Abstract
The transcriptional activity of the vitamin D receptor (VDR) is regulated by a number of coactivator and corepressor complexes, which bind to the VDR in a ligand (1,25(OH)2D3) dependent (coactivators) or inhibited (corepressors) process. In the keratinocyte the major coactivator complexes include the vitamin D interacting protein (DRIP) complex and the steroid receptor coactivator (SRC) complexes. These coactivator complexes are not interchangeable in their regulation of keratinocyte proliferation and differentiation. We found that the DRIP complex is the main complex binding to VDR in the proliferating keratinocyte, whereas SRC2 and 3 and their associated proteins are the major coactivators binding to VDR in the differentiated keratinocyte. Moreover, we have found a specific role for DRIP205 in the regulation of β-catenin pathways regulating keratinocyte proliferation, whereas SRC3 uniquely regulates the ability of 1,25(OH)2D3 to induce more differentiated functions such as lipid synthesis and processing required for permeability barrier formation and the innate immune response triggered by disruption of the barrier. These findings provide a basis by which we can understand how one receptor (VDR) and one ligand (1,25(OH)2D3) can regulate a large number of genes in a sequential and differentiation specific fashion.
Keywords: Vitamin D receptor, coactivators, keratinocytes, differentiation, proliferation, β-catenin, innate immunity, permeability barrier
1.1 INTRODUCTION
The epidermis is composed of four layers of keratinocytes at different stages of differentiation (Fig. 1). The basal layer (stratum basale, SB) rests on the basal lamina separating the dermis and epidermis. Within this layer are the stem cells. These cells proliferate, providing the cells for the upper differentiating layers. They contain an extensive keratin network comprised of keratins K5 and K14. The layer above the basal cells is the spinous layer (stratum spinosum, SS). These cells initiate the production of the keratins K1 and K10, which are the keratins characteristic of the more differentiated layers of the epidermis. Cornified envelope precursors such as involucrin also appear in the spinous layer as does the enzyme transglutaminase, responsible for the ε-(γ-glutamyl) lysine cross-linking of these substrates into the insoluble cornified envelope. The granular layer (stratum granulosum,SG), lying above the spinous layer, is characterized by electron-dense keratohyalin granules containing profilaggrin, the precursor of filaggrin, a protein thought to facilitate the aggregation of keratin filaments, and loricrin, a major component of the cornified envelope. The granular layer also contains lamellar bodies, lipid-filled structures that fuse with the plasma membrane, divesting their contents into the extracellular space between the SG and stratum corneum (SC) where the lipid contributes to the permeability barrier of skin. The outer layer of the epidermis provides both a barrier to water loss (permeability barrier) and a barrier to invasion by infectious organisms (innate immunity).
Figure 1. A model showing the differential expression of various markers and functions in the epidermis with differentiation.
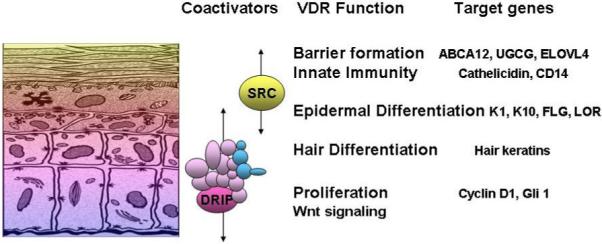
DRIP205 is most highly expressed in the stratum basale and spinosum where it participates with VDR in regulating proliferation. SRC3 on the other hand is found in highest concentration in the stratum granulosum where it participates with VDR in the regulation of terminal differentiation including formation of the permeability barrier and innate immune responsiveness.
The keratinocytes not only produce vitamin D3 from 7-dehydrocholesterol (7-DHC) but convert D3 to its active metabolite 1,25(OH)2D3. These cells also possess the receptor for 1,25(OH)2D3 (VDR), enabling them to respond to 1,25(OH)2D3 in an intracrine, autocrine or paracrine fashion [1]. 1,25(OH)2D3 increases involucrin, transglutaminase activity, loricrin, filaggrin and cornified envelope formation at subnanomolar concentrations [2-7], while inhibiting proliferation at least at concentrations above 1nM. In addition, 1,25(OH)2D3 induces the enzymes responsible for the processing the long chain glycosylceramides that are critical for permeability barrier formation [8] and the toll like receptors (TLR2), coreceptor CD14, and defensins critical for the innate immune response in skin [9] .
The process of epidermal differentiation is sequential. 1,25(OH)2D3 and VDR regulate all steps from the control of proliferation in the SB, to the regulation of K1, K10, involucrin, and transglutaminase production in the SS, to the regulation of loricrin and filaggrin production in the SG, to the synthesis of lipids required for the permeability barrier in the SC, and to the development of the innate immune system [1, 7, 9]. The major question being addressed in our current studies is how does this occur? In previous studies we noted the differential distribution of VDR coactivators DRIP 205 and SRC2,3 within the epidermis, and their differential binding to VDR depending on the state of differentiation of the keratinocyte [10-12]. In this study we show that in addition to differential distribution, these coactivator complexes are differentially selective for genes regulated by VDR. Our data support the hypothesis that SRC facilitates the ability of VDR to regulate the more differentiated functions of the keratinocyte including barrier formation and the innate immune response, whereas DRIP facilitates the ability of VDR to regulate proliferation.
1.2 MATERIALS AND METHODS
Keratinocyte cultures
Normal epidermal keratinocytes were isolated from neonatal human foreskin, and grown in 154CF medium (KGM, Cascade). The cells were cultured with KGM containing 0.03mM calcium to obtain proliferating keratinocytes; differentiation was induced with 1.2mM calcium, and in some experiments with ascorbic acid and serum to maximize lamellar body formation [13] .
VDR and coactivator knockdown and overexpression
Four individual siOligos (Dharmacon) were initially tested alone and in combination for VDR and the coactivators (except for SRC3 which used a previously published sequence), and the best combination was chosen for efficient knockdown of gene expression. For long term knockdown studies, the most efficient oligonucleotide was subcloned into an adenovirus [7]. Constitutive expression of VDR was achieved by cloning a translational fusion of VDR to green fluorescent protein (GFP) into pDNR-CMV (BD Biosciences) for subsequent cloning into the adenoviral host [7]. Primary second passage human keratinocytes were transfected at a rate of 75-250 ifu/cell and grown for 3 days in 0.03m Ca2+ KGM media. Depending on the experiment they were then transferred into media containing 1.2mM Ca2+ and/or treated with vehicle or 10−8M 1,25(OH)2D3.
Morphologic assessment
Routine phase microscopy was used to visualize cell morphology. Confocal microscopy was used to colocalize β-catenin and E-cadherin in cellular membranes. Ultrastructural examination was performed to look for changes in cell-cell adhesion, lamellar body (LB) secretion, and barrier disruption. LB density and its content, lipid secretion and its location, and lipid processing were assessed visually in randomly photographed micrographs.
Cell proliferation and apoptosis
Proliferation was assessed by BrdU incorporation using the Zymed BrdU staining kit according to the manufacturer’s instructions. The identification of apoptotic cells was determined using TACS™ 2 TdT-DAB In Situ Apoptosis Detection Kit from Trevigen.
mRNA and protein levels
The mRNA levels of various genes critical for keratinocyte proliferation and differentiation were measured by quantitative real time PCR using standard procedures as we have previously described [7]. The protein levels were analyzed by western blots using standard techniques available in our laboratory [14] .
Lipid analysis
Epidermal specific lipids were measured in the epidermis and cultured keratinocytes using high performance thin-layer chromatography as we have recently published [8] .
Innate immune response
Skin wounds were generated by a 5 mm full thickness incision under aseptic conditions. A small biopsy was subsequently obtained and processed by qRTPCR, western analysis and immunohistochemistry for cathelicidin, CD14, and toll like receptors using methods described above.
1.3 RESULTS
The role of VDR and its coactivators in regulating proliferation and apoptosis
The function of DRIP205 and SRC3 in regulating keratinocyte proliferation and apoptosis was examined using siRNA-silencing technology. Human epidermal keratinocytes were transfected with siRNA for VDR, DRIP205, or SRC3 to block their expression. Efficiency of knockdown was at least 75% (Fig 2A,B). Both DRIP205 and VDR silencing resulted in changes in keratinocyte morphology (from cuboidal to spindle-shaped cells), but knockdown of SRC3 did not (Fig. 2C, phase contrast). Keratinocyte proliferation was then assessed by BrdU incorporation demonstrating that knockdown of VDR and DRIP205 increased proliferation, whereas knockdown of SRC3 had the opposite effect (Fig. 2C,D). Apoptosis as assessed by TUNEL staining, on the other hand, was decreased by knockdown of VDR and DRIP, whereas SRC3 knockdown increased apoptosis (Fig 2E).
Figure 2. Impact of VDR, DRIP205, and SRC3 knockdown on keratinocyte morphology, proliferation and apoptosis.
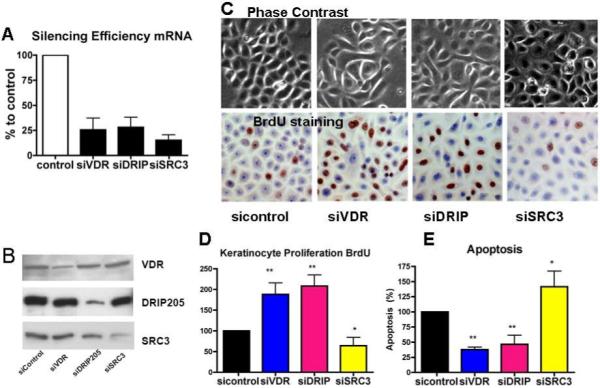
The keratinocytes were transfected with siRNAs to VDR, DRIP205, and SRC3, respectively. The efficiency and specificity of the knockdowns are shown at both the mRNA (A) and protein (B) level. Phase contrast was used to assess morphology (C). Proliferation was evaluated using BrdU (C,D), and apoptosis was determined by TUNEL staining (E). Statistical significance is shown by asterisks (*P<0.05 **P<0.01).
β-catenin, by inducing genes such as cyclin D1 and Gli1 (an important transcription factor in the hedgehog pathway), promotes the proliferation of keratinocytes. These genes are known to contain both LEF/TCF and VDR response elements [15]. Therefore, to explore the mechanism by which silencing of DRIP205 and VDR results in hyperproliferation, we investigated their roles in β-catenin signaling. Initial experiments demonstrated that 1,25(OH)2D3 and VDR overexpression inhibited the expression of β-catenin reporter constructs (cyclin D1 promoter, TOPGlow) (data not shown). Following these studies we examined whether DRIP205 played a role in the regulation of cyclin D1 and Gli1 in keratinocytes. These results are shown in Fig. 3. The data demonstrated that silencing of DRIP205 increased the expression of both cyclin D1 and Gli1, like that seen with the silencing of VDR. SRC3 knockdown did not show this response (data not shown). Although 1,25(OH)2D3 tended to suppress cyclin D1 and Gli1 expression in the cells in which VDR and DRIP205 had been silenced, presumably because the knockdown was incomplete, these effects of 1,25(OH)2D3 were no longer significant. Using confocal microscopy, we then observed that knockdown of VDR and DRIP205 but not SRC3 blocked calcium induced formation of the E-cadherin/β-catenin complex in the membrane, suggesting that by keeping β-catenin anchored to the membrane, less was available to stimulate proliferation through its nuclear interactions with LEF/TCF (data not shown). These results indicated that DRIP205 played a greater role than SRC3 in mediating VDR regulation of keratinocyte proliferation at least in part through regulation of β-catenin signaling.
Figure 3. Regulation by VDR and DRIP205 of cyclin D1 and Gli1 expression.
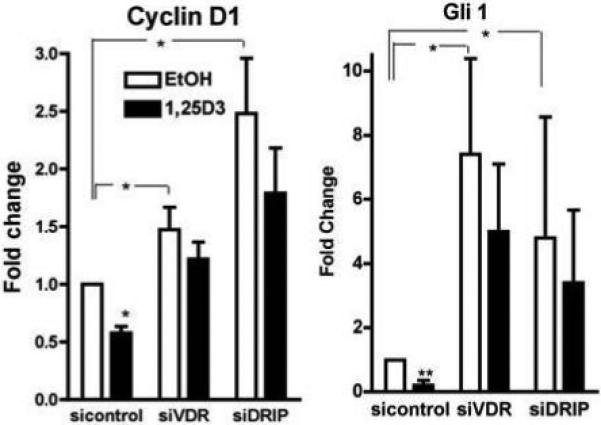
The keratinocytes were transfected with the specific and control siRNAs to VDR, DRIP205, and SRC3. The cells were treated with 10nM 1,25(OH)2D3 or vehicle as in Fig. 2. The mRNA levels of cyclin D1, Gli1, and control L19 were measured by real time PCR. The expression is shown as % of the sicontrol vehicle cultures. The error bars enclose mean +/− SD of triplicate cultures. Statistical significance is shown by asterisks (*P<0.05 **P<0.01).
Effect of VDR and coactivators on differentiation markers
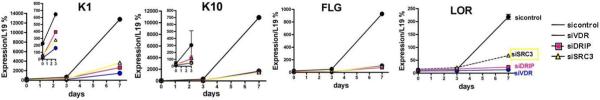
To evaluate the role of these coactivators in keratinocyte differentiation keratinocytes were maintained in high calcium for up to 7 days. Differentiation was evaluated by measuring mRNA levels of various differentiation markers. These results are found in Fig. 4. After 3 days of culture, levels of the early markers keratin 1 (K1) and keratin 10 (K10) increased. When DRIP205, SRC3 or VDR was silenced, the induction of K1 and K10 was significantly inhibited. Late differentiation markers, filaggrin (FLG) and loricrin (LOR) were induced after 7 days of culture. As for the early differentiation markers, silencing of DRIP205, SRC3 or VDR markedly inhibited their induction by calcium. These results indicated that both DRIP205 and SRC3 play critical roles in the induction of these markers of keratinocyte differentiation.
Figure 4. Impact of DRIP205, SRC3, and VDR silencing on calcium induced expression of keratinocyte differentiation markers.
The keratinocytes were transfected with siRNAs to VDR, DRIP205, and SRC3, respectively. The cells were then switched to 1.2mM calcium, and the mRNA levels of the indicated differentiation markers determined at 3 and 7 days by qRTPCR. Silencing of both DRIP205 and SRC3, as well as VDR inhibited the calcium induced expression of these markers. The error bars enclose mean +/−SD of triplicate cultures, and in most cases are within the symbol.
Effect of VDR on permeability barrier formation
One of the most differentiated functions of the keratinocyte is the formation of the permeability barrier. This involves the formation of the cornified envelope, a process in which proteins such as involucrin and loricrin are crosslinked into an insoluble matrix, and the production and secretion of long chain lipids, glucosylceramides in particular, into this matrix to water proof it. To examine whether DRIP and SRC complexes differ in their roles in the production and secretion of the lipids for barrier formation, a vitamin C and serum supplemented submerged culture system was employed, in which keratinocytes form barrier layers with lamellar bodies resembling those of the epidermis in vivo. The expression of DRIP205, SRC3, and VDR was blocked using adenovirus encoding shRNA for each coactivator (75 ifu/cell) or the control adenovirus. The blocking efficiency for VDR and the coactivators was VDR 70%, DRIP 90%, SRC3 80% as assessed by protein levels [7]. The infected keratinocytes were maintained in vitamin C supplemented serum for an additional 5-10 days to allow formation of the multilayered, epidermis-like tissue. The results are shown in Fig. 5.
Figure 5. VDR and SRC3 but not DRIP205 regulate production of epidermal specific GlcCer species, the formation of lamellar bodies, and the key enzymes and transporter involved.
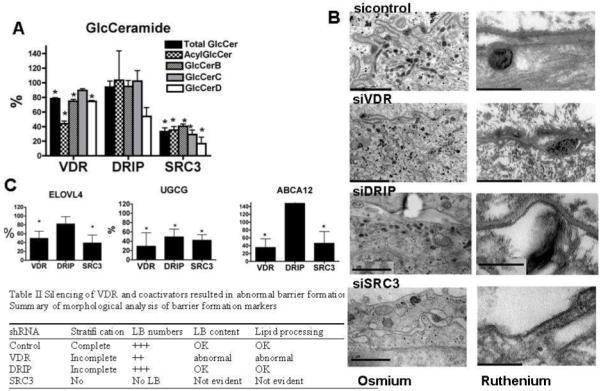
Human epidermal keratinocytes were infected with shRNA adenovirus for VDR and its coactivators to block their expression. Keratinocytes were maintained in medium supplemented with vitamin C and serum to induce LB production. A. The levels of key lipids were determined expressed as % of control. Data are represented as mean +/− SD of three measurements. (* p<0.03). B. The tissues were examined by electron microscopy to determine number of LBs and their content (left panels). The lipid secretion and processing were evaluated using ruthenium staining (right panels). Bars represent 1.0 μm (left panels) and 0.2 μm (right panels). The table summarizes the results. C. The transcription of lipid synthesis enzymes was evaluated by qRTPCR. The mRNA levels were normalized to control gene L19, and relative expression to control group was expressed as a percentage (%). Only the significantly affected genes are shown (ELOVL4, UGCG, and ABCA12). Data are represented as mean +/− SD of at least three independent experiments (* p<0.01).
We first assessed the relative levels of the immediate precursors of the barrier ceramides, i.e., the glucosylated ceramides (GlcCer) (Fig. 5A). When VDR was silenced, total GlcCer and epidermis specific GlcCer (acylGlcCer) levels decreased. Silencing SRC3, but not DRIP also decreased total GlcCer and epidermis specific acylCer species. We then used electron microscopy to examine these cells for their ability to carry out three key steps in barrier formation: i) stratification, ii) lamellar body (LB) formation, iii) lipid secretion processing. The results are shown in Fig. 5B. Stratification was assessed as the extent of formation of organelle depleted flattened corneocyte layers enclosed by a cornified envelope. LB formation was evaluated as the number, density (per μm2 cytosol) and lamellar contents of individual LBs in osmium tetroxide post-fixed samples. LB secretion and processing of secreted lipids into lamellar bilayers within the extracellular spaces were assessed by post-fixation with ruthenium tetroxide. Control tissues infected with control adenovirus showed significant stratification and cornification, as well as a reasonable density of LBs, some of which secreted their contents into the extra-cellular spaces (Fig. 5B, sicontrol). Most of these organelles exhibited internal lamellar contents. In addition, organelle secretion and post-secretory processing into mature extracellular lamellar membrane structures were readily observed. In contrast, when VDR is silenced, both incomplete stratification and abnormal lamellar bilayer formation were observed (Fig. 5B, siVDR). In these VDR silenced tissues, we also found decreased numbers of LBs, and individual LBs often lacked typical lamellar contents. In addition, very little LB secretion appeared to occur into the extracellular spaces in VDR silenced cells. SRC3 silenced cells showed more marked abnormalities, with no stratification, no LB formation, and no lamellar bilayers (Fig. 5B, siSRC3). When DRIP205 was blocked, stratification was incomplete, but a reasonable number of LBs, replete with internal lamellar content and relatively normal appearing lamellar bilayers, were observed (Fig. 5B, siDRIP). The results are summarized in the table contained in Fig. 5. To examine the mechanism by which VDR and SRC3 regulate glucosylceramide synthesis and barrier formation in cultured keratinocytes we measured changes in the expression of the key enzymes involved (Fig. 5C). We found that the mRNA level of the fatty acid elongase ELOVL4, essential for N-acyl fatty acid chain elongation and a key step in acylCer and acylGlcCer synthesis, was significantly decreased when either VDR or SRC3 was silenced but not when DRIP205 was silenced (Fig. 5C). Furthermore, the expression and enzyme activity of ceramide glucosyl transferase (UGCG) and the lipid transporter, ABCA12, responsible for transporting lipids into LB, were selectively reduced by silencing VDR and SRC3, but not DRIP205 (Fig. 5C). Together, these results indicate that VDR and SRC3 but not DRIP205 regulate specific enzymes in the acylCer/GlcCer synthetic pathway, including steps involved in the elongation of fatty acids (ELOVL4), Cer glucosylation (UGCG) and lipid loading into LB (ABCA12).
Role of 1,25(OH)2D3/VDR and its coactivators in the innate immune response of the epidermis
The barrier function of the epidermis includes its resistance to pathogenic organisms. Part of this defense involves the innate immune system. Innate immune responses involve the activation of toll-like receptors (TLRs) that interact with specific membrane patterns (PAMP) shed by infectious agents that trigger the innate immune response in the host. Activation of TLRs leads to the induction of antimicrobial peptides such as cathelicidin and reactive oxygen species, which kill the organism (Fig 6 model). In previous studies we (9, 12) have shown that 1,25(OH)2D3 is critical for this system to work. In these studies skin was wounded with a small incision. This induced the expression of TLR2 and CD14 (key receptors in the innate immune response), cathelicidin, and CYP27b1 (the enzyme producing 1,25(OH)2D3) in the skin (Fig. 6A). The induction of CD14 and cathelicidin by wounding the skin of mice lacking CYP27b1 did not occur (CD14) or was blunted (cathelicidin) (Fig. 6B). We then showed in human keratinocytes that 1,25(OH)2D3 induced TLR2, CD14, and cathelicidin (Fig. 6C,D), and finally, that this action of 1,25(OH)2D3/VDR was blocked in keratinocytes in which SRC3 was silenced, although not in keratinocytes lacking DRIP205 (Fig. 6D). These results suggest that like formation of the lipids required for the permeability barrier, the innate immune response requires SRC3, not DRIP205.
Figure 6. The innate immune response in the epidermis requires 1,25(OH)2D3 and the coactivator SRC3.
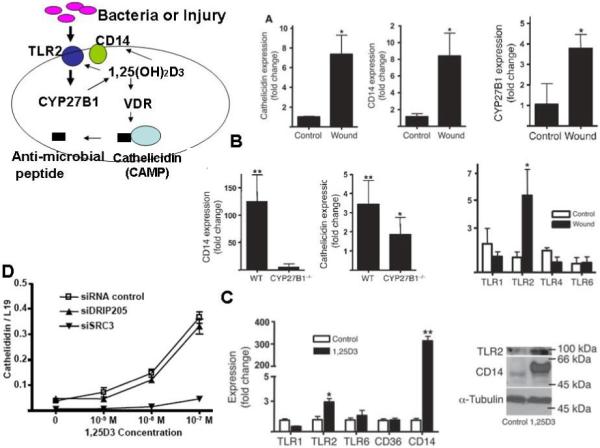
A. The expression of cathelicidin, CD14, CYP27b1, and TLR2 (but not other TLRs) in human skin were increased by wounding. B. This experiment was repeated in CYP27b1 null mice demonstrating that CYP27b1 was required for the induction of CD14 and cathelicidin. C. Cultured human keratinocytes were used to demonstrate the ability of 1,25(OH)2D3 (100nM) to stimulate TLR2, CD14, and cathelicidin. D. Silencing of SRC3 but not of DRIP205 blocked the ability of 1,25(OH)2D3 to induce cathelicidin expression in these cultured human keratinocytes. All data are expressed as mean +/− SD of triplicate experiments.
1.4 DISCUSSION
In this study we explored the different roles of the coactivators DRIP205 and SRC3 in modulating 1,25(OH)2D3/VDR transcriptional activity for a number of genes involved with proliferation and differentiation of keratinocytes. This approach was motivated by our initial findings that DRIP binding to VDR dominated extracts from proliferating keratinocytes, whereas SRC 3 binding to VDR dominated extracts from differentiated keratinocytes. We then proceeded to demonstrate that DRIP205 was mainly expressed in proliferating keratinocytes, whereas SRC3 was mainly expressed in differentiated keratinocytes both in vitro and in vivo. This differential distribution has functional consequences. Although some genes or keratinocyte functions such as CYP24 expression and a number of differentiation markers appeared to be regulated equally well whether DRIP205 or SRC3 was the coactivator, other genes/functions clearly showed preferences. For example, proliferation of keratinocytes and the regulation of genes associated with proliferation such as cyclin D1 and Gli1 clearly favored DRIP205, in that knockdown of SRC3 unlike that of DRIP205 failed to have a significant effect on proliferation. On the other hand, more differentiated functions such as formation of the permeability barrier and development of the innate immune response favored SRC3, in that knockdown of DRIP205 unlike that of SRC3 failed to alter these processes or the induction by 1,25(OH)2D3/VDR of the genes involved in these processes. These results support our hypothesis that DRIP205 and SRC3 differentially regulate 1,25(OH)2D3/VDR actions in keratinocytes such that the appropriate actions are accomplished in the appropriate cell—ie. proliferation is regulated in proliferating keratinocytes using DRIP205 as coactivator whereas terminal differentiation and maintenance of the protective barrier is regulated in differentiated keratinocytes using SRC3 as coactivator.
At this point these studies have involved keratinocytes in vitro. We have developed mice selectively null for DRIP205 or SRC3 in their keratinocytes. DRIP null mice show hyperproliferation of both hair follicles and epidermis similar to VDR null mice during first 3 weeks of life, but with time develop alopecia as the hair follicles subsequently atrophy again like VDR null mice. SRC3 null mice do not show such changes. On the other hand SRC3 null mice have both a defective permeability barrier due to changes in lipid processing in the epidermis, and fail to mount an innate immune response to wounding. Thus the in vitro experiments reported here have successfully predicted the changes observed in vivo.
1.5 ACKNOWLEDGEMENTS
The authors acknowledge the financial support of NIH PO1AR39448, RO1AR050023, VA Merit Review, and American Institute of Cancer Research 07A140 and the administrative support from Ms Teresa Tong and Victoria Lee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1.6 REFERENCES
- 1.Bikle DD, Pillai S. Vitamin D, calcium, and epidermal differentiation. Endocrine Review. 1993;14:3–19. doi: 10.1210/edrv-14-1-3. [DOI] [PubMed] [Google Scholar]
- 2.Pillai S, Bikle DD. Role of intracellular-free calcium in the cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol. 1991;146(1):94–100. doi: 10.1002/jcp.1041460113. [DOI] [PubMed] [Google Scholar]
- 3.Bikle DD, Pillai S, Gee E. Squamous carcinoma cell lines produce 1,25 dihydroxyvitamin D, but fail to respond to its prodifferentiating effect. J Invest Dermatol. 1991;97(3):435–441. doi: 10.1111/1523-1747.ep12481267. [DOI] [PubMed] [Google Scholar]
- 4.Hosomi J, Hosoi J, Abe E, et al. Regulation of terminal differentiation of cultured mouse epidermal cells by 1 alpha,25-dihydroxyvitamin D3. Endocrinology. 1983;113(6):1950–1957. doi: 10.1210/endo-113-6-1950. [DOI] [PubMed] [Google Scholar]
- 5.Smith EL, Walworth NC, Holick MF. Effect of 1 alpha,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986;86(6):709–714. doi: 10.1111/1523-1747.ep12276343. [DOI] [PubMed] [Google Scholar]
- 6.McLane JA, Katz M, Abdelkader N. Effect of 1,25-dihydroxyvitamin D3 on human keratinocytes grown under different culture conditions. In Vitro Cell Dev Biol. 1990;26(4):379–387. doi: 10.1007/BF02623829. [DOI] [PubMed] [Google Scholar]
- 7.Hawker NP, Pennypacker SD, Chang SM, et al. Regulation of Human Epidermal Keratinocyte Differentiation by the Vitamin D Receptor and its Coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol. 2007;127:874. doi: 10.1038/sj.jid.5700624. [DOI] [PubMed] [Google Scholar]
- 8.Oda Y, Uchida Y, Moradian S, et al. Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol. 2009;129(6):1367–1378. doi: 10.1038/jid.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oda Y, Sihlbom C, Chalkley RJ, et al. Two distinct coactivators, DRIP/mediator and SRC/p160, are differentially involved in vitamin D receptor transactivation during keratinocyte differentiation. Mol Endocrinol. 2003;17(11):2329–2339. doi: 10.1210/me.2003-0063. [DOI] [PubMed] [Google Scholar]
- 11.Oda Y, Ishikawa MH, Hawker NP, et al. Differential role of two VDR coactivators, DRIP205 and SRC-3, in keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol. 2007;103(3-5):776–780. doi: 10.1016/j.jsbmb.2006.12.069. [DOI] [PubMed] [Google Scholar]
- 12.Schauber J, Oda Y, Buchau AS, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128(4):816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 13.Uchida Y, Behne MJ, Quiec D, et al. Vitamin C Stimulates Sphingolipid Production and Markers of Barrier Formation in Submerged Human Keratinocyte Cultures. J Invest Dermatol. 2001;117:1307–1313. doi: 10.1046/j.0022-202x.2001.01555.x. [DOI] [PubMed] [Google Scholar]
- 14.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282(12):8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 15.Palmer HG, Anjos-Afonso F, Carmeliet G, et al. The Vitamin D Receptor Is a Wnt Effector that Controls Hair Follicle Differentiation and Specifies Tumor Type in Adult Epidermis. PLoS ONE. 2008;3(1):e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]