Abstract
Here we describe the detection and identification of a yellow chlorophyll catabolite (Cj-YCC) in fresh extracts of senescent leaves of Cercidiphyllum japonicum. In addition, we report its partial synthesis by oxidation of Cj-NCC-1, the major (colourless) “nonfluorescent” chlorophyll catabolite (NCC) found in degreened leaves of C. japonicum. The spectroscopic analysis and structural characterization indicated Cj-YCC to be a simple dehydrogenation product of Cj-NCC-1 (by formal removal of a hydrogen atom at the C(20)- and C(1)-positions). Indeed, NCCs are easily oxidized and were first called “rusty pigments”, as they had a tendency to turn brown upon storage on a dry silica gel plate. The yellow tetrapyrrole Cj-YCC may thus come about by oxidation of Cj-NCC-1 in the leaves. Its presence in the yellow leaves of a deciduous tree provides the first evidence for the contribution of a coloured chlorophyll catabolite to the fall colours.
Introduction
The appearance of the fall colours is commonly associated with chlorophyll breakdown,1 a phenomenon in higher plants that, for a long time, was considered enigmatic.2,3 In spite of intensive search for coloured chlorophyll catabolites in senescent higher plants, such compounds have remained elusive.3 Indeed, the first chlorophyll catabolites from higher plants, to be discovered and structurally characterized, were colourless (and nonfluorescent) linear tetrapyrroles, called “non-fluorescing” chlorophyll catabolites (NCCs, such as Cj-NCC-1 (1), see Fig. 1).4,5 In the time since, much of the basic biochemical processes involved in chlorophyll breakdown in higher plants has been uncovered.6–10 Indeed, the enzymatic degradation of chlorophylls in senescent higher plants is now known to rapidly progress to the colourless NCCs, which are commonly considered to be the “final” tetra-pyrrolic products of chlorophyll catabolism in higher plants, and which are found in degreened leaves6–10 and in ripened fruit.8,11
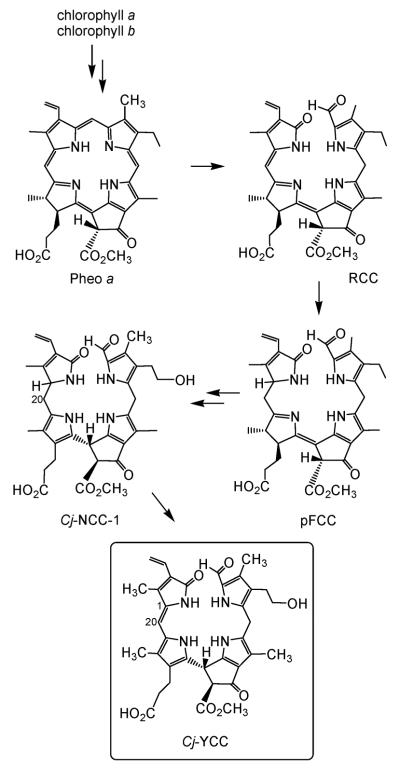
Fig. 1.
Outline of key steps in breakdown of chlorophylls in senescent leaves:7 structural formulae of pheophorbide a (Pheo a), of red chlorophyll catabolite (RCC), of primary fluorescent chlorophyll catabolite (pFCC) and constitutional formulae of Cj-NCC-1 (1),12,13 a ‘nonfluorescent’ chlorophyll catabolite (NCC) from senescent leaves of Cercidiphyllum japonicum, and of Cj-YCC (2).
Here, we report on the identification of a yellow chlorophyll catabolite (YCC) in freshly harvested senescent leaves of a Katsura tree (Cercidiphyllum japonicum), on the structural characterization of this natural Cj-YCC and on its partial synthesis from Cj-NCC-1 (1), the major NCC found in freshly senescent leaves of C. japonicum.12,13 Our findings, which were guided by the original attribute of the NCCs as being “rusty pigments” (which decomposed with appearance of brown colour),4,14 indicate chlorophyll breakdown in senescent plants to proceed to a yellow catabolite, likely to be formed from an NCC. This study provides the first evidence for an active contribution by a (yellow) chlorophyll catabolite to the appearance of the fall colours.
Results
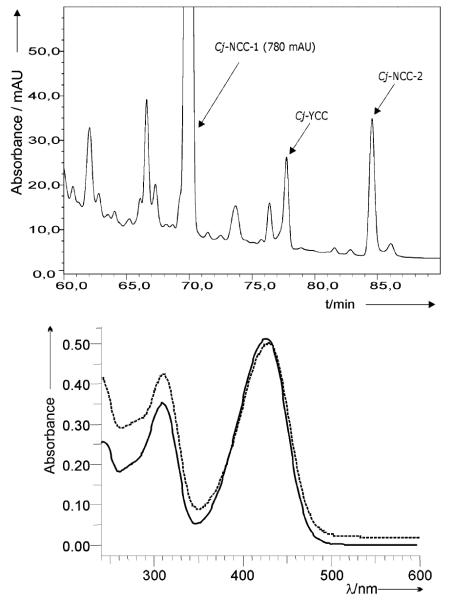
Yellow fresh leaves (500 g, fresh weight) of Cercidiphyllum japonicum were harvested from the tree in early senescence and were directly deposited into liquid nitrogen. Extracts of the frozen leaves were prepared and were analyzed directly by high performance liquid chromatography (HPLC) (see Fig. 2, top). HPLC analysis of the extract, and on-line UV/Vis-spectroscopy, revealed the extract to contain a yellow compound (see Fig. 2, bottom). By comparison (HPLC, UV/Vis) with authentic material, the yellow compound was identified as Cj-YCC (2). Authentic Cj-YCC (2) was prepared from Cj-NCC-1 (1) by chemical oxidation, as described further below.
Fig. 2.
Top: HPLC-analysis of a freshly prepared extract of senescent leaves of C. japonicum, with fractions of Cj-NCC-1 (1), Cj-YCC (2) and Cj-NCC-2 (3) highlighted (detection at 320 nm). Bottom: UV/Vis-spectrum of semi-synthetic Cj-YCC (2) in MeOH (c 15.9 = μM, full line) and of a sample of 2, isolated from freshly degreened leaves of C. japonicum (dashed line).
In parallel to the analytical experiment, the yellow tetrapyrrole Cj-YCC (2) was isolated from the fresh extract of 500 g of senescent leaves of C. japonicum, which was worked-up by chromatography on a silica gel column. A slightly orange solid sample (56.9 mg) of a raw mixture of Cj-NCC-2 (3) and Cj-YCC (2) was further purified by MPLC to give samples of 27 mg of pure Cj-YCC (2) and of 25 mg of Cj-NCC-2 (3). Both samples were identified by spectroscopic means with semi-synthetic 2 and with the earlier characterized 3.13 Similarly, the chromatographic fractions containing Cj-NCC-1 (1) were collected separately and worked-up by precipitation from dichloromethane solution by addition of hexane. The colourless catabolite Cj-NCC-1 (1) was obtained in a yield of 479 mg (see Experimental).
In an alternative (synthetic) procedure, Cj-YCC (2) was prepared selectively and in 48% yield from oxidation of Cj-NCC-1 (1) with dicyano-dichloro-quinone (DDQ) (see Fig. 3): a solution of 50 mg of 1 in deoxygenated acetone was treated at −70 °C with a small amount of acetic acid and about 1.1 molequivalents of DDQ. Work-up of the resulting red solution gave a dark solid residue. This was re-dissolved in methanol and treated with trifluoro-acetic acid at room temperature and for 4 min. The mixture was then neutralized with phosphate buffer pH 7 and the solid raw reaction mixture was purified by MPLC on a reversed phase column (RP-18). Two major fractions were collected, from which 5.2 mg (about 10.5%) of Cj-NCC-1 (1), and 24.1 mg (48%) of Cj-YCC (2) were obtained.
Fig. 3.
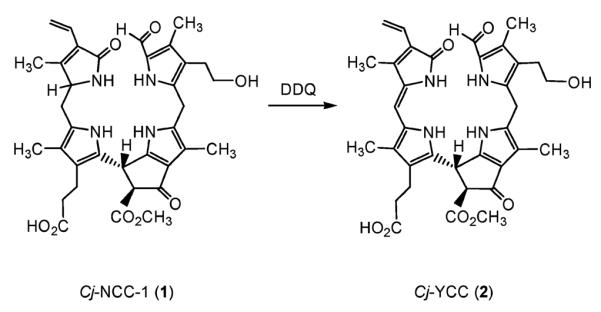
Structural outline of the oxidation of Cj-NCC-1 (1) by dicyano-dichloro-quinone (DDQ) to give Cj-YCC (2).
The yellow tetrapyrrolic catabolite Cj-YCC (2) exhibited a UV/Vis-spectrum in methanol with a characteristic maximum at 426 nm, followed by two maxima at 310 and 244 nm, with relative intensities of 1.00:0.69:0.50, respectively (see Fig. 2, bottom). As revealed by the structural analysis, delineated below, the chromophore relevant for the absorption band in the visible comprises the ‘western’ rings (the A/D-segment) and corresponds to that present (twice) in bilirubin.15 For structural analysis and spectroscopic characterization, mass spectrometry was used to determine the molecular formula of Cj-YCC (2), which was deduced (from the pseudo-molecular ion at m/z = 643.2) to be C35H38N4O8. This indicated the yellow tetrapyrrole 2 to have two H-atoms less per molecule than the colourless parent, Cj-NCC-1 (1). The constitution of the yellow catabolite Cj-YCC (2) was then deduced from 1H-NMR-spectra, as well as homo- and heteronuclear 2-D spectra (ROESY, 1H,13C-HSQC and 1H,13C-HMBC, see Fig. 4 and Experimental): In the 1H-NMR spectra of Cj-YCC (2, in CD3OD, at 25 °C) the signals of all 32 carbon bound hydrogen atoms could be observed. Among these, the singlet of CH(5)=O at low field stood out, the spin system for a peripheral vinyl group at an intermediate field, and five singlets, of four methyl groups at high field and of one near 3,7 ppm. From 1H,13C heteronuclear NMR correlations (HSQC- and g-HMBC-spectra16) of 2, complete assignment of the 1H and 13C signals could be achieved and the constitution of 2 could be assigned as that of an oxidation product of 1, resulting from removal of two hydrogen atoms from the C1- and C20-positions of 1. The structure of 2 was further characterized by the use of 1H-ROESY spectra. They helped to establish the Z-configuration of the new double bond between C20 and C1. On that basis and assuming, that the stereo-chemistry of 2 would correspond to that of Cj-NCC-1 (1) elsewhere, the yellow oxidation product 2 is thus assigned the structure of the (132S,15R,20Z)-31,32 -didehydro-4,5,10,15-(22,24H)-hexahydro-132-methoxy-carbonyl-4,5-seco-4,5-dioxo-phytoporphyrinate (see Fig. 3 and 4).
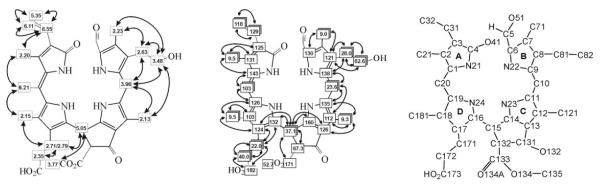
Fig. 4.
Left: Assignments for 1H-signals from 500 MHz 1H-NMR spectra of Cj-YCC (2) in CD3OD and correlations from H,H-COSY- and ROESY-spectra16 (solid lines and arrows). Centre: Assignment of 13C-signals from 1H,13C-heteronuclear correlations in HSQC-and HMBC-spectra.16 Right: Atom-numbering for 1 and 2, based on that proposed by IUPAC for the chlorophylls; however, simple 2- and 3-digit numbers are used here (e.g. 172) (in the text, an upper case number indicates the position on the side chain, e.g. 172, as suggested by IUPAC, see e.g. ref. 19).
Discussion
Our studies reveal the occurrence of a yellow chlorophyll catabolite (YCC) in senescent leaves of the higher plant C. japonicum. Cj-YCC (2) is indicated to result from further (oxidative) transformation of Cj-NCC-1 (1), the main NCC in the leaves.12,13 Thus, the tetrapyrrole 2 is suggested to be a (non-enzymatic) product of natural chlorophyll breakdown in the senescent leaves. Aside from such yellow tetrapyrroles, as analyzed here, other studies had revealed the existence of colourless “urobilinogenoidic” chlorophyll catabolites in senescent leaves of barley,17 indicated to arise from oxidative deformylation of the main NCC from degreened primary leaves of barley, named Hv-NCC-1.4
The molecular formula of the yellow tetrapyrrole Cj-YCC (2) clearly indicates it to be a formal oxidation product of Cj-NCC-1 (1) and 2 is obtained readily and effectively by an oxidation of 1 with the quinone DDQ. Indeed, the NCC Cj-NCC-1 (1) was recently shown to be oxidized easily and to be an effective antioxidant (with activity) similar to that of bilirubin.11 Antioxidants are suggested to be particularly important in plants, to prolong viability, not only of the leaves, but also of fruit. The abundant NCCs may thus have relevant physiological effects in senescent higher plants, rather than being mere detoxification products.11 Our data now provide evidence for the presence of YCC 2, a yellow linear tetrapyrrole, in freshly senescent leaves. This YCC is likely to be the result from further endogenous oxidation of the NCC 1. Our experiments indicate this to occur readily under oxidative conditions and involvement of an enzyme not to be required (it cannot be ruled out, at this stage). Such products of oxidative breakdown of the colourless NCCs, as YCC 2, may thus contribute actively (as yellow pigments) to the (appearance of the) fall colours. However, in freshly degreened leaves of C. japonicum Cj-YCC-2 (2) is indicated to be only a minor contributor to the yellow colour: preliminary experiments indicate about 5–10% of the yellow colour to be due to the YCC 2 (from HPLC analysis and integration of absorbance at wave lengths >400 nm of 2, of carotenoid fractions and of the major unidentified, polar fractions, that also absorb light at wave lengths >400 nm).
Very recently, bilirubin was reported to be a cytoprotective component, relevant in the reduction of coronary heart diseases, of retinal damage and of cancer mortality.18 Exploratory experiments along these lines, but with Cj-NCC-1 (1) have shown this colourless tetrapyrrolic breakdown product of chlorophyll to inhibit the proliferation of cancer cell lines. The structure of the characteristic chromophore, that extends over rings A and D of a YCC (such as Cj-YCC; for nomenclature, used here, see ref .19), is remarkably similar to that of bilirubin.15 This structural relationship of Cj-YCC (2) with bilirubin may also suggest closely similar and physiologically relevant chemical properties. Experiments are currently underway to test the antioxidant properties of Cj-YCC (2) and its photochemical and photophysical behaviour.
Conclusion
All in all, our studies add another piece of evidence that the various NCCs20 produced in senescing plants and ripening fruit may not be merely the “final products of detoxification” of chlorophyll (as is typically considered, for senescent leaves10). As seen here, NCCs are easily converted to YCCs by oxidation. Such catabolites from chlorophylls are linear tetrapyrroles15 that may possibly have relevant physiological effects in senescent higher plants and in higher animals. It will be of interest to study chemical reactivities of these products of endogenous breakdown of chlorophylls in senescent leaves and ripening fruit,8 and to explore their possible physiological effects.
Experimental
Materials
Solvents for extractions were regent-grade commercials and distilled before use. HPLC grade methanol was from Merck (Darmstadt, Germany) and Acros Organics (Geel, Belgium). Potassium dihydrogen phosphate puriss.p.a. and potassium phosphate dibasic-anhydrous puriss.p.a. were from Fluka (Buchs, Switzerland). Sep-Pak-C18 cartridges were obtained from Waters Associates. pH values were measured with a WTW Sentix 21 electrode connected to a WTW pH535 digital pH meter. Leaves of the Cercidiphyllum japonicum tree were collected in late September/October 2006 in the Botanical Garden of the University of Innsbruck and were stored at −80 °C in a deep freezer. Leaves for immediate analysis were picked likewise, frozen with liquid nitrogen and analyzed directly by HPLC.
HPLC
Gynkotek HPLC System with manual sampler, M480 pump (analytical), M300 pump (preparative), Phenomenex DG-301 online degasser, Gynkotek UVD 340 diode array detector and Jasco FP-920 fluorescence detector. Data were collected with Gynkosoft 5.50 and processed with Chromeleon V6.50. Analytical HPLC: Hypersil ODS 5 μm 250 × 4.6 mm i.d. column at r.t. protected with a Phenomenex ODS 4 mm × 3 mm i.d. pre-column, flow rate 0.5 ml min−1, 20 μl injection volume. Solvent A: 100 mM potassium phosphate (pH 7.0), solvent B: methanol.
UV/Vis: HITACHI U-3000 spectrophotometer; λmax in nm (ε) - 1H-NMR. Varian Unity plus 500 MHz; δ in ppm referenced to δ (C1HD2OD) = 3.31 ppm and δ (13CD3OD) = 49.0 ppm, coupling constants J in Hz, spectra were recorded at 26 °C. ESI-MS. Finigan MAT 95-S double-focussing sector field instrument with ESI source, pos. ions, flow rate 2 mlmin−1, spray voltage 3.0 kV, solvent water/methanol 1:1 (v/v).
Analysis of NCCs and YCCs in senescent leaves of Cercidiphyllum japonicum
Four yellow leaves of C. japonicum (collected in October 2006 in the Botanical Garden in Innsbruck and stored at −80 °C) were ground with liquid nitrogen in a mortar. 200 μl of methanol and 100 μl of potassium-phosphate buffer (100 mM, pH 7.0) were added to the pulverized yellow leaves in an Eppendorf reaction vessel; the vessel was shaken well and afterwards centrifuged for 5 minutes at 13000 rpm. The solution was pipetted into a viva-pure mini spin column and spun again for 5 minutes in the centrifuge. 20 μl of the obtained yellow solution were applied to analytical HPLC. Column: Thermo, ODS Hypersil, 250 × 4.5; particle size (μ) 5; flow rate: 500 μl min−1; eluent: t = 0–10 min: 80% buffer pH = 7 (100 mM KH2PO4/K2HPO4), 20% methanol; t = 10–70 min: linear gradient up to 40% buffer, 60% methanol; t = 70–94 min: 40% buffer, 60% methanol.
Isolation of Cj-NCCs and of Cj-YCC from fresh senescent leaves of Cercidiphyllum japonicum
500 g (fresh weight) yellow Cercidiphyllum japonicum leaves were blended with 600 ml of dichloromethane/methanol 95/5 (v/v) by a Braun hand blender Model MR 5000 in a 5 l stainless steel beaker. The solvent of the slurry was removed by squeezing it through a narrow pore net (the procedure was repeated seven times with 400 ml portions of dichloromethane/methanol 95/5 (v/v)). The combined extracts were filtered through a plug of dry cotton-wool. The combined filtrates (about 3.5 l) were applied to a silica column (190 mm diameter, 160 mm length, 430 g silica 60). The column was then washed with 500 ml dichloromethane. Liquid chromatography using 500 ml dichloromethane/methanol 98/2 (v/v), 500 ml dichloromethane/methanol 95/5 (v/v), 250 ml dichloromethane/methanol 90/10 (v/v), 1 l dichloromethane/methanol 80/20 (v/v), 500 ml dichloromethane/methanol 60/40 (v/v) and 500 ml methanol resulted in raw fractions of Cj-NCCs and Cj-YCC, which were combined and extracted with an equal amount of 0.1 M potassium phosphate buffer (pH 5.2). The organic phase was separated and the aqueous phase was extracted twice with 250 ml/250 ml of dichloromethane. The combined dichloromethane extracts were then filtered through a plug of dry cotton-wool, the solvent was evaporated, and the residue was dried in vacuo to give 1.897 g of raw product.
The raw product was dissolved by 10 ml methanol and diluted by 40 ml 0.1 M potassium phosphate buffer (pH 7.0). The deep orange suspension was centrifuged (4 min, 4500 × g) and filtered through a glass micro-fibre filter (1.6 μm pore size; 25 mm diameter). The filtrate was immediately applied to MPLC (50 ml injection, RP-18 column, i.d. 45 mm, length 480 mm, flow rate 8 ml min−1, gradient: 5 min at 0.1 M potassium phosphate buffer (pH 7.0)/methanol 80/20 (v/v), then within 20 min to 0.1 M potassium phosphate buffer (pH 7.0)/methanol 40/60 (v/v)). The collected NCC- and YCC-fractions were then desalted by using a Sep Pak cartridge and were eluted with methanol. The solvent was evaporated and dried in vacuo to give 56.9 mg of a mixed sample of Cj-YCC (2) and Cj-NCC-2 (3), and 478.8 mg Cj-NCC-1 (1).
The solid mixture of Cj-YCC and Cj-NCC-2 was re-dissolved by 10 ml methanol and diluted by 40 ml 0.1 M potassium phosphate buffer (pH 7.0). The deep yellow solution was separated by MPLC (50 ml injection, i.d. 45 mm, length 480 mm, flow rate 8 ml min−1, gradient: 5 min at 0.1 M potassium phosphate buffer (pH 7.0)/methanol 80/20 (v/v), then within 15 min to 0.1 M potassium phosphate buffer (pH 7.0)/methanol 50/50 (v/v)). The collected NCC- and YCC-fractions were then desalted by using a Sep Pak cartridge, the fractions were eluted with methanol. The solvent was evaporated and the residues were dried in vacuo to give 27.0 mg of Cj-YCC (2) and 24.9 mg Cj-NCC-2 (3).
Preparation of Cj-YCC (2) by oxidation of Cj-NCC-1 (1) with DDQ
50 mg (77.6 μmol) of Cj-NCC-1 (1) were dissolved in 5 ml of deoxygenated acetone and the solution was cooled to −70 °C, when 44 μl of acetic acid were added. A solution of 19.5 mg (85.9 μmol) DDQ in 2.5 ml of cold acetone was slowly added to the reaction mixture with a syringe. The resulting red reaction mixture was kept under argon and was allowed to warm up to about −50 °C over a time of 2 hours. The cold reaction mixture was treated with 192 mg solid sodium acetate and kept for an additional time of 30 min at a temperature of about −50 °C. The reaction mixture was then treated with 250 ml of 0.1 M potassium phosphate buffer pH 7 and filtered through a Sep Pak cartridge. The reaction products were eluted with 50 ml of methanol and dried. The raw product mixture was dissolved in methanol and treated with 2 ml of trifluoro-acetic acid at room temperature. After 4 min, the mixture was diluted with 250 ml of 0.1 M potassium phosphate buffer pH 7 and desalted by filtration through a Sep Pak cartridge. After elution with 50 ml methanol, the raw reaction product was dried (its weight was determined as 52.2 mg). The reaction product was purified by MPLC on a reversed phase column (RP-18). Aside of 5.2 mg of re-isolated 1, the main yellow fraction (of Cj-YCC, 2) was collected and dried. It contained 24.1 mg of 2 (48.2% yield), which was identified by HPLC, and its UV/Vis- and 1H-NMR-spectra.
Spectroscopic data of Cj-YCC (2)
UV/Vis (λmax, log ε, MeOH, c = 1.59 × 10−5 M): 426 (4.51), 310 (4.35), 244 (4.22) (see Fig. 2, bottom). 1H-NMR (500 MHz, in CD3OD): δ = 2.20 (s, H3C(21)), 2.13 (s, H3C(121)), 2.15 (s, H3C(181)), 2.23 (s, H3C(71)), 2.35 (m, H2C(172)), 2.63 (m, H2C(81)), 2.71 (m, HAC(171)), 2,79 (m, HBC(171)), 3.48 (m, H2C(82)), 3.77 (s, H3C(135)), 3.96 (s, H2C(10)), 5.05 (s, HC(15)), 5.35(dd, J = 2/11.5 Hz, HAC(32)), 6.11 (d broad, J = 17.5 HZ, HBC(32)), 6.55 (m, HC(31)). 13C-NMR (in CD3OD, signal assignment from 1H13C-HSQC and 1H13C-HMBC experiments): δ 9.01 (71), 9.28 (121), 9.49 (21), 9.52 (181), 22.0 (171), 23.6 (10), 28.0 (81), 37.1 (15), 40.0 (172), 52.7 (135), 62.6 (82), 67.3 (132), 102.6 (18), 102.6 (20), 112.2 (12), 118.1 (32), 120.7 (8), 124.1 (17), 125.0 (3),125.5 (19),125.8 (13), 129.3 (31), 129.6 (6), 131.4 (2), 131.6 (16), 134.9 (11), 138.4 (9), 143.1 (1), 159.9 (14), 171.1 (133), 182.0 (173). ESI-MS: m/z = 1323.43 (18, [2M + K + H]+), 1307.58 (20, [2M + Na + H]+), 683.20 (10), 682.20 (16), 681.21 (30, [M + K]+), 680.16 (9), 645.20 (17), 644.20 (39), 643.21 (100, [M + H]+), 642.17 (38), 641.18 (16), 613.17 (7), 612.18 (21), 611.19 (43), 610.20 (27), 609.17 (14), 520.11 (14, [M + H-Ring A]+), 490.12 (13, [M + H-Ring B]+), 458.09 (25), 357.07 (25), 325.15 (50).
Acknowledgements
We thank Nigel Whittle, Sonja Berger and Gerhard Scherzer for technical support and would like to acknowledge financial support by the Austrian National Science Foundation (FWF projects P-19596 and L-472).
Footnotes
This paper was published as part of the themed issue in honour of Nicholas Turro.
References
- 1.Bell CR, Lindsey AH. Fall Colors and Woodland Harvests. Laurel Hill Press; Chapel Hill, USA: 1990. [Google Scholar]
- 2.Brown SB, Houghton JD, Hendry GAF. In: Chlorophylls. Scheer H, editor. CRP-Press; Boca Raton, USA: 1991. pp. 465–489. [Google Scholar]
- 3.Matile P. Senescence in Plants and Its Importance for Nitrogen-Metabolism. Chimia. 1987;41:376–381. [Google Scholar]
- 4.Kräutler B, Jaun B, Bortlik K, Schellenberg M, Matile P. On the Enigma of Chlorophyll Degradation - the Constitution of a Secoporphinoid Catabolite. Angew. Chem., Int. Ed. 1991;30:1315–1318. [Google Scholar]
- 5.Matile P, Hörtensteiner S, Thomas H, Kräutler B. Chlorophyll breakdown in senescent leaves. Plant Physiol. 1996;112:1403–1409. doi: 10.1104/pp.112.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kräutler B, Matile P. Solving the riddle of chlorophyll breakdown. Acc. Chem. Res. 1999;32:35–43. [Google Scholar]
- 7.Kräutler B, Hörtensteiner S. In: Chlorophylls and Bacteriochlorophylls. Grimm B, Porra R, Rüdiger W, Scheer H, editors. Springer; Dordrecht, The Netherlands: 2006. pp. 237–260. [Google Scholar]
- 8.Kräutler B. Chlorophyll breakdown and chlorophyll catabolites in leaves and fruit. Photochem. Photobiol. Sci. 2008;7:1114–1120. doi: 10.1039/b802356p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matile P. In: Regulation of Photosynthesis. Aro E-M, Andersson B, editors. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. pp. 277–296. [Google Scholar]
- 10.Hörtensteiner S. Chlorophyll Degradation During Senescence. Annu. Rev. Plant Biol. 2006;57:55–77. doi: 10.1146/annurev.arplant.57.032905.105212. [DOI] [PubMed] [Google Scholar]
- 11.Müller T, Ulrich M, Ongania KH, Kräutler B. Colorless Tetrapyrrolic Chlorophyll Catabolites Found in Ripening Fruit Are Effective Antioxidants. Angew. Chem., Int. Ed. 2007;46:8699–8702. doi: 10.1002/anie.200703587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curty C, Engel N. Chlorophyll catabolism. 9. Detection, isolation and structure elucidation of a chlorophyll a catabolite from autumnal senescent leaves of Cercidiphyllum japonicum. Phytochemistry. 1996;42:1531–1536. [Google Scholar]
- 13.Oberhuber M, Berghold J, Breuker K, Hörtensteiner S, Kräutler B. Breakdown of chlorophyll: A nonenzymatic reaction accounts for the formation of the colorless “nonfluorescent” chlorophyll catabolites. Proc. Natl. Acad. Sci. USA. 2003;100:6910–6915. doi: 10.1073/pnas.1232207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bortlik K, Peisker C, Matile P. A Novel Type of Chlorophyll Catabolite in Senescent Barley Leaves. J. Plant Physiol. 1990;136:161–165. [Google Scholar]
- 15.Falk H. Chemistry of Linear Oligopyrroles and Bile Pigments. Springer Verlag; Wien: 1989. [Google Scholar]
- 16.Kessler H, Gehrke M, Griesinger C. Two-Dimensional NMR-Spectroscopy - Background and Overview of the Experiments. Angew. Chem. Int. Ed. 1988;27:490–536. [Google Scholar]
- 17.Losey FG, Engel N. Isolation and characterization of a urobilinogenoidic chlorophyll catabolite from Hordeum vulgare. J. Biol. Chem. 2001;276:8643–8647. doi: 10.1074/jbc.M009288200. [DOI] [PubMed] [Google Scholar]
- 18.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: A major physiologic cytoprotectant. Proc. Natl. Acad. Sci. USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheer H. In: Chlorophylls and Bacteriochlorophylls. Biochemistry, Biophysics, Functions and Applications. Grimm B, Porra RJ, Rüdiger W, Scheer H, editors. Springer; Dordrecht: 2006. pp. 1–26. [Google Scholar]
- 20.Kräutler B. In: Progress in the Chemistry of Organic Natural Products. Herz W, Falk H, Kirny GW, Moore RE, Tann C, editors. Springer Verlag; Wien: 2008. [Google Scholar]