Abstract
Objective
Adenosine and the activation of specific adenosine receptors are implicated in the attenuation of inflammation and organ ischemia-reperfusion (IR) injury. We hypothesized that activation of A1, A2A, or A3 adenosine receptors would provide protection against lung IR injury.
Methods
Using an isolated, ventilated, blood-perfused rabbit lung model, lungs underwent 18 hours cold ischemia followed by 2 hours reperfusion. Lungs were administered either vehicle, adenosine, or selective A1, A2A, or A3 receptor agonists (CCPA, ATL-313, or IB-MECA, respectively) alone or with their respective antagonists (DPCPX, ZM241385, or MRS1191) during reperfusion.
Results
Compared to the vehicle-treated control group, treatment with A1, A2A, or A3 agonists significantly improved function (increased lung compliance and oxygenation and decreased pulmonary artery pressure), decreased neutrophil infiltration by myeloperoxidase activity, decreased edema, and reduced TNF-α production. Adenosine treatment was also protective but not to the level of the agonists. When each agonist was paired with its respective antagonist, all protective effects were blocked. The A2A agonist reduced pulmonary artery pressure and myeloperoxidase activity and increased oxygenation to a greater degree than the A1 or A3 agonists.
Conclusions
Selective activation of A1, A2A, or A3 adenosine receptors provides significant protection against lung IR injury. The decreased elaboration of the potent proinflammatory cytokine, TNF-α, and decreased neutrophil sequestration likely contribute to the overall improvement in pulmonary function. These results provide evidence for the therapeutic potential of specific adenosine receptor agonists in lung transplant recipients.
Introduction
Primary graft dysfunction (PGD), the most severe form of ischemia-reperfusion (IR) injury, continues to be a major cause of morbidity and mortality after lung transplantation. The most recent report from the International Society for Heart and Lung Transplantation reported that PGD was the leading cause of death (28.8% of patient deaths) within the first 30 days after transplant (1). Although the overall incidence of lung IR injury has remained fairly constant (just under 25%), the 30-day mortality after transplant has improved over the past two decades primarily due to reductions in PGD (2). The clinical significance of lung IR injury is further magnified by its association with the development of bronchiolitis obliterans (3). Improvements in outcomes after lung transplantation as well as extending the donor pool and recipient criteria are predicated on the ability to minimize the deleterious inflammatory responses that occur with lung IR injury.
Adenosine is an endogenous mediator which typically serves as a cytoprotective modulator in response to stress. Many studies have demonstrated the protective effects of adenosine in the setting of organ IR injury. Adenosine signals through four subtypes of the G protein-coupled receptor, A1, A2A, A2B and A3, all of which are expressed in the lung. Classically, protective effects of adenosine receptor signaling occur through second messenger pathways such as cAMP production or the phospholipase C pathway. Our laboratory has extensively studied selective A2A receptor activation and have shown it to provide significant protection against lung IR injury (4-6). However, the effects of specific activation of A1, A2B, and A3 adenosine receptors in lung IR injury remain poorly understood. Previous studies have provided evidence that A1 and A3 receptors may primarily be involved in anti-inflammatory actions (7, 8) while the A2B receptor may have more pro-inflammatory actions (9) in the lung. The objective of this study is to further define the protective effects of adenosine receptor subtypes, using specific agonists and antagonists, on lung IR injury. We hypothesize that specific activation of A1, A2A, or A3 receptors provides significant protection from lung injury and dysfunction after IR. Use of specific adenosine receptor agonists and antagonists in an isolated, blood-perfused rabbit model of lung IR will be utilized to test this hypothesis.
Materials and Methods
Animal Care
New Zealand white rabbits (Burleson Enterprises, Inc.) of both sexes (3.0-3.5 kg) were utilized in this study and received humane care in accordance with the “Guide for care and use of laboratory animals” published by the National Institute of Health (National Institutes of Health publication no.85-23, revised 1995). The study protocol was reviewed and approved by The Animal Care and Use Committee at the University of Virginia.
Experimental protocol
Eight experimental groups of lungs (n=6/group) were compared utilizing an isolated, whole blood-perfused, ventilated rabbit lung model of IR (Kent Scientific, Model TIS3862, Litchfield, CT) as previously described (4). All lungs were reperfused for 120 min following 18 hrs of cold ischemic storage, time periods which we have previously demonstrated to result in reproducible injury versus sham lungs (4). Lungs received either vehicle (DMSO), adenosine, or a specific adenosine receptor agonist (with or without its respective antagonist) as summarized in Table 1. Each of the agonists used in this study are very potent and specific for their target. IB-MECA is reported to be approximately 50-fold selective for A3 versus A1 or A2A (10). ATL-313 is very selective for A2A versus A1 (81-fold) and A3 (350-fold) (11). CCPA is reported to be approximately 53-fold selective for A1 versus A2A or A3 (12). Each receptor agonist or antagonist was added to the whole blood perfusate at the beginning of reperfusion (See Table 1 for specific doses). The doses used were based upon well-established doses utilized in previous studies which do not result in significant cardiovascular effects (4, 13, 14). Adenosine was administered by constant infusion (0.75mg/kg/min) during reperfusion due to its very short half-life of several seconds in whole blood.
Table 1.
Adenosine receptor agonists and antagonists utilized
| Adenosine Receptor | Agonist (dose, source) | Antagonist (dose, source) |
|---|---|---|
| A1 | CCPA (10nM, Sigma) | DPCPX (100nM, Sigma) |
| A2A | ATL-313 (100nM, Dr. J. Linden) | ZM241385 (100nM, Tocris Bioscience) |
| A3 | IB-MECA (60 nM, Sigma) | MRS1191 (10nM, Sigma) |
| All | Adenosine (0.75mg/kg/min, Sigma) | NA |
CCPA, 2-chloro-N(6)-cyclopentyladenosine; ATL-313, 4-{3-[6-amino-9-(5-cyclopropylcarbamoyl-3,4-dihydroxy-tetrahydro-furan-2-yl)-9H-purin-2-yl]-prop-2-ynyl}-piperidine-1-carboxylic acid methyl ester; IB-MECA: 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide; DPCPX: 8-cyclopentyl-1,3-dipropylxanthine; ZM 241385, 4-(2-[7-Amino-2-[2-furyl][1,2,4]triazolo[2,3-a][1,3,5]triazin-5-yl-amino]ethyl); MRS 1191, 3-ethyl-5-benzyl-2-methyl-6-phenyl-4-phenylethynyl-1, 4-(±)-dihydropyridine-3,5-dicarboxylate.
Harvest Procedure
Animals were randomly assigned to each experimental group. Each animal was anesthetized with intramuscular ketamine (50mg/kg) and xylazine (5 mg/kg). Tracheal intubation was performed via a tracheostomy, and mechanical ventilation (Kent Scientific, Model RSP1002, Litchfield, CT) was instituted with room air at a respiratory rate of 30 breaths/min. Median sternotomy and thymectomy were performed. The aorta and pulmonary artery (PA) were encircled, and 1000 U/kg heparin was administered intravenously. Prostaglandin E1 (30μg) was then administered via the PA, and the vena cavae were ligated to initiate ischemia five minutes after heparin administration. The PA was then cannulated through a ventriculotomy made within a purse-string placed in the right ventricular outflow tract. After the left ventricle was vented through a left ventriculotomy and the aorta was ligated, 100 ml/kg of buffered Perfadex® (Vitrolife, Kungsbacka, Sweden) preservation solution was infused into the PA at a pressure of 30cm H2O at 4°C. Topical cooling was achieved with cold sa line solution slush. The left atrium was cannulated through the left ventriculotomy with an outflow catheter. The lung-heart block was excised. The lungs were stored inflated at 4 °C for 18 hrs.
Reperfusion procedure
A force transducer suspended the heart-lung block, and ventilation was initiated with 95% oxygen and 5% carbon dioxide gas mixture (Kent Scientific, Model RSP1002, Litchfield, CT). All groups underwent 120 min whole-blood perfusion at 37°C. Atelectasis was grossly eliminated by administering one breath of approximately 30cm H2O positive end expiratory pressure once per min during the first 5 min of the stabilization period. Lungs were ventilated at a constant tidal volume of 10cc/kg with 3cm H2O of positive end expiratory pressure at a rate of 30 breaths/min. The PA and the outflow catheters connected the lung-heart block to a venous blood reperfusion circuit. New Zealand white rabbits served as fresh venous blood donors. Blood was circulated through a pediatric oxygenator set to deoxygenate the blood and add carbon dioxide in order to simulate venous blood (PO2 = 60mmHg/PCO2 = 60mmHg). The lungs were subsequently perfused via the PA cannula at 60 mL/min with “venous” blood at 37°C.
Lung physiology
A dynamic data acquisition program (DASYLab, DASYTEC, USA, Bedford, NH) recorded PA pressure and pulmonary compliance. Pulmonary venous blood samples were collected for blood gas analysis (Bayer 348 pH/Blood Gas Analyzer, Bayer Corp., E. Walpole, MA) at 15, 30, 60, 90, and 120 min after initiation of reperfusion.
Lung wet/dry weight
The lung wet/dry weight ratio was used to assess pulmonary edema. After the 120 min reperfusion period, fresh samples of lung tissue were collected from the right lower lobe of the left lung. Fresh lung samples were blotted to remove excess blood, weighed, and desiccated under vacuum at 55°C until a stable dry weight was achieved.
Myeloperoxidase (MPO) activity
Neutrophil sequestration was assessed by measuring MPO activity in left lung tissue as previously described by our laboratory (5).
Bronchoalveolar lavage (BAL)
BAL was performed on all lungs at the end of the reperfusion period. The right upper and middle lobes were isolated and lavaged with 10 mL normal saline. The BAL fluid was then centrifuged at 1500g for 5 min at 4°C. The supernatant was stored at -80°C until analysis.
Measurement of TNF-α
Protein levels of TNF-α in BAL fluid were measured using a TNF-α ELISA kit (BD Biosciences, San Diego, CA) as instructed by the manufacturer. Samples were assessed in triplicate.
Statistics
Values are expressed as the mean ± standard error of the mean. ANOVA was used to determine if significant differences existed between groups. Tukey's honest significant difference multiple-comparison test was used to determine which groups were significantly different when the ANOVA results were significant. Repeated measures analysis of variance was performed and ultimately allowed us to conclude that PA pressure, lung compliance, and oxygenation changes over time and depends upon group. Reported p-values are considered significant when less than 0.05.
Results
Lung physiology
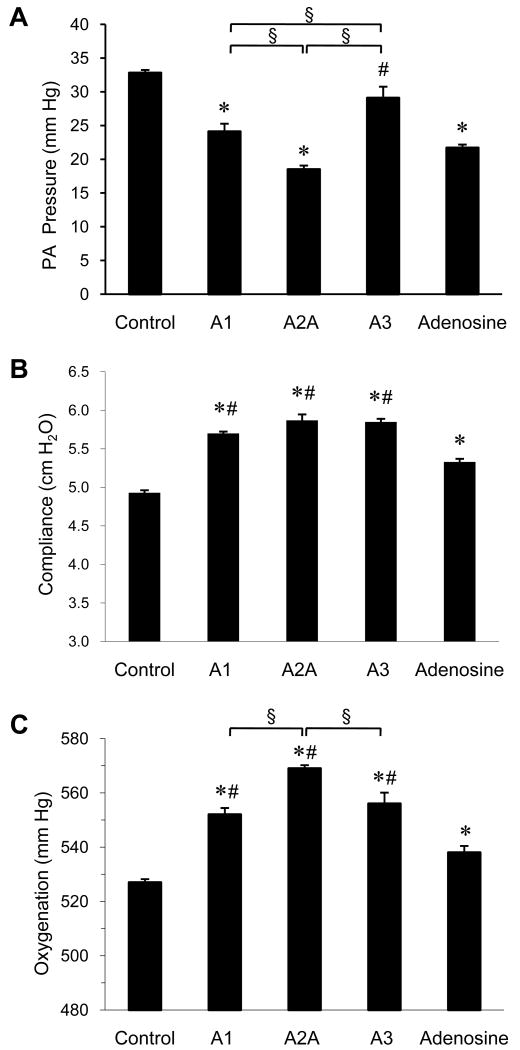
Selective activation of A1 and A2A receptors during reperfusion resulted in significant decreases in PA pressure versus Control at the end of the 120 min reperfusion period (Figure 1A). In addition, significant decreases in PA pressure were observed in the A2A agonist-treated group relative to treatment with A1 or A3 agonists (Figure 1A). The A3 receptor agonist reduced PA pressure somewhat, but this was not significant. The A1, A2A, and A3 receptor agonists resulted in significant increases in lung compliance (Figure 1B) and oxygenation (Figure 1C) compared to Control at 120 min reperfusion. Oxygenation was significantly higher in the A2A agonist-treated group versus treatment with A1 or A3 agonists (Figure 1C). The improvements in the aforementioned physiologic measurements by each agonist were all blocked by the concomitant administration of antagonist (Table 2). While adenosine administration resulted in significant improvement of PA pressure, lung compliance, and oxygenation, these improvements were generally not as great as was observed for the receptor agonists.
Figure 1. Lung function.
Comparison of pulmonary artery (PA) pressure (A), pulmonary compliance (B) and oxygenation (C) between groups at the end of the 120-min reperfusion period. *p<0.0002 vs. Control, #p<0.02 vs. Adenosine, §p<0.02.
Table 2. Pulmonary function at the end of the 120-min reperfusion period.
| Group | PA Pressure (mm Hg) | Compliance (cm H2O) | Oxygenation (mm Hg) |
|---|---|---|---|
| Control | 32.83 ± 0.48 | 4.93 ± 0.03 | 527.7 ± 1.1 |
| A1 agonist | 24.17 ± 1.19 a, d | 5.70 ± 0.03 a, b | 551.7 ± 2.2 a, b |
| A1 agonist + antagonist | 31.67 ± 0.56 b | 5.07 ± 0.03 b | 527.0 ± 2.1 |
| A2A agonist | 18.50 ± 0.62 a, c, d | 5.87 ± 0.08 a, b | 569.2 ± 1.1 a, b, c, d |
| A2A agonist + antagonist | 31.33 ± 0.84 b | 5.12 ± 0.48 b | 525.3 ± 2.9 b |
| A3 agonist | 29.17 ± 1.66 b, c | 5.85 ± 0.04 a, b | 556.2 ± 4.0 a, b |
| A3 agonist + antagonist | 32.00 ± 1.59 b | 5.07 ± 0.06 b | 530.0 ± 3.5 |
| Adenosine | 21.67 ± 0.49 a | 5.33 ± 0.04 a | 538.3 ± 2.5 a |
p<0.0002 vs. Control,
p<0.02 vs. Adenosine,
p<0.01 vs. A1 agonist,
p<0.02 vs. A3 agonist
Production of TNF-α
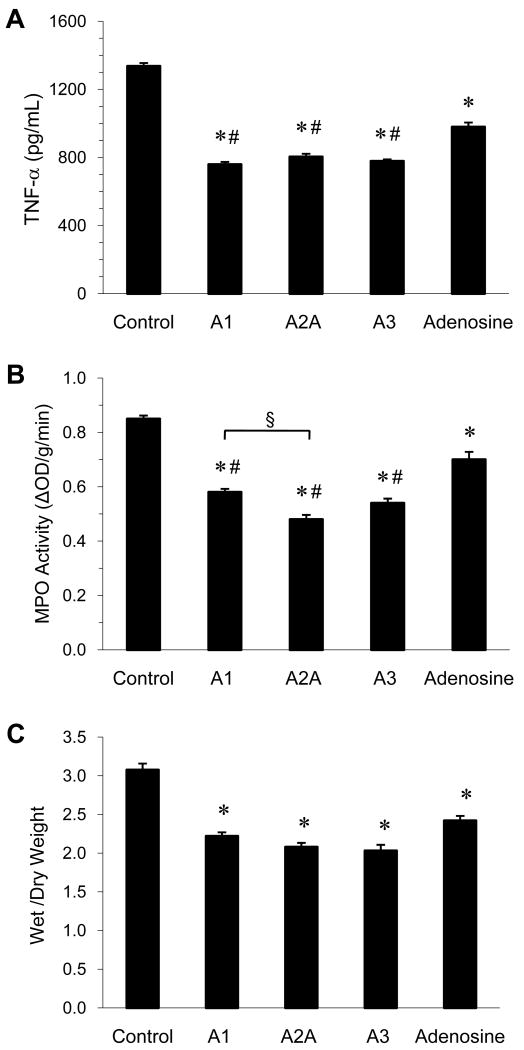
Treatment with A1, A2A, and A3 receptor agonists as well as adenosine resulted in significantly less production of the pro-inflammatory cytokine TNF-α versus Control (Figure 2A). The administration of each receptor agonist with its respective antagonist blocked the decrease in TNF-α (Table 3). No significant difference in TNF-α production occurred between the agonist-treated groups. Each receptor agonist resulted in significantly lower TNF-α production compared to adenosine alone.
Figure 2. Lung injury.
Comparison of TNF-α levels in BAL fluid (A), myeloperoxidase (MPO) activity (B), and lung wet/dry weight ratio (C) between groups at the end of the 120-min reperfusion period. *p<0.0002 vs. Control, #p<0.01 vs. Adenosine, §p<0.01.
Table 3. Pulmonary inflammation at the end of the 120-min reperfusion period.
| Group | TNF-α (pg/ml) | MPO Activity (ΔOD/g/min) | Wet/Dry Weight |
|---|---|---|---|
| Control | 1336 ± 18.9 | 0.858 ± 0.012 | 3.07 ± 0.08 |
| A1 agonist | 759 ± 14.7 a, b | 0.582 ± 0.013 a, b | 2.22 ± 0.05 a |
| A1 agonist + antagonist | 1308 ± 18.7 b | 0.810 ± 0.015 b | 2.98 ± 0.09 b |
| A2A agonist | 804 ± 17.6 a, b | 0.482 ± 0.017 a, b, c | 2.08 ± 0.05 a |
| A2A agonist + antagonist | 1326 ± 18.7 b | 0.847 ± 0.026 b | 2.90 ± 0.18 b |
| A3 agonist | 778 ± 10.5 a, b | 0.540 ± 0.016 a, b | 2.03 ± 0.08 a |
| A3 agonist + antagonist | 1287 ± 13.8 b | 0.843 ± 0.150 b | 3.00 ± 0.06 b |
| Adenosine | 978 ± 26.0 a | 0.697 ± 0.028 a | 2.41 ± 0.06 a |
p<0.0002 vs. Control,
p<0.01 vs. Adenosine,
p<0.01 vs. A1 agonist
Myeloperoxidase (MPO) activity
MPO activity was used as an indicator of neutrophil sequestration into the lung. Treatment with A1, A2A, and A3 receptor agonists as well and adenosine resulted in significant decreases in MPO activity versus Control (Figure 2B). The administration of each receptor agonist with its respective antagonist blocked the decrease in MPO activity (Table 3). Selective A2A receptor activation resulted in the greatest decrease in MPO activity (44% reduction versus control). Each receptor agonist resulted in significantly less MPO activity compared to adenosine alone.
Wet/dry weight ratio
Lung wet/dry weight ratio was measured to assess pulmonary edema. Treatment with A1, A2A, and A3 receptor agonists as well and adenosine resulted in significantly less wet/dry weight versus Control (Figure 2C). The administration of each receptor agonist with its respective antagonist blocked the decrease in wet/dry weight (Table 3). No significant difference in wet/dry weight occurred between the agonist-or adenosine-treated groups.
Discussion
The results from this study suggest that pharmacologic modulation of specific adenosine receptors may potentially be a useful therapeutic method for attenuating lung IR injury and thus improving outcomes in patients after lung transplantation. While the anti-inflammatory effects of adenosine are well-described, the ability to translate adenosine-related therapeutics into clinical practice requires better understanding of the relationship between adenosine receptor subtypes and end-organ effect. The protective as well as deleterious profile of adenosine receptors with respect to lung IR injury is poorly understood. A modest number of studies have begun to evaluate the role of single adenosine receptors in lung IR injury (4, 7, 15). However, it is difficult to compare and contrast the role of different adenosine receptor subtypes between these various studies and models. The present study better characterizes and compares the effects of specific activation of A1, A2A, and A3 receptors in an accepted, reproducible model of lung IR injury.
Of the adenosine receptor subtypes, A2A receptor activation has most consistently demonstrated potent anti-inflammatory properties and has repeatedly attenuated lung IR injury in various studies. For example, A2A agonists have been associated with inhibition of inflammatory cytokine release, reduction of IR-induced apoptotic injury, and diminution of free radical production (4, 16). In addition, neutrophil-induced IR injury is directly abrogated by selective A2A receptor activation (17). The attenuation of lung dysfunction and injury after IR by A2A agonists observed in prior studies was confirmed in the present study. Compared to A1 and A3 receptor agonists, the A2A agonist provided greater protection (e.g. lower PA pressure, improved oxygenation, and reduced MPO activity). This may be due to the fact that A2A receptors are predominantly expressed on inflammatory cells including neutrophils, mast cells, macrophages, monocytes, and T cells, and it has been shown that A2A receptor activation prevents leukocyte adhesion to endothelial cells as well as inhibiting the release of toxic oxygen products (18).
Studies on the role of A1 receptor activation in lung IR injury have been somewhat confusing. Although the A1 receptor has been shown to play a protective role after IR in the heart (19), intestine (20), liver (8), and kidney (21), an early study by Neely et al. concluded that A1 receptor antagonists block lung IR injury (15). Paradoxically, A1 receptor activation with CCPA was shown to exert a cytoprotective role against IR injury in skeletal muscle; an effect which was blocked by the A1 receptor antagonist DPCPX (22). In the current study, A1 receptor activation by CCPA improved lung function in association with decreased neutrophil infiltration (MPO activity), inflammatory cytokine production (TNF-α), and edema (wet/dry weight). Importantly, these protective effects were blocked by DPCPX. One possible explanation for the differing conclusions between our study and the study by Neely et al. could involve differences in the model. Neely et al. utilized an in vivo model in which the left lower lobe in intact-chest cats was rendered ischemic for 2 hours and reperfused for 2 hours. A more likely explanation could involve a difference in DPCPX administration where we used DPCPX at a concentration of 100nM in the whole blood reperfusate while Neely et al. utilized a bolus dose of 6mg/kg DPCPX to the cat either 30 min prior to ischemia or 1 hour after reperfusion. We estimate that this dose resulted in a blood-concentration of DPCPX at least 700-fold greater than our dose of 100nM, a dose that could lead to unwanted side effects such as the non-specific antagonism of other (anti-inflammatory) adenosine receptors. Although 100nM DPCPX was quite efficient in blocking the protective effects of the A1 agonist CCPA in our study, we did not test the effect of DPCPX alone.
Proinflammatory properties of the A3 receptor have been demonstrated by mast-cell degranulation resulting from administration of IB-MECA (23). On the other hand, A3 receptor activation reduces degranulation and free radical formation from eosinophils (24). More anti-inflammatory effects; however, have been associated with A3 receptor activation. For example, activation of the A3 receptor was shown to suppress superoxide production and chemotaxis in mouse neutrophils (25). Moreover, various studies have demonstrated that A3 agonists reduce IR-associated myocardial injury (26, 27). In a study of feline lung IR injury, Rivo et al. showed that A3 receptor activation with IB-MECA reduced alveolar injury, wet/dry weight, MPO activity, and apoptosis (7). In support of this data, our current study found that IB-MECA provided significant protection against lung dysfunction and injury after IR. However, A3 receptor activation differed from A1 and A2A receptor activation in that it did not significantly decrease PA pressure versus Control.
The administration of adenosine during reperfusion reduced PA pressure and increased lung compliance versus Control but failed to significantly improve oxygenation. The A1, A2A, and A3 receptor agonists all improved oxygenation and lung compliance to a greater extent than adenosine alone. Although adenosine infusion did decrease TNF-α production and MPO activity versus Control, this was not as great of a decrease as what occurred with the A1, A2A, and A3 agonists. This may account for the poorer lung function with adenosine-treatment compared to the agonists. Adenosine has also been shown to enhance the release of histamine from mast cells and potentiate the bronchoconstrictor response in patients with COPD and asthma (24). Interactions and crosstalk between the adenosine receptors themselves also remain largely unknown and could account for differences in end-organ effect between adenosine administration and selective adenosine receptor activation. Finally, adenosine could also be activating the A2B receptor, a pathway which may be proinflammatory in this setting.
Although small but significant differences in oxygenation were observed as summarized above, all of the pO2 levels were above 500 mm Hg. This high degree of oxygenation would likely not make a large difference clinically. However, we reported pO2 levels in this study for completion of the data and to illustrate that the observed significant differences in oxygenation did correlate well with other differences in physiologic parameters such as PA pressure and pulmonary compliance.
As stated in the Methods, the doses of agonist used in our study were based upon well-established doses utilized in previous studies which do not result in significant cardiovascular effects. Thus it is unlikely that the protective effects of the agonists were primarily physiologic. The reductions in PA pressure observed with the use of the agonists are likely secondary to the anti-inflammatory affects of the agonists. The mechanism responsible for these anti-inflammatory effects are little understood. Adenosine receptors couple, via G proteins, to an intricate network of second messenger signaling pathways such as modulation of cAMP production or phospholipase C. However, the effects of G protein coupling can differ substantially between receptor subtypes. In addition, they are known to couple to mitogen-activated protein kinases (MAPK), indicating a possible role in cell growth, differentiation, survival, and death. Current studies in our laboratory are evaluating mechanisms of action and what cell types are most responsible for the protective effects of adenosine receptors in the setting of lung IR injury.
The role of the A2B receptor in lung IR injury remains largely unknown as no selective, potent A2B agonists yet exist. Functional A2B receptors are widely distributed on cells including fibroblasts, various vascular beds, hematopoietic cells, mast cells, and endothelium (28), and have recently been shown to be highly expressed on mouse alveolar epithelial cells (29). Activation of A2B receptors has been associated with the release of proinflammatory mediators by activated human mast cells and subsequent release of IL-8 (30). In addition, results from Sun et al. suggest that A2B receptor signaling influences pathways critical for pulmonary inflammation and injury in vivo (9). With the recent development of A2B receptor knockout mice, the role of the A2B receptor in lung injury will be better understood. In fact, our laboratory has observed that A2B receptor knockout mice have less lung injury after IR compared to wild-type mice (unpublished data), further supporting a proinflammatory role of the A2B receptor in lung injury.
The current study contained several limitations. One limitation of the isolated rabbit lung model is in the length of study. It is difficult to perfuse the lungs beyond 2 hours and maintain stable lungs, and thus we limited the reperfusion time to 2 hours. However, we feel that the acute period of reperfusion (i.e. the first several hours) is a very critical time of injury that determines whether a transplant recipient will experience primary graft dysfunction, which is why we focused on this acute time period. It is possible that longer reperfusion times might lead to different results, and we speculate that some of the parameters used to assess lung protection by adenosine receptor agonists, such as oxygenation, would be even more convincing after longer reperfusion.
Another limitation of our study is that we did not utilize any mechanical maneuvers such as controlled low pressure reperfusion which is often utilized in the clinical setting. One reason for this is because we wanted to remain consistent with the model used in our previous studies which produces significant and reproducible injury. It is possible that such mechanical maneuvers would mask the protection offered by adenosine receptor agonists. However, despite such maneuvers used in transplant recipients, there will always be some level of inflammation induced upon reperfusion. It is likely that a combination of mechanical maneuvers as well as adenosine receptor agonists would prove most effective at preventing both immediate endothelial injury and inflammation as well later injury and infiltration of inflammatory cells. One of the goals of our study was to directly compare the protective effects of the different adenosine agonists. Some of these differences were small but significant, and these differences may not have been detected if we utilized mechanical maneuvers to blunt injury.
In conclusion, results from the present study suggest that selective activation of A1, A2A, or A3 adenosine receptors provides protection after lung IR by significantly attenuating both lung dysfunction and injury. As better adenosine receptor agonists are developed and the interactions between adenosine receptors are further elucidated, the pharmacologic modulation of adenosine receptor signaling will likely play an important role in minimizing the vast deleterious effects of IR injury after lung transplantation. Further characterization of protective and also potentially detrimental effects of adenosine receptor subtypes is required to maximize the possibilities of adenosine-related therapeutics translating to clinical improvements in patient care.
Acknowledgments
This study was supported by NIH R01HL092953 (VEL and ILK), NIH T32HL007849 (ILK), and the Roche Organ Transplant Research Foundation (VEL).
Footnotes
Disclosures: Drs. Linden and Kron were shareholders in Adenosine Therapeutics, LLC., the corporation that provided ATL-313, at the time of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, et al. The registry of the international society for heart and lung transplantation: twenty-sixth official adult lung and heart-lung transplantation Report-2009. J Heart Lung Transplant. 2009 Oct;28(10):1031–49. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Ailawadi G, Lau CL, Smith PW, Swenson BR, Hennessy SA, Kuhn CJ, et al. Does reperfusion injury still cause significant mortality after lung transplantation? J Thorac Cardiovasc Surg. 2009 Mar;137(3):688–94. doi: 10.1016/j.jtcvs.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002 Apr;73(4):1041–7. doi: 10.1016/s0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- 4.Gazoni LM, Laubach VE, Mulloy DP, Bellizzi A, Unger EB, Linden J, et al. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J Thorac Cardiovasc Surg. 2008 Jan;135(1):156–65. doi: 10.1016/j.jtcvs.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 5.Reece TB, Ellman PI, Maxey TS, Crosby IK, Warren PS, Chong TW, et al. Adenosine A2A receptor activation reduces inflammation and preserves pulmonary function in an in vivo model of lung transplantation. J Thorac Cardiovasc Surg. 2005 May;129(5):1137–43. doi: 10.1016/j.jtcvs.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Sharma AK, Linden J, Kron IL, Laubach VE. Protection from pulmonary ischemia-reperfusion injury by adenosine A2A receptor activation. Respir Res. 2009;10:58. doi: 10.1186/1465-9921-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivo J, Zeira E, Galun E, Matot I. Activation of A3 adenosine receptor provides lung protection against ischemia-reperfusion injury associated with reduction in apoptosis. Am J Transplant. 2004 Dec;4(12):1941–8. doi: 10.1111/j.1600-6143.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- 8.Todo S, Zhu Y, Zhang S, Jin MB, Ishizaki N, Tanaka H, et al. Attenuation of ischemic liver injury by augmentation of endogenous adenosine. Transplantation. 1997 Jan 27;63(2):217–23. doi: 10.1097/00007890-199701270-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, et al. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006 Aug;116(8):2173–82. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998 May;19(5):184–91. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005 Apr 15;174(8):5040–6. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 12.Cappellacci L, Franchetti P, Vita P, Petrelli R, Lavecchia A, Costa B, et al. 5′-Carbamoyl derivatives of 2′-C-methyl-purine nucleosides as selective A1 adenosine receptor agonists: affinity, efficacy, and selectivity for A1 receptor from different species. Bioorg Med Chem. 2008 Jan 1;16(1):336–53. doi: 10.1016/j.bmc.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 13.Brambilla R, Cattabeni F, Ceruti S, Barbieri D, Franceschi C, Kim YC, et al. Activation of the A3 adenosine receptor affects cell cycle progression and cell growth. Naunyn Schmiedebergs Arch Pharmacol. 2000 Mar;361(3):225–34. doi: 10.1007/s002109900186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaar R, Jones MR, Chen JF, Ravid K. Animal models for the study of adenosine receptor function. J Cell Physiol. 2005 Jan;202(1):9–20. doi: 10.1002/jcp.20138. [DOI] [PubMed] [Google Scholar]
- 15.Neely CF, Keith IM. A1 adenosine receptor antagonists block ischemia-reperfusion injury of the lung. Am J Physiol. 1995 Jun;268(6 Pt 1):L1036–46. doi: 10.1152/ajplung.1995.268.6.L1036. [DOI] [PubMed] [Google Scholar]
- 16.Rivo J, Zeira E, Galun E, Einav S, Linden J, Matot I. Attenuation of reperfusion lung injury and apoptosis by A2A adenosine receptor activation is associated with modulation of bcl-2 and bax expression and activation of extracellular signal-regulated kinases. Shock. 2007 Mar;27(3):266–73. doi: 10.1097/01.shk.0000235137.13152.44. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan GW. Adenosine A2A receptor agonists as anti-inflammatory agents. Curr Opin Investig Drugs. 2003 Nov;4(11):1313–9. [PubMed] [Google Scholar]
- 18.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–87. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Cerniway RJ, Byford AM, Berr SS, French BA, Matherne GP. Cardiac overexpression of A1-adenosine receptor protects intact mice against myocardial infarction. Am J Physiol Heart Circ Physiol. 2002 Mar;282(3):H949–55. doi: 10.1152/ajpheart.00741.2001. [DOI] [PubMed] [Google Scholar]
- 20.Kaminski PM, Proctor KG. Attenuation of no-reflow phenomenon, neutrophil activation, and reperfusion injury in intestinal microcirculation by topical adenosine. Circ Res. 1989 Aug;65(2):426–35. doi: 10.1161/01.res.65.2.426. [DOI] [PubMed] [Google Scholar]
- 21.Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004 Feb;286(2):F298–306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- 22.Zheng J, Wang R, Zambraski E, Wu D, Jacobson KA, Liang BT. Protective roles of adenosine A1, A2A, and A3 receptors in skeletal muscle ischemia and reperfusion injury. Am J Physiol Heart Circ Physiol. 2007 Dec;293(6):H3685–91. doi: 10.1152/ajpheart.00819.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, et al. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003 Jul 1;171(1):338–45. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 24.Polosa R. Adenosine-receptor subtypes: their relevance to adenosine-mediated responses in asthma and chronic obstructive pulmonary disease. Eur Respir J. 2002 Aug;20(2):488–96. doi: 10.1183/09031936.02.01132002. [DOI] [PubMed] [Google Scholar]
- 25.van der Hoeven D, Wan TC, Auchampach JA. Activation of the A(3) adenosine receptor suppresses superoxide production and chemotaxis of mouse bone marrow neutrophils. Mol Pharmacol. 2008 Sep;74(3):685–96. doi: 10.1124/mol.108.048066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cross HR, Murphy E, Black RG, Auchampach J, Steenbergen C. Overexpression of A(3) adenosine receptors decreases heart rate, preserves energetics, and protects ischemic hearts. Am J Physiol Heart Circ Physiol. 2002 Oct;283(4):H1562–8. doi: 10.1152/ajpheart.00335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tracey WR, Magee WP, Oleynek JJ, Hill RJ, Smith AH, Flynn DM, et al. Novel N6-substituted adenosine 5′-N-methyluronamides with high selectivity for human adenosine A3 receptors reduce ischemic myocardial injury. Am J Physiol Heart Circ Physiol. 2003 Dec;285(6):H2780–7. doi: 10.1152/ajpheart.00411.2003. [DOI] [PubMed] [Google Scholar]
- 28.Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997 Dec;49(4):381–402. [PubMed] [Google Scholar]
- 29.Cagnina RE, Ramos SI, Marshall MA, Wang G, Frazier CR, Linden J. Adenosine A2B receptors are highly expressed on murine type II alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009 Jul 2;297:L467–L74. doi: 10.1152/ajplung.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest. 1995 Oct;96(4):1979–86. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]