Abstract
γδ T cells develop in the thymus before entering the periphery. Recent work suggests that thymic development does little to constrain γδ T cell antigen specificities, but instead determines their effector fate. When triggered through the T cell receptor, ligand-naïve γδ T cells produce IL-17, ligand-experienced cells make IFN-γ and those that are strongly self-reactive make IL-4. Importantly, γδ T cells are able to make cytokines immediately upon TCR engagement. These characteristics allow γδ T cells to initiate an acute inflammatory response to pathogens and to host antigens revealed by injury. These advances warrant a fresh look at how γδ T cells may function in the immune system.
Keywords: γδ T cells, thymocyte development, ligand recognition
Introduction
γδ T cells, together with αβ T cells and B cells, are present in all but the most primitive vertebrates. Each population contributes to host immune defense uniquely. However, most γδ T cells and αβ T cells produce similar cytokines and mount cytotoxic responses. Other than in the murine skin, αβ T cells are always found alongside and usually in excess to γδ T cells. Therefore, the major difference in how γδ T cells and αβ T cells contribute to host immune competence is likely because these two types of cells are triggered differently. Indeed, γδ T cells and αβ T cells have different antigen recognition requirements and recognize different sets of antigens [1], but it is unclear how a functional γδ T cell antigen-specific repertoire develops. The antigen-specific repertoire of peripheral αβ T cells is the outcome of thymic selections. γδ T cells, like αβ T cells, require development in the thymus prior to entering the periphery [2]. This review examines recent studies on thymic ligand recognition and γδ T cell repertoire and function development.
I. γδ T cells recognize diverse ligands and MHC molecules are not obligatory components of γδ T cell antigens
γδ T cells, αβ T cells, and B cells are the only types of cells that use V(D)J rearrangement to generate diverse receptors capable of recognizing different antigens. Although the basic organization of the γδ TCR loci is similar to that of the αβ TCR and immunoglobulin (Ig) loci, γδ TCRs are generated using much fewer V genes than αβ TCRs and most Igs. However, the potential diversity generated at the combined V(D)J (CDR3) junctions of γδ TCRs (approximately 1018 combinations) is much higher than that of αβ TCRs (~1016 combinations) and Igs (~1011 combinations) [1]. While it is generally accepted that multiple V genes confer evolutionary advantages on the immune system, the CDR3 formed by V(D)J recombination appears to be more important in determining antigen specificity. Even though the Ig heavy chain locus contains the highest number of V gene segments, mice constrained to use a single heavy chain V gene (VH), but with full CDR3 diversity potential, were able to generate antibody responses to a variety of protein and hapten antigens [3]. The CDR3 junctions are also important for γδ TCR antigen recognition. The antigen recognition determinants of the closely related nonclassical MHC class I T10 and T22-specific γδ TCRs from normal mice are localized on the TCR δ chain CDR3 junction [4]. Also, the TCRs of LBK5 (specific for IE b, k, s) and LKD1 (specific for IAd) showed identical Vγ and Vδ sequences, which differ only in the CDR3 region of the γ and the δ chain [5]. Thus, through their capacity for great CDR3 variety, γδ TCRs have the potential to recognize a diverse set of antigens, despite the comparatively small number of V gene segments.
Indeed, γδ T cell antigens include both host and pathogen-derived molecules. These include T10, T22 and a complex of F1-ATPase with apolipoprotein A–I. Direct binding between these antigens and their respective murine [6] and human [7] γδ TCRs has been demonstrated. Other γδ T cell specificities include HSV glycoprotein gI, recognized by the murine γδ T cell clone Tg14.4 [8], and MHC class I-related molecules MICA and MICB recognized by some human γδ T cell clones [9]. T10, T22, MICA and MICB have MHC-like structures, but do not bind peptide or other moieties [10–11]. γδ T cells can recognize MHC molecules, as is the case for the γδ T cell clones LKD1 and LBK5 [5]. However, detailed analysis showed that IEk recognition by the LBK5 TCR differs from αβ TCR recognition of peptide/IE complexes. In particular, peptides bound to IEk do not confer specificity to LBK5 stimulation. Additionally, LBK5 reactivity is not sensitive to mutations on IE molecules which affect αβ TCR binding of peptides in association with IEk [12–13].
Length distributions of CDR3 loops are consistent with these experimental observations and reflect the differences between αβ and γδ TCR antigen recognition. The length distributions of CDR3 loops for γδ TCRs are more similar to those of antibodies, where the CDR3 loops of the TCR δ chain and the Ig heavy chain tend to be long and variable, while the CDR3 loops of the TCR γ chain and Ig light chain are short and constrained. In contrast, the lengths of the CDR3 loops of the TCR α and β chains are similar in length and highly constrained, likely reflecting the structural requirement for interaction with peptide-MHC complexes [14]. Based on this structural requirement of antigen receptors for ligand binding and the antigens that have been identified, it is clear that MHC is not an obligatory component of γδ TCR ligands. Thus, it is improbable that γδ T cells undergo selection in the thymus based on TCR interactions with peptide-MHC complexes as αβ T cells do.
II. γδ thymocyte development and exit into the periphery is not contingent on encountering cognate antigen in the thymus
Early analysis of the role of thymic selection in the establishment of a functional γδ T cell repertoire has focused on the studies of KN6 and G8 γδ T cell receptor (TCR) transgenic mice. G8 and KN6 are two independently isolated γδ T cell clones that happen to have the same specificity—T10 and T22 of the b haplotype, but not the d haplotype [12, 15–16]. In both experimental systems, TCR transgenic mice were crossed to C57BL/6 (B6) mice, which express both T10 and T22; BALB/c mice, which only express T10; or to β2-microglobulin-deficient mice (B2m−/−), which do not have cell-surface T10 or T22 expression. When the TCR transgenes were expressed in the B6 or B2m−/− background, there were fewer or no transgenic T cells in the thymus and/or periphery as compared to transgenic T cells in the BALB/c background. These transgenic T cells also showed a reduced ability to secrete IL-2 and to proliferate when stimulated in vitro [17–20]. These results were taken to suggest that antigen-specific γδ T cells are eliminated by encountering their agonist antigen in the thymus and that these cells require an encounter with cognate ligand for thymic maturation and function in the periphery. It was concluded that γδ T cells are subject to ligand-driven thymic positive and negative selection much like αβ T cells. However, in subsequent experiments with the same lines of G8 transgenic mice, Schweighoffer and Fowlkes [21] showed that the G8 transgenic T cells could mature in B2m−/− mice; this contradicts the conclusion that positive selection is a requirement for γδ development.
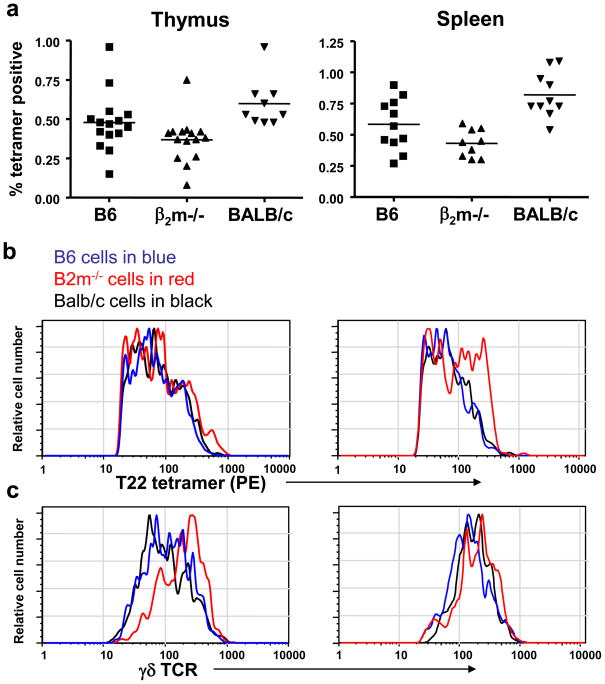
These initial conclusions regarding the effect of thymic ligand exposure on γδ T cell development were further called into question from studies examining the T10/T22-specific γδ T cell populations in non-transgenic mice. Using T22 tetramers to stain γδ T cells from naïve, normal mice, it was found that the frequency of T10/T22-specific γδ T cells in B6, BALB/c, and B2m−/− mice is roughly in the same range, 0.1–1% of the total γδ T cell population, as summarized in Figure 1 [22]. This was the case for γδ T cells from the thymus, spleen, and intestinal inter-epithelial lymphocyte compartments from each strain of mouse [4]. T22 tetramer staining of γδ T cells and single cell TCR analysis indicated T10/T22-specific γδ T cells from different mouse strains show a range of ligand binding affinity. Thus, the presence or absence of an endogenous ligand during development does little to affect the T10/T22-specific γδ T cell repertoire. In fact, ~0.85% of the TCRδ sequences from out of frame rearrangements and CD3ε−/− thymocytes have a CDR3 motif that is necessary and sufficient for T10/T22 binding [4]. This frequency is well within the range of the normal frequency of T10/T22-specific γδ T cells in the periphery. Furthermore, the frequency of T10/T22-specific γδ T cells was unaffected by the absence of β2m and class II MHC molecules, with or without cyclosporin A treatment. Cyclosporin A is a calcineurin inhibitor which blocks positive selection of αβ T cells. This indicates that development of γδ T cells in general and the T10/T22-specific γδ T cells in particular is not inhibited by or dependent on the expression of T10 or T22, class II MHC or other β2-microglobulin-associated proteins, and that development of this population proceeds independently of αβ T cell selection signaling pathways.
Figure 1. Frequency of T10/T22-Specific γδ T cells from Mice with and without Endogenous T10 and T22 Expression.
Frequency (a), tetramer staining intensity (b), and TCR surface expression (c) on T10/T22-specific γδ thymocytes (left column) and splenocytes (right column) from C57BL/6 (B6) (T10+T22+) (blue), B2m−/−(T10+T22+) (red), and BALB/c (T10+T22+) (black) mice. Each symbol or histogram represents the result of one mouse.
TCR dimerization may be sufficient to induce signaling for γδ T cells to develop in the thymus
γδ thymocyte maturation requires signaling through the TCR [2]; the activation of the mitogen-activated protein (MAP) kinase (Raf-MEK-ERK) pathway is downstream of TCR signaling. Exit of mature thymocytes from the thymus requires up-regulation of sphingosine-1-phosphate receptor 1 (S1P1) [23]. Regardless of the genetic background and the endogenous T10/T22 expression pattern of the host, T10/T22-specific γδ thymocytes had similar levels of phosphorylated ERK1 and/or ERK2 (extracellular signal-regulated kinase) (pERK1/2), CD5, a stable indicator of TCR signaling strength [24–25], and S1P1 expression as compared to other γδ thymocytes. These findings illustrate that γδ thymocyte development and exit into the periphery is not contingent on encountering cognate antigen in the thymus [22]. In fact, it was demonstrated that VγVδ pairs, except the Vγ5 Vδ1 TCR from dendritic epidermal T cells (DETCs), were able to dimerize and mediate signal(s) for BaF3 cells to grow without IL-3 when expressed as the extracellular domains of the erythropoietin receptor (EPOR). This experimental system has been used to demonstrate that the pre-Tα TCR can dimerize and mediate signals without ligand engagement [26]. Although it is unclear what DETC TCRs recognize, all experiments indicate that these cells need to encounter thymic ligand to develop [27–29]. Since γδ thymocytes have a low threshold for signaling (high levels of pERK1/2 [22] and mir181 expression (C–Z Chen and Y-h Chien, unpublished observation)), it is possible that TCR dimerization alone can drive the completion of their thymic development. After development, the ability of the γδ TCR to dimerize could also aid in improving signaling efficiency of γδ T cells upon antigen recognition; indeed, despite the absence of the coreceptors CD4 and CD8, γδ T cells have been shown to signal more efficiently than αβ T cells [30].
The majority of γδ T cells in the thymus have not encountered ligand and an antigen naïve γδ T cell repertoire is actively maintained in the periphery
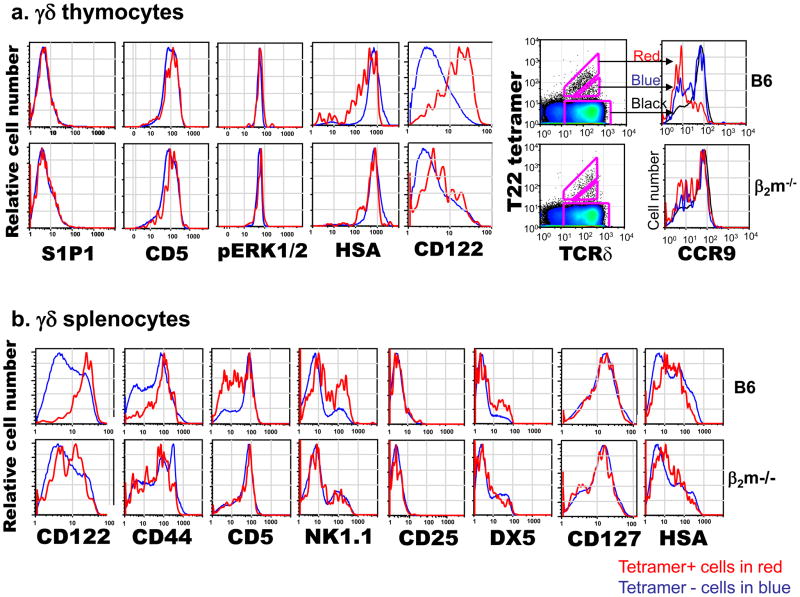
T10/T22-specific γδ thymocytes in B6, BALB/c, and B2m−/− mice were similar in number and had comparable tetramer staining intensities, but the expression range of cell surface markers reflecting TCR signaling suggested that the majority of them from B6 and BALB/c mice have experienced stronger signaling through TCR, likely from encountering antigen, while those from B2m−/− mice had not [22]. In particular, T10/T22-specific cells from B6 and BALB/c mice expressed lower levels of TCR and heat stable antigen (HSA, or J11D), but higher levels of the IL-2 and IL-15 receptor common β chain (CD122) than those from B2m−/− mice. αβ thymocytes that encounter ligand express lower levels of HSA, and an HSAlo profile has also been reported for G8 γδ TCR transgenic thymocytes that develop in the presence of ligand [20–21]. Similarly, the up-regulation of CD122 has been used as an indicator of self-ligand recognition in αβ thymocytes [31] and during the development of murine skin γδ dendritic epidermal cells [29]. In addition, the majority of the T10/T22-specific cells in B2m−/− mice were CD44lo,int, CD122lo,int and expressed high levels of the chemokine receptor CCR9, while those from B6 mice were CD44hi, CD122hi, and a greater fraction of these cells were CCR9lo [32]. Importantly, the staining patterns of total γδ T cells in all strains of mice are more similar to T10/T22-specific γδ T cells from B2m−/− mice than those from B6 mice. Taken together, these results (summarized in Figure 2) suggest that the majority of the γδ T cells have not encountered ligand during development.
Figure 2. Surface Phenotypes of T10/T22-Specific γδ T Cells from Mice with and without Endogenous T10 and T22 Expression.
γδ thymocytes (a) or γδ splenocytes (b) from B6 and B2m−/− mice were analyzed for the expression of cell surface markers commonly associated with TCR signaling and antigen recognition. T22-tetramer positive cells in red, T22-tetramer negative cells in blue.
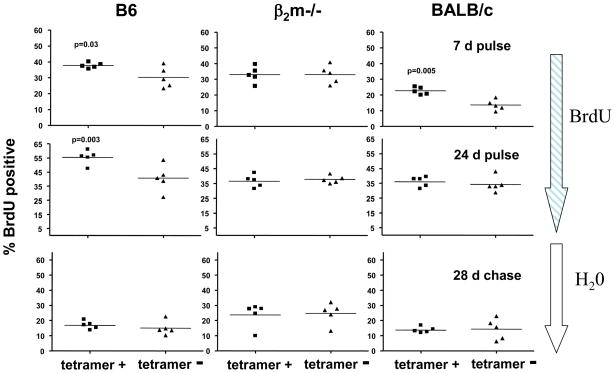
Consistent with this supposition, peripheral T10/T22-specific and the rest of the γδ T cells from B2m−/− mice have a similar turn-over rate as evaluated by the levels of intracellular bromodeoxyuridine (BrdU) incorporation, whether it is short term labeling, long term labeling or long chase after long term labeling. In contrast, T10/T22-specific γδ T cells from B6 and BALB/c mice incorporated significantly more BrdU after 7-day labeling than the vast majority of γδ T cells. In addition, the turnover rate of the majority of the splenic γδ T cells from B6 mice was similar to that of T10/T22-specific γδ T cells, which developed in B2m−/− mice (B6 background). However, the difference in the turnover rates was no longer apparent after a 28-day chase preceded by 24-day labeling. Thus, as summarized in Figure 3, encountering antigen increased the turnover of T10/T22-specific γδ T cells, but did not ‘fix’ this specificity in the repertoire. Indeed, there were no significant differences in the frequency of T10/T22-specific γδ T cells from any strain of mice between two and twenty weeks of age. This suggests that an antigen-naïve γδ T cell repertoire is actively maintained in peripheral lymphoid organs by turning over rapidly and by not prolonging the life-span of γδ T cells that have encountered antigen [22].
Figure 3. Host T10 and T22 Expression Enhances T10/T22-Specific γδ T Cell Turnover but Does Not Fix this Specificity in the Repertoire.
The percentage of BrdU+ cells among tetramer-positive and negative γδ splenocytes from B6, B2m−/−, and BALB/c mice were analyzed by intracellular BrdU staining. Mice were fed with BrdU in their drinking water for 7 days (upper panels) or 24 days (middle panels) or chased with normal drinking water for 28 days after the 24 day labeling phase (lower panels). Each dot represents the analysis of one mouse. Data were analyzed by a paired, two-tailed student t test.
III. TCR signaling strength determines the functional specification of γδ T cells: antigen-naïve γδ T cells make IL-17, antigen-experienced γδ T cells make IFN-γ, and weakened TCR signaling leads to the overt appearance of IL-4-producing cells
To test whether encountering ligand during thymic development is required or inhibitory for γδ T cells to function in the periphery, Jensen et al. used CD122 expression to approximate γδ T cells that have and have not encountered ligand and analyzed CD122hi and CD122lo γδ T cells isolated from the thymus, spleen and lymph nodes. They found that upon TCR cross-linking, CD122lo cells produced IL-17, but not IFN-γ. Conversely, CD122hi cells produced IFN-γ and not IL-17 [22], indicating that antigen-naïve γδ T cells make IL-17 and antigen-experienced γδ T cells make IFN-γ. Indeed, they went on to show that T10/T22-specific γδ T cells from B2m−/− lymph nodes made IL-17, and T10/T22-specific thymocytes and splenocytes in YETI mice (B6 background), which express an IFN-γ-yellow fluorescence protein (YFP) bicistronic reporter, expressed YFP. These results indicate that γδ T cells that have developed in the presence or absence of ligand are not inactivated or anergic, but have different effector functions and suggest a correlation between γδ TCR signaling strength and functional specifications. Importantly, regardless of their effector functions, γδ T cells responded to TCR triggering readily. This is in stark contrast to the activation requirements of αβ T cells, which require an initial antigen-specific priming event by professional antigen presenting cells before developing into effector cells with the capability to secrete cytokines days later.
IL-17 is a T cell cytokine which regulates the expansion and recruitment of neutrophils and monocytes to initiate the inflammatory response [33–34]. In acute inflammation, a swift IL-17 response must be elicited without prior antigen exposure. Given the ability of γδ T cells to produce cytokine immediately upon TCR stimulation, they may be uniquely suited to produce IL-17 at the onset of the inflammatory response. Indeed, a large fraction of γδ T cells from the draining lymph nodes become IL-17+ immediately after myelin oligodendrocyte glycoprotein (MOG) peptide immunization in Complete Freund’s adjuvant (CFA), days before the emergence of MOG specific IL-17+αβ T cells [22]. γδ T cells are the major early producers of IL-17 in several murine models of infection [35].
There have been other correlations of cell surface marker expression with the differential ability of γδ T cells to produce either IFN-γ or IL-17. Kisielow and colleagues identified SCART1 and SCART2 as novel scavenger receptor proteins which are highly expressed on γδ lymphocytes. Upon strong TCR stimulation in the presence of IL-2, SCART2 levels on SCART2hi cells decrease considerably. Interestingly, SCART2hi, but not SCART2lo, γδ T cells make IL-17 [36]. Ribot et al. demonstrated that γδ T cells expressing the TNF receptor family member CD27 make IFN-γ, while CD27+ cells produce IL-17. Although it is unclear how CD27 expression on γδ T cells is induced, treatment of fetal thymic organ cultures with anti-CD3 or cyclosporin A increases or decreases the fraction of CD27+ cells respectively, according to the altered strength of TCR signal [37]. In addition, Haas et al. showed that γδ thymocytes that are CD24loCD44hi and CCR6+ produce IL-17A, and those that are CD24loCD44hi and NK1.1+ make IFN-γ [38]. Intriguingly, CCR6+ but not NK1.1+ γδ thymocytes expressed CCR9, and T10/T22-specific γδ thymocytes in B2m−/−, but not in B6 mice showed CCR9hi expression. Thus, CCR6+ T cells may be less antigen-experienced than the NK1.1+ γδ T cells. Furthermore, Shibata et al. reported that fetal γδ thymocytes that are CD25+CD122− make IL-17 [39]. In addition to these markers, Vγ gene segment usage has also been used to correlate with IL-17 and IFN-γ production [40–41]. However, in these latter cases, it is unclear whether and how Vγ gene segment usage and CD25 expression can be related to TCR signaling strength and/or environmental cues.
Although most γδ T cells make IL-17 or IFN-γ, some γδ T cells instead make IL-4. Interestingly, IL-4-producing γδ T cells seem to be over-represented in mice with defective TCR signaling. Pereira and colleagues described a small population of Thy-1dull γδ thymocytes (~5% of the total γδ thymocytes), which are the major IL-4 producing γδ T cells in normal mice [42–43]. While most of the γδ T cells are CD4+8+, ~40–50% of these cells are CD4+, and a similar fraction of these cells also express NK1.1. This population of cells expresses very restricted TCRs, which are encoded by Vδ6.3/6.4, Vγ1.1, Jγ4 with nearly identical CDR3 sequences. Mice expressing a mutant allele of the adapter protein linker of activated T cells (LAT) lack αβ T cell development, but have elevated levels of serum IgE and a large number of Vγ1.1-expressing CD4+ γδ T cells; many of them secrete IL-4, rather than IFN-γ. These altered γδ T cells are the source of T cell help for B cell activation and IgE class switching [44]. ITK (inducible T cell kinase) is a component of the αβ TCR signaling pathway. ITK+/+ mice have defective Th2 αβ T cell development and an increased number of γδ T cells that express CD4 and produce Th2-associated cytokines (IL-4, 5 and 13). These γδ T cells express CD4, NK1.1, Vγ1.1 and Vδ6.3 and are responsible for the spontaneously elevated levels of serum IgE and increased numbers of germinal center B cells [45–46]. Thus, weakened TCR signaling can be correlated with the emergence of this population of γδ T cells. The most straightforward interpretation would be that that Vγ1+Vδ6.3+ γδ thymocytes recognize a thymic antigen and receive a strong TCR signal during development. Indeed, von Boehmer and colleagues showed that Vγ1+Vδ6.3+ γδ thymocytes on average express twice as high CD5 levels as Vg1+Vd6.3+γδ thymocytes do. Cell surface CD5 levels have been used as measure of TCR signal strength [24–25]. In addition, Thy-1dull Vγ1+Vδ6.3+ γδ T cells express the BTB-zinc finger transcription factor PLZF (promyelocytic leukemia zinc finger protein) [45, 47]. von Boehmer and colleagues showed that TCR crosslinking could induce PLZF expression in γδ thymocytes, but IL-17-producing γδ T cells do not express PLZF [47].
Summary
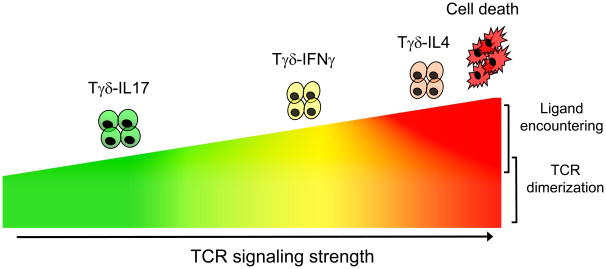
Clearly, to determine whether the development of T10/T22-specific γδ T cells is typical of the entire adult γδ T cell repertoire will require further studies when additional γδ T cell antigens are identified. Until then, these recent developments seem to indicate that γδ T cells differ from αβ T cells in how thymic development influences their TCR specificities and effector-fate development. In particular, encountering ligand is not necessary for γδ T cell development. Thus, once the antigen specificity repertoire is generated by V(D)J rearrangement, it is only marginally modified by thymic selection. In fact, a large fraction of peripheral γδ T cells have not encountered ligand either in the thymus or in the periphery, and this antigen-naïve population is actively maintained by rapidly turning over, and cells that have encountered self-ligands do not accumulate [22]. While ligand expression does little to constrain antigen specificities of the γδ T cell repertoire, it does play a role in endowing lymphoid γδ T cells with different functional programs. There is very clear evidence for distinct functional subsets of lymphoid γδ T cells. Those that are antigen-naive make IL-17 (Tγδ-IL-17); those that are antigen-experienced make IFN-γ (Tγδ-IFNγ) and those that are strongly self-reactive make IL-4 (Tγδ-IL-4) (as illustrated in Figure 4). Importantly, regardless of ligand experience, γδ T cells are able to make cytokines immediately upon TCR engagement. γδ T cells are different from αβ T cells in both their requirements for antigen recognition and activation, and also in their development of TCR repertoire and effector fates. These characteristics make γδ T cells ideal to function in the first line of defense at the onset of an acute inflammatory response to new pathogens that the host encounters, as well as to host antigens that are only revealed by injury. In addition, by acting early in the inflammatory response, γδ T cells may modulate and shape the subsequent αβ T cell and B cell responses that develop during the inflammatory process and thus may play a much larger role in the adaptive immune response than previously recognized. This may be the key to understanding how γδ T cells contribute to host immune competence and why these cells have been maintained throughout vertebrate evolution.
Figure 4. Effect of Differential TCR Signaling Strength on γδ T cell Effector Fates.
TCR signaling occurs at a baseline level through spontaneous TCR dimerization. Upon antigen binding, TCR signaling strength determines the effector fate of γδ thymocytes. With increasing TCR signaling strength, γδ thymocytes develop into mature γδ T cells secreting either IL-17 (Tγδ-IL17), IFN-γ (Tγδ-IFNγ), or IL-4 (Tγδ-IL-4). Stronger TCR signaling will lead to cell death.
Abbreviations
- TCR
T cell receptor
- IFN-γ
interferon gamma
- IL
interleukin
- MHC
major histocompatability complex
- Ig
immunoglobulin
- B2m
beta-2-microglobulin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christina Meyer, Email: christina.meyer@stanford.edu.
Xun Zeng, Email: xunzeng@stanford.edu.
Yueh-hsiu Chien, Email: chien@stanford.edu.
References
- 1.Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev. 2007;215:46–58. doi: 10.1111/j.1600-065X.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 2.Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 3.Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13:37–45. doi: 10.1016/s1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 4.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, et al. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252–255. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 5.Matis LA, Fry AM, Cron RQ, Cotterman MM, Dick RF, Bluestone JA. Structure and specificity of a class II MHC alloreactive gamma delta T cell receptor heterodimer. Science. 1989;245:746–749. doi: 10.1126/science.2528206. [DOI] [PubMed] [Google Scholar]
- 6.Crowley MP, Fahrer AM, Baumgarth N, Hampl J, Gutgemann I, Teyton L, et al. A population of murine gammadelta T cells that recognize an inducible MHC class Ib molecule. Science. 2000;287:314–316. doi: 10.1126/science.287.5451.314. [DOI] [PubMed] [Google Scholar]
- 7.Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M, et al. Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein AI. Immunity. 2005;22:71–80. doi: 10.1016/j.immuni.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Sciammas R, Bluestone JA. HSV-1 glycoprotein I-reactive TCR gamma delta cells directly recognize the peptide backbone in a conformationally dependent manner. J Immunol. 1998;161:5187–5192. [PubMed] [Google Scholar]
- 9.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 10.Li P, Willie ST, Bauer S, Morris DL, Spies T, Strong RK. Crystal structure of the MHC class I homolog MIC-A, a gammadelta T cell ligand. Immunity. 1999;10:577–584. doi: 10.1016/s1074-7613(00)80057-6. [DOI] [PubMed] [Google Scholar]
- 11.Wingren C, Crowley MP, Degano M, Chien Y, Wilson IA. Crystal structure of a gammadelta T cell receptor ligand T22: a truncated MHC-like fold. Science. 2000;287:310–314. doi: 10.1126/science.287.5451.310. [DOI] [PubMed] [Google Scholar]
- 12.Schild H, Mavaddat N, Litzenberger C, Ehrich EW, Davis MM, Bluestone JA, et al. The nature of major histocompatibility complex recognition by gamma delta T cells. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 13.Hampl J, Schild H, Litzenberger C, Baron M, Crowley MP, Chien YH. The specificity of a weak gamma delta TCR interaction can be modulated by the glycosylation of the ligand. J Immunol. 1999;163:288–294. [PubMed] [Google Scholar]
- 14.Rock EP, Sibbald PR, Davis MM, Chien YH. CDR3 length in antigen-specific immune receptors. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K, Van Kaer L, Bonneville M, Hsu S, Murphy DB, Tonegawa S. Recognition of the product of a novel MHC TL region gene (27b) by a mouse gamma delta T cell receptor. Cell. 1990;62:549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 16.Bluestone JA, Cron RQ, Cotterman M, Houlden BA, Matis LA. Structure and specificity of T cell receptor gamma/delta on major histocompatibility complex antigen-specific CD3+, CD4−, CD8minus; T lymphocytes. J Exp Med. 1988;168:1899–1916. doi: 10.1084/jem.168.5.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonneville M, Ishida I, Itohara S, Verbeek S, Berns A, Kanagawa O, et al. Self-tolerance to transgenic gamma delta T cells by intrathymic inactivation. Nature. 1990;344:163–165. doi: 10.1038/344163a0. [DOI] [PubMed] [Google Scholar]
- 18.Dent AL, Matis LA, Hooshmand F, Widacki SM, Bluestone JA, Hedrick SM. Self-reactive gamma delta T cells are eliminated in the thymus. Nature. 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 19.Pereira P, Zijlstra M, McMaster J, Loring JM, Jaenisch R, Tonegawa S. Blockade of transgenic gamma delta T cell development in beta 2-microglobulin deficient mice. EMBO J. 1992;11:25–31. doi: 10.1002/j.1460-2075.1992.tb05023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells FB, Gahm S-J, Hedrick SM, Bluestone JA, Dent A, Matis LA. Requirement for Positive Selection of γδ Receptor-Bearing T Cells. Science. 1991;253:903–905. doi: 10.1126/science.1831565. [DOI] [PubMed] [Google Scholar]
- 21.Schweighoffer E, Fowlkes BJ. Positive selection is not required for thymic maturation of transgenic gamma delta T cells. J Exp Med. 1996;183:2033–2041. doi: 10.1084/jem.183.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 24.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 Expression Is Developmentally Regulated By T Cell Receptor (TCR) Signals and TCR Avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Müller W, Kileen N, et al. A Role for CD5 in TCR-Mediated Signal Transduction and Thymocyte Selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 26.Yamasaki S, Ishikawa E, Sakuma M, Ogata K, Sakata-Sogawa K, Hiroshima M, et al. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nature immunology. 2006;7:67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JM, Girardi M, Roberts SJ, Barbee SD, Hayday AC, Tigelaar RE. Selection of the cutaneous intraepithelial gammadelta+ T cell repertoire by a thymic stromal determinant. Nature immunology. 2006;7:843–850. doi: 10.1038/ni1363. [DOI] [PubMed] [Google Scholar]
- 28.Mallick-Wood CA, Lewis JM, Richie LI, Owen MJ, Tigelaar RE, Hayday AC. Conservation of T cell receptor conformation in epidermal gammadelta cells with disrupted primary Vgamma gene usage. Science. 1998;279:1729–1733. doi: 10.1126/science.279.5357.1729. [DOI] [PubMed] [Google Scholar]
- 29.Xiong N, Kang C, Raulet DH. Positive Selection of Dendritic Epidermal [gamma][delta] T Cell Precursors in the Fetal Thymus Determines Expression of Skin-Homing Receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Hayes SM, Love PE. Distinct Structure and Signaling Potential of the γδTCR Complex. 2002;16:827–838. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 31.Hanke T, Mitnacht R, Boyd R, Hunig T. Induction of interleukin 2 receptor beta chain expression by self-recognition in the thymus. J Exp Med. 1994;180:1629–1636. doi: 10.1084/jem.180.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen KD, Shin S, Chien YH. Cutting edge: Gammadelta intraepithelial lymphocytes of the small intestine are not biased toward thymic antigens. J Immunol. 2009;182:7348–7351. doi: 10.4049/jimmunol.0900465. [DOI] [PubMed] [Google Scholar]
- 33.Ley K, Smith E, Stark M. IL-17A-producing neutrophil-regulatory Tn lymphocytes. Immunologic research. 2006;34:229–242. doi: 10.1385/IR:34:3:229. [DOI] [PubMed] [Google Scholar]
- 34.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien RL, Roark CL, Born WK. IL-17-producing gammadelta T cells. European journal of immunology. 2009;39:662–666. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kisielow J, Kopf M, Karjalainen K. SCART scavenger receptors identify a novel subset of adult gammadelta T cells. J Immunol. 2008;181:1710–1716. doi: 10.4049/jimmunol.181.3.1710. [DOI] [PubMed] [Google Scholar]
- 37.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas JD, González FHM, Schmitz S, Chennupati V, Föhse L, Kremmer E, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. European journal of immunology. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 39.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ {gamma}{delta} T Cells As Fetal Thymus-Derived Naturally Occurring IL-17 Producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 40.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of Collagen-Induced Arthritis by Oligoclonal, IL-17-Producing {gamma}{delta} T Cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 42.Azuara V, Levraud JP, Lembezat MP, Pereira P. A novel subset of adult gamma delta thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 43.Gerber DJ, Azuara V, Levraud JP, Huang SY, Lembezat MP, Pereira P. IL-4-producing gamma delta T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J Immunol. 1999;163:3076–3082. [PubMed] [Google Scholar]
- 44.Nunez-Cruz S, Aguado E, Richelme S, Chetaille B, Mura AM, Richelme M, et al. LAT regulates gammadelta T cell homeostasis and differentiation. Nature immunology. 2003;4:999–1008. doi: 10.1038/ni977. [DOI] [PubMed] [Google Scholar]
- 45.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in γδT cells is pivotal for controlling IgE production in vivo. Proceedings of the National Academy of Sciences. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi Q, Xia M, Hu J, Hicks E, Iyer A, Xiong N, et al. Enhanced development of CD4+ {gamma}{delta} T cells in the absence of Itk results in elevated IgE production. Blood. 2009;114:564–571. doi: 10.1182/blood-2008-12-196345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, et al. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of gammadelta T cells with restricted TCR diversity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]