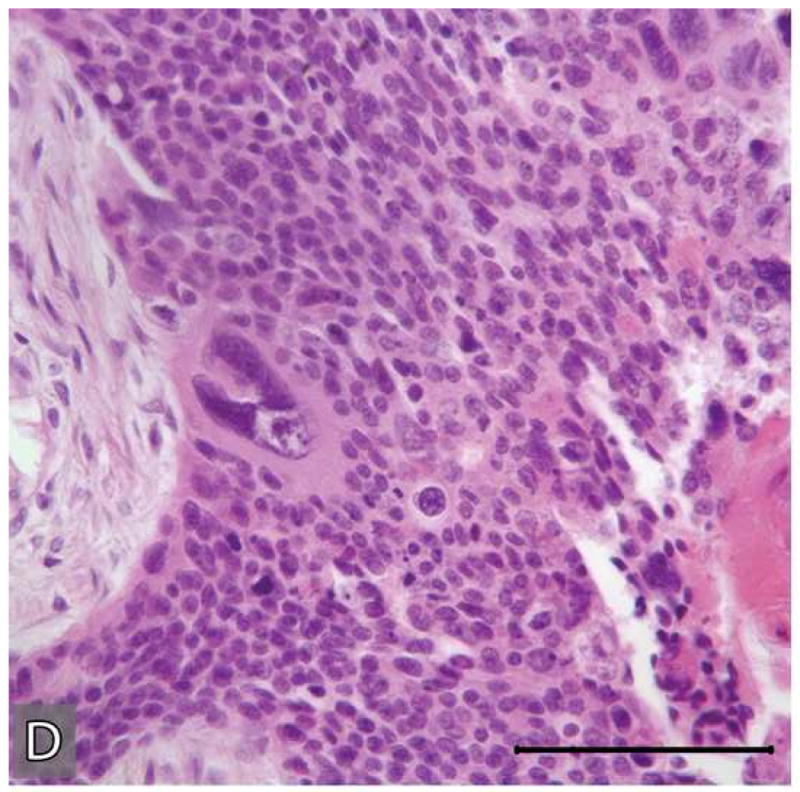
Microscopic appearances of the tumor in the resected specimen. The resected tumor revealed two distinct components, one component with histopathologic features of a benign CEOT (A&B) the other component, an odontogenic carcinoma displaying the histologic features of a poorly differentiated squamous cell carcinoma (C&D)). The malignant portion of the tumor revealed necrosis (*), marked nuclear and cellular pleomorphism and areas of squamoid differentiation (→). (Hematoxylin-eosin stain, Original magnifications × 40 A & C), × 100 (B) and × 200 (D) Bar: 100 μM)
Microscopic features of the benign CEOT component were identical to the previous incisional biopsy of this tumor. There was no significant cytologic atypia or increased mitoses (Figure 6: A-B). The benign CEOT component occupied approximately one third of the anterior portion of the tumor mass. The predominant component, however, was an odontogenic carcinoma, composed of poorly differentiated malignant squamoid epithelial cells with eosinophilic cytoplasm, large pleomorphic and hyperchromatic nuclei with prominent nucleoli (Figure 6: C-D). These tumor islands revealed dyskeratosis, increased and abnormal mitotic figures and focal areas of comedo-necrosis. The posterior, inferior and medial resection margins of the specimen were positive for tumor involvement.
Immunohistochemical studies
Representative tissue sections containing the areas of benign CEOT and odontogenic carcinoma were immunohistochemially stained for Ki-67 and p53 antigens. Tumor proliferative activity determined by the Ki-67-labeling index (%) was assessed by counting 1000 tumor cells per tissue section. Tumor proliferation rate as defined by Ki-67-labeling index was less than 1% in the benign CEOT component, but was markedly higher, up to 42%, in the odontogenic carcinoma component (Figure 7: A,B). Nuclear p53 staining was detectable in the tumor cells of the benign CEOT component (Figure 7 C. On the other hand, odontogenic carcinoma cells were virtually devoid of p53 expression, while scattered p53 positive cells were found within the surrounding stroma and served as built-in positive control for the specificity of p53 immunostaining (Figure 7 D).
Figure 7.
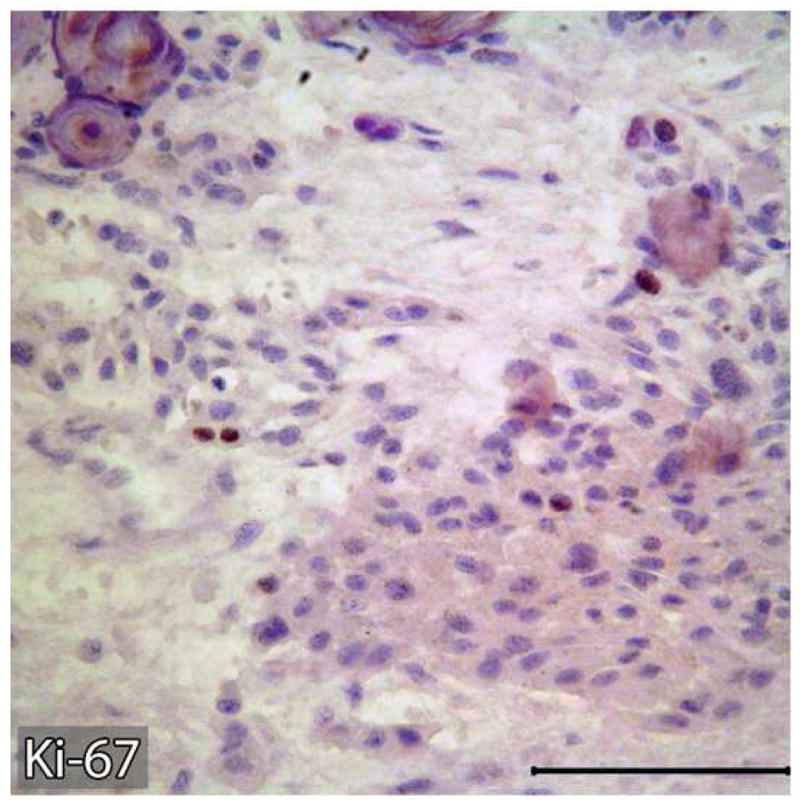
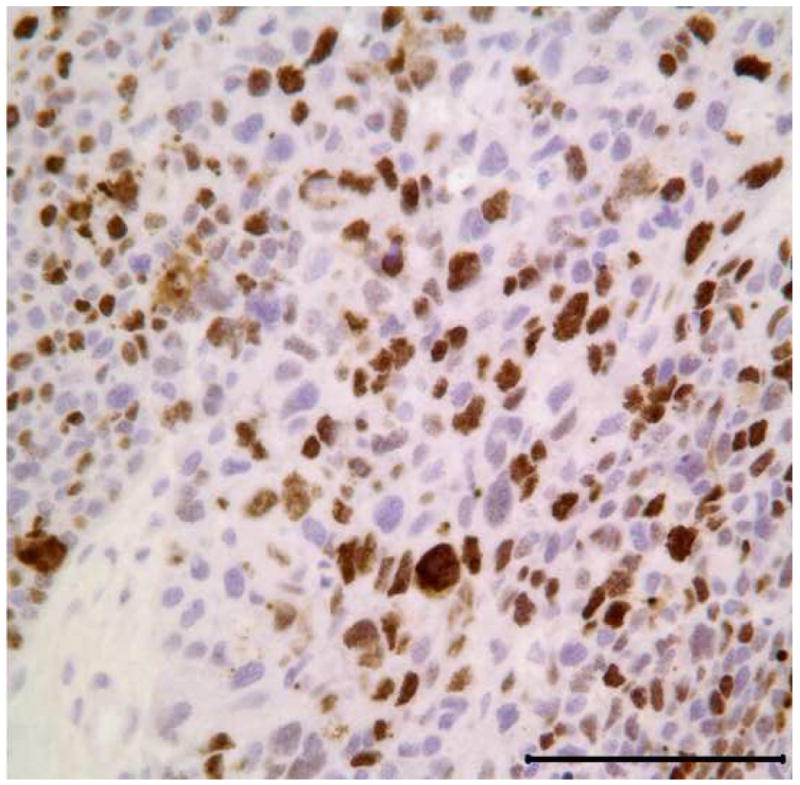
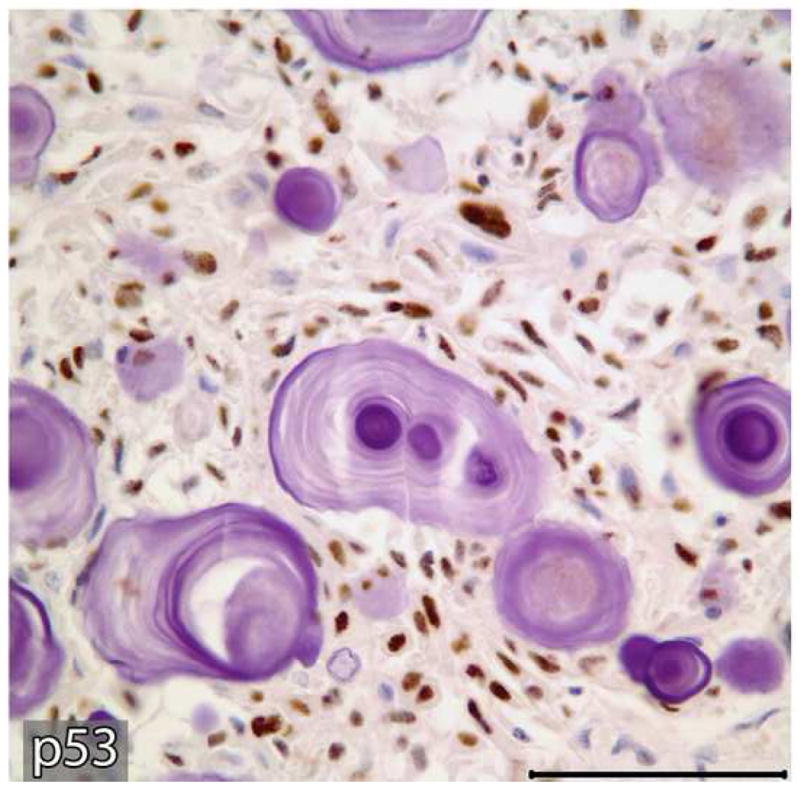
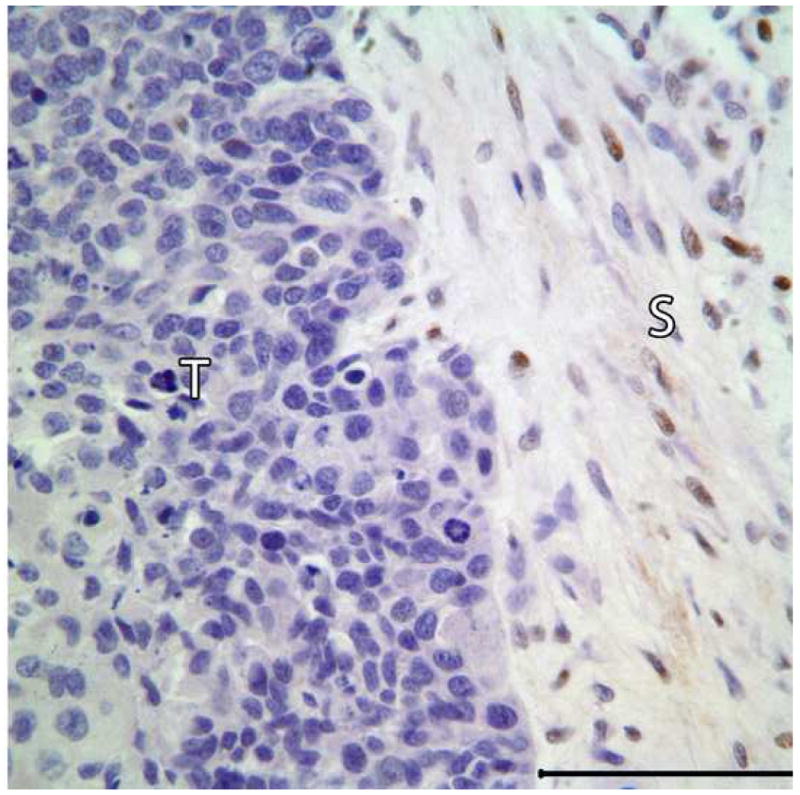
Expression patterns of tumor cell proliferation marker Ki-67 (A&B) and tumor suppressor p53 protein (C&D) in benign and malignant components of the resected tumor. Markedly higher proliferative activity (Ki-67 immunolabeling) is noted in the malignant portion of the tumor (B) compared to the benign CEOT component (Left). Nuclear immunoreactivity for p53 antigen is noted in the tumor cells of benign CEOT portion (C) but not in the malignant tumor cells (D). Stromal cells (S) in the malignant portion of the tumor demonstrate nuclear p53 reactivity. (Immunohistochemical staining, Original magnification × 200 Bar: 100 μM)
Discussion
We report a case of malignant CEOT which metastasized to the base of the skull causing death of the patient less than two years after initial diagnosis and treatment. The initial incisional biopsy specimen was diagnosed as CEOT, but subsequent resection of the tumor showed both benign CEOT and a malignant de-differentiated odontogenic carcinoma. This case also highlights pitfalls in the surgical management of a clinically aggressive odontogenic tumor whose initial incisional biopsy was not representative of the tumor as a whole.
Calcifying epithelial odontogenic tumor (CEOT; ICD-O code: 9340/0) was first described by Pindborg in 1955 as a benign but locally invasive tumor, accounting for less than 1% of all odontogenic tumors [3, 4]. CEOT is distinguished from other odontogenic tumors because of its unique histopathologic features characterized by the presence of amyloid deposits around the sheets of tumor cells which often become calcified in the form of concentric rings. Although most CEOT present clinically as asymptomatic, slow-growing intra- or extraosseous tumors of the jaw, CEOT of the posterior maxilla may present with nasal stuffiness, headache and epistaxis [1, 2, 6]. CEOT are three times more likely to occur in the mandible than in the maxilla [2, 6].
As in the current case, intraosseous variants of CEOT commonly occur in association with an unerupted tooth, most commonly the mandibular 3rd molar. CEOT associated with an unerupted tooth commonly presents as unior multilocular radiolucency with scattered flecks of calcification in close proximity to the impacted tooth. CEOT occurs between the ages of 8 and 92 years with a mean age at diagnosis of 40 years [2, 6]. There is no racial or gender predilection for CEOT [2, 6]. Besides the classic form, three additional histologic variants of CEOT have been described which may have bearing on their prognosis and treatment [2, 6]. Non-calcifying CEOT with Langerhans cells and CEOT with cementum- and bone-like material are known to be less aggressive and are amenable to more conservative surgical treatment [2, 6]. The third variant, clear cell CEOT is more aggressive and has a higher rate of recurrence than conventional CEOT[2, 6].
Ameloblastic carcinomas and clear cell odontogenic carcinomas are the two most common malignancies of odontogenic epithelial origin [1, 16]. Ameloblastic carcinomas frequently occur de novo and rarely from a preexisting ameloblastomas. Ameloblastic carcinoma typically exhibits histological features of ameloblastoma with cytologic atypia and increased mitotic activity [20]. Clear cell odontogenic carcinoma occurs as a primary de novo jaw malignancy which has biphasic tumor cell population consisting of sheets of clear cells interspersed with cords and strands of dark, basaloid cells [21]. Other less common odontogenic malignant tumors include ghost cell odontogenic carcinoma and primary intraosseous squamous cell carcinomas [1, 16].
There are previous reports of ameloblastic carcinoma misdiagnosed as ameloblastoma and treated more conservatively, leading to poor treatment outcome [22]. However, re-evaluation of the initial biopsies in these cases proved that these biopsies were misread [22]. In contrast, in the current case the initial diagnosis of CEOT remains unchanged upon re-evaluation of the initial biopsy by a second group of oral pathologists. Hence, the poor surgical treatment outcome in the current case is attributable to sampling error of the initial incisional biopsy which is unavoidable in large tumors. In contrast to ameloblastomas, the majority of CEOT are slow-growing, non-aggressive tumors that rarely extend into the intratrabecular spaces and generally do not cause perforation of the cortical plate[2, 6, 20]. Thus, CEOT are considered less aggressive than solid ameloblastomas and conservative surgery has been advocated as the treatment of choice [2, 6, 20]. Therefore, conservative surgery with preservation of mandibular function was planned at the early surgical phase of this case based on the initial diagnosis of CEOT. Malignancy was not considered in the initial clinical evaluation and treatment planning stage of our case because (1) the patient fit the classical presentation of CEOT associated with an impacted mandibular 3rd molar; (2) the initial biopsy result did not indicate it; (3) there was neither inferior alveolar nerve involvement nor regional lymphadenopathy.
Malignant variants of CEOT are extremely rare and display wide variations in their clinical course (Table 1). Here, we report the first clearly documented case of malignant transformation in a CEOT resulting in skull base metastasis with intracranial extension within one year of diagnosis, leading to the patient's death two years after the initial diagnosis. Basu, et. al. described the first case of malignant transformation in CEOT in a 75-year -old male which metastasized to the regional lymph nodes [13]. Since then a total of eight cases, including our own, have been reported in the English literature (Table 1). Malignant transformation occurred in the primary tumor in five cases and in recurrent tumors in the remaining three cases (Table 1). Overall ages ranged from 40 to 83 years with a mean of 57.25 years; five of these patients were male and three were female. All but one of these malignant CEOTs occurred in the mandible (Table1). Of these eight cases, two had regional lymph node metastasis and three presented with distant bone (n=2) and lung metastasis (n=1). Unlike the current case, tumor related death was not recorded in any of the previously reported cases of malignant CEOT. Hence, the current case is unique because of the aggressive biologic behavior of the tumor resulting in the death of the patient due to malignant CEOT a little more than a year after initial diagnosis.
The aggressive nature of this tumor is reflected in its poor histologic differentiation, the presence of necrosis and a high proliferation rate as assessed by Ki-67 labeling index. The Ki-67 antigen is a nonhistone protein, is a DNA-binding nuclear protein expressed in proliferating cells (G1, S, G2 and M phases), but not in quiescent (G0) cells and hence is widely used to determine tumor proliferation rate [23]. Another critical finding of our case is that malignant evolution of CEOT is linked to the loss of the tumor suppressor gene p53 transcriptional activity. The p53 protein which is commonly known as the “guardian of the genome” is the most frequently mutated gene in human cancers, including oral cancers [24-26]. To prevent replication of cells with damaged DNA, p53 protein causes cell cycle arrest for DNA repair or apoptosis at the G1-S boundary [24, 25]. Hence, the loss of p53 function in cells makes them susceptible to accumulate variety of genetic defects at an elevated rate resulting in malignant transformation and progression [24, 25]. In malignant tumor cells, p53 gene abnormalities lead to either loss of the p53 translational product, as in our case, or overexpression of functionally inactive mutant p53 protein[25]. Loss of p53 transcriptional activity is one of the earliest events in the natural history of cancer which confers tumor cells the two most important phenotypes: (1) a growth advantage for clonal expansion by escaping G1 checkpoint arrest and apoptosis; (2) genomic instability and acquisition of more oncogenic mutations. Our finding that the expression of p53 protein in the nuclei of tumor cells found in the benign portion of this tumor but not in its malignant component indicates that loss p53 function may play a significant role in the malignant transformation of CEOT.
In conclusion, we present a case of malignant evolution of a CEOT with histologic evidence of de-differentiation into a poorly differentiated squamous cell carcinoma. Histologic and immunohistochemical observations support the presence of two distinct components representing a benign CEOT and a malignant odontogenic carcinoma within the same tumor. Unfortunately, this led to sampling error at the initial incisional biopsy which resulted in dramatically different disease course and treatment outcome from previously reported cases of malignant CEOT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neville B, Damm D, Allen C, Bouquot J. Odontogenic Cysts and Tumors. In: Neville B, Damm D, Allen C, Bouquot J, editors. Oral & Maxillofacial Pathology. St. Lousi, Missouri: SAUNDERS/ELSEVIER; 2009. p. 716. [Google Scholar]
- 2.Philipsen HP, Reichart PA. Calcifying epithelial odontogenic tumor: biological profile based on 181 cases from the literature. Oral Oncol. 2000;36:17. doi: 10.1016/s1368-8375(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 3.Pindborg J. Calcifying Epithelial Odontogenic Tumors. Acta Pathologica Microbiologica Scandinavica. 1955;111(suppl) doi: 10.1111/apm.1966.68.2.169. [DOI] [PubMed] [Google Scholar]
- 4.Pindborg JJ. A calcifying epithelial odontogenic tumor. Cancer. 1958;11:838. doi: 10.1002/1097-0142(195807/08)11:4<838::aid-cncr2820110423>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Houston GD, Fowler CB. Extraosseous calcifying epithelial odontogenic tumor: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:577. doi: 10.1016/s1079-2104(97)90123-2. [DOI] [PubMed] [Google Scholar]
- 6.Reichart PA, Philipsen HP. Odontogenic Tumors and Allied Lesions. London: Quintessence Publishing Co Ltd; 2004. Calcifying Epithelial Odontogenic Tumor; p. 93. [Google Scholar]
- 7.Baunsgaard P, Lontoft E, Sorensen M. Calcifying epithelial odontogenic tumor (Pindborg tumor): an unusual case. Laryngoscope. 1983;93:635. doi: 10.1002/lary.1983.93.5.635. [DOI] [PubMed] [Google Scholar]
- 8.Ching AS, Pak MW, Kew J, Metreweli C. CT and MR imaging appearances of an extraosseous calcifying epithelial odontogenic tumor (Pindborg tumor) AJNR Am J Neuroradiol. 2000;21:343. [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CY, Mohammadi H, Mostofi R, Habibi A. Calcifying epithelial odontogenic tumor of the maxillary sinus. J Oral Maxillofac Surg. 1992;50:1326. doi: 10.1016/0278-2391(92)90237-t. [DOI] [PubMed] [Google Scholar]
- 10.Mohtasham N, Habibi A, Jafarzadeh H, Amirchaghmaghi M. Extension of Pindborg tumor to the maxillary sinus: a case report. J Oral Pathol Med. 2008;37:59. doi: 10.1111/j.1600-0714.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakano H, Ota Y, Yura Y. Calcifying epithelial odontogenic tumor of the maxilla with ulcerative stomatitis: A case report. Br J Oral Maxillofac Surg. 2008 doi: 10.1016/j.bjoms.2008.07.201. [DOI] [PubMed] [Google Scholar]
- 12.Nelson SR, Schow SR, Read LA, Svane TJ. Treatment of an extensive calcifying epithelial odontogenic tumor of the mandible. J Oral Maxillofac Surg. 1992;50:1126. doi: 10.1016/0278-2391(92)90507-v. [DOI] [PubMed] [Google Scholar]
- 13.Basu MK, Matthews JB, Sear AJ, Browne RM. Calcifying epithelial odontogenic tumour: a case showing features of malignancy. J Oral Pathol. 1984;13:310. doi: 10.1111/j.1600-0714.1984.tb01429.x. [DOI] [PubMed] [Google Scholar]
- 14.Bouckaert MM, Raubenheimer EJ, Jacobs FJ. Calcifying epithelial odontogenic tumor with intracranial extension: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:656. doi: 10.1067/moe.2000.106577. [DOI] [PubMed] [Google Scholar]
- 15.Cheng YS, Wright JM, Walstad WR, Finn MD. Calcifying epithelial odontogenic tumor showing microscopic features of potential malignant behavior. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:287. doi: 10.1067/moe.2002.121991. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg D, Sciubba J, Koch W, Tufano RP. Malignant odontogenic tumors: a 22-year experience. Laryngoscope. 2004;114:1770. doi: 10.1097/00005537-200410000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Kawano K, Ono K, Yada N, et al. Malignant calcifying epithelial odontogenic tumor of the mandible: report of a case with pulmonary metastasis showing remarkable response to platinum derivatives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:76. doi: 10.1016/j.tripleo.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Kumar M, Fasanmade A, Barrett AW, et al. Metastasising clear cell odontogenic carcinoma: a case report and review of the literature. Oral Oncol. 2003;39:190. doi: 10.1016/s1368-8375(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 19.Veness MJ, Morgan G, Collins AP, Walker DM. Calcifying epithelial odontogenic (Pindborg) tumor with malignant transformation and metastatic spread. Head Neck. 2001;23:692. doi: 10.1002/hed.1097. [DOI] [PubMed] [Google Scholar]
- 20.Sciubba JJ, Eversole LR, Slootweg PJ. Odontogenic/Ameloblastic Carcinomas. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. p. 287. [Google Scholar]
- 21.Bang G, Koppang H. Clear Cell Odontogenic Carcinoma. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. p. 292. [Google Scholar]
- 22.Smith B, Mulligan J, Wagner E. Ameloblastic carcinoma and relationships with ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;108:526. [Google Scholar]
- 23.Gerdes J. Ki-67 and other proliferation markers useful for immunohistological diagnostic and prognostic evaluations in human malignancies. Semin Cancer Biol. 1990;1:199. [PubMed] [Google Scholar]
- 24.Gorgoulis VG, Vassiliou LV, Karakaidos P, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 25.Harris CC. p53 tumor suppressor gene: from the basic research laboratory to the clinic--an abridged historical perspective. Carcinogenesis. 1996;17:1187. doi: 10.1093/carcin/17.6.1187. [DOI] [PubMed] [Google Scholar]
- 26.Tsantoulis PK, Kastrinakis NG, Tourvas AD, et al. Advances in the biology of oral cancer. Oral Oncol. 2007;43:523. doi: 10.1016/j.oraloncology.2006.11.010. [DOI] [PubMed] [Google Scholar]


