Abstract
Numerous synthetic vitamin D analogs have been studied for their effects on the prevention and treatment of breast cancer. However, the inhibitory effects of naturally occurring 1α,25-dihydroxyvitamin D3 or its synthetic analogs on ErbB2 overexpressing mammary tumorigenesis have not been reported. Gemini vitamin D analogs are novel synthetic vitamin D derivatives with a unique structure of two six-carbon chains at C-20. We have previously shown that Gemini vitamin D analogs significantly inhibited carcinogen-induced estrogen receptor (ER)-positive mammary tumorigenesis and reduced ER-negative MCF10DCIS.com xenograft tumor growth without hypercalcemic toxicity. In the present study, we have determined the inhibitory effect of a potent Gemini vitamin D analog BXL0124 (1α,25-dihydroxy-20R-21(3-hydroxy-3-deuteromethyl-4,4,4-trideuterobutyl)-23-yne-26,27-hexafluoro-cholecalciferol) on the ErbB2/Her-2/neu overexpressing mammary tumorigenesis. The Gemini BXL0124 inhibits ErbB2-positive mammary tumor growth and down-regulates the phosphorylation of ErbB2, ERK and AKT in tumors of MMTV-ErbB2/neu transgenic mice. These effects of Gemini BXL0124 in vivo were confirmed by using the ErbB2 overexpressing tumor cells derived from the mammary tumors of MMTV-ErbB2/neu mice. In conclusion, the Gemini vitamin D analog BXL0124 inhibits the growth of ErbB2 overexpressing mammary tumors through regulating the ErbB2/AKT/ERK signaling pathways, suggesting that Gemini vitamin D analog may be considered for translational studies.
Keywords: Gemini Vitamin D, ErbB2, neu, mammary tumorigenesis
1. INTRODUCTION
The hormonally active ligand for vitamin D receptor (VDR), 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), formed from vitamin D3 predominantly through hydroxylation by a 25-hydroxylase in the liver and a 1α-hydroxylase in the kidney, is known to play a major role in maintaining calcium/phosphate homeostasis [1, 2]. 1α-Hydroxylase in non-renal tissues, such as breast, colon and prostate, also produces the active form 1α,25(OH)2D3, which is important for the inhibition of cell proliferation by cell cycle arrest in the G0/G1 phase, induction of apoptosis and increased cell differentiation in cancer cells [3–6]. Despite the anti-cancer activities of 1α,25(OH)2D3, the use of 1α,25(OH)2D3 for the prevention and treatment of cancer is limited due to its hypercalcemic toxicity. Therefore, numerous synthetic vitamin D analogs have been developed for better efficacy with decreased calcemic toxicity [2].
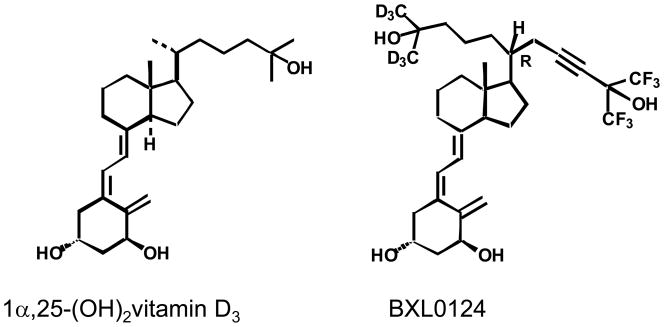
Gemini vitamin D analogs are novel synthetic vitamin D analogs that have a unique structure of two six-carbon chains at C-20 (see Fig. 1). In colon cancer models where MC-26 mouse colorectal cancer cells are implanted in BALB/c mice, certain Gemini vitamin D analogs were reported to exert greater activity than 1α,25(OH)2D3 in inhibiting tumor growth and in reducing the invasive spread of tumor cells into surrounding muscle without inducing hypercalcemic toxicity [7, 8]. We have previously reported that certain Gemini vitamin D analogs exert better growth inhibitory effect than 1α,25(OH)2D3 in MCF10 breast cancer cells [9]. In addition, we have demonstrated that Gemini vitamin D analogs inhibit tumor growth in ER-positive N-methyl-N-nitrosourea (NMU)-induced mammary tumorigenesis and in ER-negative MCF10DCIS.com xenograft breast cancer, where cyclin-dependent kinase inhibitor, p21, and insulin-like growth factor binding protein 3 (IGFBP3) were shown as common molecular targets of certain Gemini vitamin D analogs [10, 11]. These findings have led us to study Gemini vitamin D analogs on another major subtype, ErbB2 overexpressing breast cancer.
Figure 1.
Structures of 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) and a Gemini vitamin D analog BXL0124 [1α,25-dihydroxy-20R-21(3-hydroxy-3-deuteromethyl-4,4,4-trideuterobutyl)-23-yne-26,27-hexafluoro-cholecalciferol].
ErbB2, also known as Her-2/neu, is an important molecular target in breast cancer prevention and therapy because ErbB2 is known to be amplified and overexpressed in 20–30% of human breast primary tumors [12]. To evaluate the efficacy of drugs and investigate the mechanism of action, the MMTV-ErbB2 transgenic mouse model overexpressing wild-type neu protooncogene has been widely used for the last two decades [12, 13]. Thus far, the potential activities of 1α,25(OH)2D3 and classic synthetic vitamin D analogs on ErbB2-overexpressing models have not been reported. In the present study, we have tested Gemini vitamin D analog BXL0124 (Fig. 1) in the MMTV-ErbB2 transgenic mouse model to determine whether the Gemini vitamin D analog BXL0124 exhibits potential suppressive effects on ErbB2 overexpressing mammary tumor growth. The detailed mechanisms of action whereby the Gemini vitamin D analog BXL0124 regulates the ErbB2-related downstream signaling pathway are investigated in vitro and in vivo.
2. MATERIALS AND METHODS
2.1. Reagents and cell culture
1α,25(OH)2D3 and Gemini vitamin D analog (BXL0124, >95% purity) (Fig. 1) were provided by BioXell, Inc. (Nutley, NJ) and dissolved in DMSO. For in vivo animal experiments, BXL0124 was diluted in cremophore/PBS (1:8, v/v). The ErbB2/Her-2/neu overexpressing cells (E18-9A-42) were derived from MMTV-ErbB2 mammary tumors [14, 15] and kindly provided by Dr. Powel Brown at Baylor College of Medicine, Houston, Texas. The cells were maintained in 10% fetal bovine serum(FBS)/DMEM medium supplemented with 1% penicillin/streptomycin at 37°C, 5% CO2.
2.2. Transgenic mice and treatment
MMTV-ErbB2/neu transgenic mice (6–7 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed and given AIN-93M diet (Research Diet Laboratory, New Brunswick, NJ). Starting at 4 months of age, mice received the treatment with either vehicle or the Gemini vitamin D analog BXL0124 in vehicle intraperitoneally three times a week. The mice weight and tumor size of each animal were measured weekly. Fifty-four weeks after BXL0124 treatment, the mice were sacrificed and tumors were weighed at autopsy. Tumors and the mammary gland were saved for further analysis and serum was collected after centrifugation of clotted blood samples. All animal studies were performed in accordance with an institutionally approved protocol.
2.3. Determination of serum calcium level
The detailed procedure for the determination of calcium concentration in serum samples (POINTE Scientific, INC. Canton, MI) was reported previously [10].
2.4. Western blot analysis
The procedure was described previously [16] and the primary antibodies, phospho-ErbB2, phospho-ERK, phospho-AKT, ERK, AKT, and PARP were from Cell Signaling (Beverly, MA), ErbB2 from NeoMarkers (Fremont, CA), Cyclin D1 from Santa Cruz Biotechnology (Santa Cruz, CA), VDR from Affinity BioReagents (Golden, CO), actin from Sigma (St. Louis, MO), and secondary antibodies were from Santa Cruz Biotechnology.
2.5. Histopathological analyses and immunohistochemistry
Mammary tumors from each group were harvested at autopsy and fixed in 10% formalin for 24 hr. They were paraffin embedded, and cut into 4 μm thick tissue sections. Individual tumors were evaluated histopathologically in hematoxylin and eosin (H&E)-stained tumor sections. For immunohistochemistry, the procedure was described previously [16], and the antibody against phospho-ErbB2, ErbB2, phospho-ERK, and phospho-AKT were from Cell Signaling (Beverly, MA).
2.6. Measurement of cell proliferation by [3H]thymidine incorporation
E18-9A-42 cells derived from MMTV-ErbB2/neu mammary tumors were treated with compounds in 10% FBS/DMEM medium for 3 days. [3H]thymidine incorporation was determined as described previously [16].
2.7. Fluorescence microscopy
E18-9A-42 cells were incubated in a collagen coated glass-bottom dish (MatTek, Ashland, MA), and treated with BXL0124 for 24 hr. Then, cells were fixed in 4% paraformaldehyde (pH 7.4) for 20 min, and blocked for 1 h with 10% BSA/0.5% Triton-X/1x PBS solution. The primary antibody, phospho-AKT (Cell Signaling Technology) and fluorophore-conjugated secondary antibody (Invitrogen, Carlsbad, CA) were probed in 10% BSA/PBS solution. The cells were irradiated with a green laser (488 nm), and UV light (364 nm) was used for DAPI staining.
2.8. Statistical analysis
Statistical significance was evaluated using the Student’s t-test.
3. RESULTS
3.1. The Gemini vitamin D analog BXL0124 inhibits the growth of mammary tumors of MMTV-ErbB2/neu transgenic mice
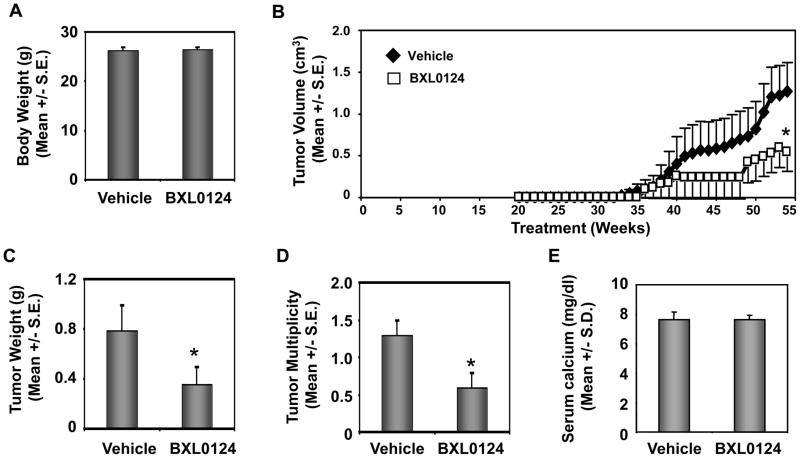
We investigated the effect of Gemini vitamin D analog BXL0124 on MMTV-ErbB2/neu transgenic mice. Because of the close association between overexpression of ErbB2 and human breast cancer, the MMTV-ErbB2 transgenic mouse model is one of the most widely used hormone-independent animal models in breast carcinogenesis [17]. The treatment with BXL0124 at the dose of 0.3 μg/kg BW had no effect on body weight (Fig. 2A). As shown in Fig. 2B, the tumor volume (cm3) in the BXL0124 treatment group was 0.54 ± 0.23, compared to 1.27 ± 0.35 in the control group, which corresponds to 57% inhibition of mammary tumor growth. The average tumor burden and tumor multiplicity at autopsy were also significantly reduced by BXL0124 treatment. The tumor weight in the BXL0124 treatment group was 0.36 ± 0.14 gram, compared to 0.79 ± 0.20 gram in the control group, which corresponds to 54% inhibition (Fig. 2C). The average tumor multiplicity was lower in BXL0124 treated group (1.3 ± 0.19), compared to the control group (0.60 ± 0.18), which was 54% reduction (Fig. 2D). The treatment with BXL0124 at the tested dose did not induce hypercalcemic toxicity (Fig. 2E).
Figure 2. Effects of the Gemini vitamin D analog BXL0124 on mammary tumorigenesis in MMTV-ErbB2/neu transgenic mice.
The MMTV-ErbB2/neu transgenic mice were treated with vehicle or BXL0124 (0.3 μg/kg body weight) intraperitoneally three times a week starting at the 4 months of age until the age of 54 weeks. The number of mice in the control and BXL0124-treated groups were 16 and 8, respectively. The tumor volume was measured weekly up to 54 weeks. A. The average body weight. B. The average tumor volume (cm3). C. The average tumor weight (gram). D. The average tumor multiplicity. E. Serum calcium level (Statistical significance, *p<0.05).
3.2. The Gemini vitamin D analog BXL0124 down-regulates the phosphorylation of ErbB2, ERK, and AKT in mammary tissues from MMTV-ErbB2/neu transgenic mice
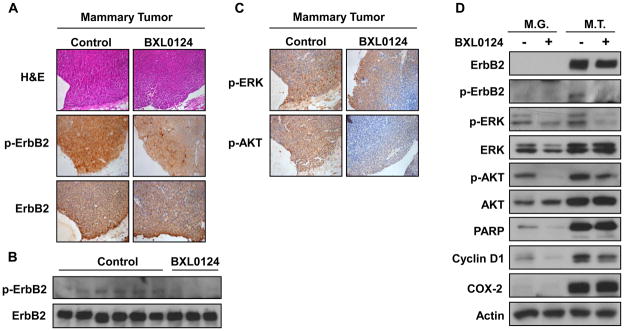
Based on histological evaluation using H&E staining in mammary tumors, we found that mammary tumors in the control and BXL0124-treated groups were adenocarcinomas and the tumor grades between groups were not different (Fig. 3A). Because the ErbB2 signaling is the main pathway driving the mammary tumorigenesis in this animal model, we determined the effect of BXL0124 on ErbB2-downstream signaling molecules. Using immunohistochemistry and Western blot analysis, we found that BXL0124 down-regulated the phosphorylation of ErbB2 without affecting the expression level of total ErbB2 protein in mammary tumors (Figs. 3A and 3B). The phosphorylation of ERK and AKT levels was also inhibited by BXL0124 treatment in mammary tumors (Fig. 3C). As shown in Fig. 3D, the expression of ErbB2 and its phospho-form were not detectable in mammary glands, while the phosphorylation of ERK and AKT were significantly down-regulated by BXL0124. In mammary tumors, BXL0124 decreased the levels of phospho-ErbB2, phospho-ERK and phospho-AKT without affecting the expression of the total proteins (Fig. 3D). Inducible cyclooxygenase (COX-2) was not detectable in mammary glands but significantly increased in mammary tumors, which was not changed by the treatment with BXL0124. BXL0124 reduced the expression of the cell proliferation marker, cyclin D1, in both mammary glands and mammary tumors (Fig. 3D), but the levels of cyclin-dependent kinases, p21 and p27, were not changed (data not shown).
Figure 3. Effect of the Gemini vitamin D analog BXL0124 on protein levels in mammary glands and mammary tumors from MMTV-ErbB2 transgenic mice.
Mammary tumor tissues were H&E stained or immunostained, and the proteins for Western blot analysis were extracted from mammary glands and mammary tumors. A. H&E staining or immunostaining with ErbB2 and phospho-ErbB2 (p-ErbB2) of mammary tumors, a representative out of tumors from three different mice. B. Western blot analysis against phospho-ErbB2 and total ErbB2 protein in mammary tumors. C. Immunostaining of ErbB2 downstream signaling molecules, phospho-ERK and phospho-AKT, in mammary tumors. D. ErbB2 related markers (ErbB2, phospho-ErbB2, ERK, phospho-ERK, AKT, phospho-AKT), cell cycle marker (cyclin D1), apoptosis marker (PARP) and COX-2 in mammary glands and mammary tumors were determined by Western blot analyses. Six different tissue samples in the control group and three different samples in BXL0124 treated group were pooled for analysis of the mammary glands or mammary tumors.
3.3. The Gemini vitamin D analog BXL0124 regulates the ErbB2-related signaling molecules and inhibits cell proliferation in mammary tumor cells derived from MMTV-ErbB2 transgenic mice
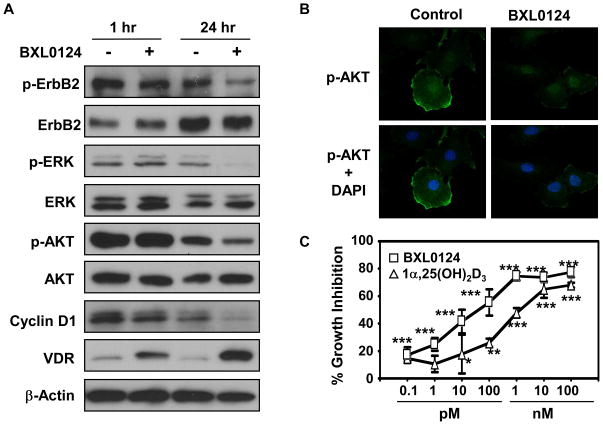
Several mammary tumor cells from MMTV-ErbB2 transgenic mice were isolated and cultured in 10% FBS/DMEM medium. One of the mammary tumor cell lines, E18-9A-42, was used for detailed analysis in cell culture. Using the E18-9A-42 cells, we determined whether BXL0124 regulates the ErbB2/AKT/ERK molecular signaling pathway in vitro. The treatment with BXL0124 decreased the levels of phospho-ErbB2, phospho-ERK, and phospho-AKT without affecting the expression level of total proteins in E18-9A-42 cells, and further down-regulated cyclin D1 expression at 24 h, although there was no change of these markers at 1 h (Fig. 4A). Using confocal microscopy, we demonstrated that the level of phospho-AKT localized in the plasma membrane was reduced by BXL0124 treatment (Fig. 4B), indicating the inhibition of AKT signaling by BXL0124 in these cells. BXL0124 suppressed the proliferation of E18-9A-42 cells in a dose-dependent manner with IC50 of 0.04 nM, compared to the naturally occurring 1α,25(OH)2D3 with IC50 of 1.5 nM (Fig. 4C). Taken together, these results suggest that BXL0124 is more active than 1α,25(OH)2D3 to inhibit cell proliferation of E18-9A-42 ErbB2 positive mammary tumor cells, which is via the regulation of the ErbB2/AKT/ERK signaling pathway.
Figure 4. Effect of the Gemini vitamin D analog BXL0124 on ErbB2-related signaling and cell proliferation in E18-9A-42 mammary tumor cells derived from MMTV-ErbB2/neu transgenic mice.
A. E18-9A-42 cells were treated with BXL0124 (10 nM) for 1 h and 24 h. The level of ErbB2 related markers (ErbB2, phospho-ErbB2, ERK, phospho-ERK, AKT, phospho-AKT), cell cycle marker (cyclin D1), and VDR were determined using Western blot analysis. B. E18-9A-42 cells were treated with BXL0124 (10 nM) for 24 h, and the localization and expression level of phospho-AKT was shown by confocal microscopy. C. E18-9A-42 cells were treated with 1α,25(OH)2D3 or BXL0124 for 3 days, and the activity in inhibiting cell proliferation was determined by [3H]-thymidine uptake assay. The IC50 values of 1α,25(OH)2D3 and BXL0124 were 1.5 nM and 0.04 nM, respectively. Statistical significance, *p<0.05, **p<0.01, ***p<0.001.
4. DISCUSSION
In human breast and colon cancer, Sjoblom et al describes 189 genes mutation at significant frequency [18], and breast cancer is considered to be a highly heterogeneous disease resulting from genetic changes, histopathological variation, and clinical outcomes [17]. These genetic and histological complexities of breast cancer indicate that multifunctional drugs need to be developed and used for the prevention and treatment of cancer [19]. Among different animal models, the MMTV-ErbB2 transgenic mouse model has been used to demonstrate the efficacy of cancer preventive agents in ER-negative/Her-2-positive subtype of breast cancer [20]. However, the efficacy of the ligands for VDR has not been reported in this model, which may indicate that the natural 1α,25(OH)2D3 and classic synthetic ligands have limited activity in ErbB2-positive breast cancer.
Our previous study demonstrated that Gemini vitamin D analogs prevented carcinogen-induced ER-positive mammary tumorigenesis and inhibited ER-negative MCF10DCIS.com xenograft tumor growth [10]. Here, we found that the Gemini vitamin D analog BXL0124 significantly inhibited mammary tumorigenesis in the MMTV-ErbB2 transgenic mouse model (Fig. 2). These studies support that Gemini vitamin D analogs have multi-functional activities in inhibiting different types of breast carcinogenesis without hypercalcemic toxicity. In the mechanistic study, BXL0124 reduced ErbB2-regulated downstream signaling, determined by down-regulation of the phosphorylation of ErbB2, AKT and ERK, without affecting the expression of total ErbB2 protein, and inhibited expression of cyclin D1 as a downstream molecular target of cell proliferation (Figs. 3 and 4). These results indicate that BXL0124 inhibits cell proliferation and tumor growth through regulating the ErbB2/AKT/ERK signaling pathway in ErbB2 overexpressing mammary tumors.
ErbB2 is a member of ErbB receptor tyrosine kinase family including epidermal growth factor receptor (EGFR, also known Her1), ErbB2 (Her2), ErbB3 (Her3) and ErbB4 (Her4) [20]. The ligand binding to the receptors induces homo- or hetero-dimerization, activates the kinase domain, and activates down-stream signaling such as the Ras/MAPK and PI3K/AKT signaling pathways [20]. Interestingly, in ErbB2 overexpressed mammary tumors, ErbB2 needs to hetero-dimerize with ErbB3 containing six docking sites for the p85 subunit of PI3K [21], and the ErbB2-ErbB3 heterodimer is the most effective ErbB dimer, which plays crucial roles in ErbB2-driven breast carcinogenesis [22]. In addition, Sergina et al reported that the limited activity of tyrosine kinase inhibitors in ErbB2-amplified breast cancer was due to the compensatory equilibrium of ErbB3 by AKT-mediated negative feedback regulation [23]. In our study, Gemini vitamin D analog BXL0124 down-regulated AKT phosphorylation as well as ErbB2/ERK activation in both ErbB2-amplified mammary tumors in vitro and in vivo (Figs. 3 and 4). This finding suggests that BXL0124 may be involved in regulating the heterodimerization of ErbB2 and ErbB3, which eventually results in the inhibition of both downstream signaling mediators, ERK and AKT.
In conclusion, the Gemini vitamin D analog BXL0124 inhibits ER-negative/ErbB2 positive mammary tumorigenesis without hypercalcemic toxicity via regulating ErbB2/ErbB3-driven downstream signaling, ERK, AKT and cell cycle regulator, cyclin D1. Taken together with our previous study [10], Gemini vitamin D analogs, especially BXL0124, may be potent agents in the prevention of different types of human breast cancer without toxicity, and should be considered for translational studies.
Acknowledgments
Grant Information: This work was supported in part by NIH R03 CA112642, NIH R01 CA127645 and the Trustees Research Fellowship Program at Rutgers, The State University of New Jersey.
The authors thank Maria Hyra and Lamberto R. Navoa of the Animal Facility in the Department of Chemical Biology for their technical assistance in taking care of the animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 2.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 3.Hussain-Hakimjee EA, Peng X, Mehta RR, Mehta RG. Growth inhibition of carcinogen-transformed MCF-12F breast epithelial cells and hormone-sensitive BT-474 breast cancer cells by 1alpha-hydroxyvitamin D5. Carcinogenesis. 2006;27(3):551–559. doi: 10.1093/carcin/bgi231. [DOI] [PubMed] [Google Scholar]
- 4.Chung I, Wong MK, Flynn G, Yu WD, Johnson CS, Trump DL. Differential antiproliferative effects of calcitriol on tumor-derived and matrigel-derived endothelial cells. Cancer Res. 2006;66(17):8565–8573. doi: 10.1158/0008-5472.CAN-06-0905. [DOI] [PubMed] [Google Scholar]
- 5.Narvaez CJ, Byrne BM, Romu S, Valrance M, Welsh J. Induction of apoptosis by 1,25-dihydroxyvitamin D3 in MCF-7 Vitamin D3-resistant variant can be sensitized by TPA. J Steroid Biochem Mol Biol. 2003;84(2–3):199–209. doi: 10.1016/s0960-0760(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 6.Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102(1–5):156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Spina C, Tangpricha V, Yao M, Zhou W, Wolfe MM, Maehr H, Uskokovic M, Adorini L, Holick MF. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J Steroid Biochem Mol Biol. 2005;97(1–2):111–120. doi: 10.1016/j.jsbmb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Spina CS, Ton L, Yao M, Maehr H, Wolfe MM, Uskokovic M, Adorini L, Holick MF. Selective vitamin D receptor modulators and their effects on colorectal tumor growth. J Steroid Biochem Mol Biol. 2007;103(3–5):757–762. doi: 10.1016/j.jsbmb.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 9.Lee HJ, Liu H, Goodman C, Ji Y, Maehr H, Uskokovic M, Notterman D, Reiss M, Suh N. Gene expression profiling changes induced by a novel Gemini Vitamin D derivative during the progression of breast cancer. Biochem Pharmacol. 2006;72(3):332–343. doi: 10.1016/j.bcp.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 10.Lee HJ, Paul S, Atalla N, Thomas PE, Lin X, Yang I, Buckley B, Lu G, Zheng X, Lou YR, Conney AH, Maehr H, Adorini L, Uskokovic M, Suh N. Gemini vitamin D analogues inhibit estrogen receptor-positive and estrogen receptor-negative mammary tumorigenesis without hypercalcemic toxicity. Can Prev Res. 2008;1(6):476–484. doi: 10.1158/1940-6207.CAPR-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maehr H, Uskokovic M, Adorini L, Penna G, Mariani R, Panina P, Passini N, Bono E, Perego S, Biffi M, Holick M, Spina C, Suh N. Calcitriol derivatives with two different side chains at C-20 III. An epimeric pair of the gemini family with unprecedented antiproliferative effects on tumor cells and renin mRNA expression inhibition. J Steroid Biochem Mol Biol. 2007;103(3–5):277–281. doi: 10.1016/j.jsbmb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Ursini-Siegel J, Schade B, Cardiff RD, Muller WJ. Insights from transgenic mouse models of ERBB2-induced breast cancer. Nat Rev Cancer. 2007;7(5):389–397. doi: 10.1038/nrc2127. [DOI] [PubMed] [Google Scholar]
- 13.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Nat Acad Sci. 1992;89(22):10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Zhang Y, Hill J, Shen Q, Kim HT, Xu X, Hilsenbeck SG, Bissonnette RP, Lamph WW, Brown PH. The Rexinoid LG100268 prevents the development of preinvasive and invasive estrogen receptor negative tumors in MMTV-erbB2 mice. Clin Cancer Res. 2007;13(20):6224–6231. doi: 10.1158/1078-0432.CCR-06-2681. [DOI] [PubMed] [Google Scholar]
- 15.Shen Q, Uray IP, Li Y, Zhang Y, Hill J, Xu XC, Young MR, Gunther EJ, Hilsenbeck SG, Colburn NH, Chodosh LA, Brown PH. Targeting the activator protein 1 transcription factor for the prevention of estrogen receptor-negative mammary tumors. Can Prev Res. 2008;1(1):45–55. doi: 10.1158/1940-6207.CAPR-08-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HJ, Ju J, Paul S, So JY, DeCastro A, Smolarek A, Lee MJ, Yang CS, Newmark HL, Suh N. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-gamma. Clin Cancer Res. 2009;15(12):4242–4249. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7(9):659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- 18.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 19.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7(5):357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 20.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 21.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Nat Acad Sci. 2003;100(15):8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9(7):463–475. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 23.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445(7126):437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]