Abstract
Total deficiency of complement factor H (CFH) is associated with dense deposit disease (DDD) and atypical haemolytic uraemic syndrome (aHUS). CFH is the major regulator of the alternative pathway of complement activation and its complete deficiency results in uncontrolled C3 activation through this pathway and secondary C3 deficiency. Plasma infusion, as a source of CFH, has been used with variable success to treat renal disease associated with CFH deficiency. However, the risks of volume and protein overload limit this therapeutic approach. In this study, we investigated the efficacy of a purified human CFH (hCFH) preparation in Cfh-gene knockout mice. These mice spontaneously develop both secondary plasma C3 deficiency and renal abnormality characterised by massive deposition of C3 along the glomerular basement membrane. The renal lesion is analogous to human dense deposit disease. Treatment of knockout mice with hCFH resulted in rapid normalization of plasma C3 levels and resolution of the glomerular basement membrane C3 deposition. Long-term assessment of mice with hCFH was not possible because of the development of an immune response against hCFH. Hence, we suggest that hCFH can be an effective alternative therapy to plasma infusions in patients with renal disease associated with CFH deficiency.
Introduction
Complete deficiency of complement factor H (CFH) is associated with dense deposit disease (DDD) and atypical haemolytic uraemic syndrome (aHUS). DDD is characterised by the presence of intramembranous electron-dense transformation of the glomerular basement membrane (GBM).1 The light microscopic features of DDD are heterogeneous but include membranoproliferative inflammation. DDD is associated with uncontrolled activation of the complement alternative pathway (AP).1 The key AP regulator is the plasma protein complement factor H (CFH). Complete genetic deficiency of CFH, results in uncontrolled AP activation and severe secondary C3 deficiency (reviewed in 2). Complete deficiency is also associated with DDD.3 DDD is also associated with other causes of AP dysregulation including autoantibodies that inhibit CFH function,4, 5 dysfunctional C3 molecules 6, 7 and autoantibodies that stabilise the AP C3 cleaving enzyme complex (C3 nephritic factors).8, 9 Animal models have reinforced the importance of uncontrolled AP activation in DDD. Gene-targeted CFH-deficient mice (Cfh−/−) spontaneously develop low plasma C3 levels and deposition of C3 along the murine GBM.10-12 This results in electron-dense GBM change and glomerular inflammation. A spontaneous mutation resulted in a breed of CFH-deficient pigs that develop both low plasma C3 levels and membranoproliferative glomerulonephritis (MPGN).13-17
Presently, there is no definitive therapy for DDD.1 The condition is associated with end-stage renal failure in a significant proportion of patients and has a very high recurrence rate in renal transplants.1, 18 Studies in Cfh−/− mice demonstrated that activation of the AP is essential for the renal disease to develop. Thus, Cfh−/− mice that are rendered deficient in the AP activation protein factor B, do not develop renal disease (reviewed in 2). Sequential histological studies in both the pig and mouse models demonstrated that the initial renal histological abnormality was deposition of C3 along the GBM.10, 16 These observations suggest that key properties of an effective therapy in DDD would include the ability to regulate AP activation and to facilitate removal of C3 along the GBM. An obvious definitive therapeutic in CFH deficiency is CFH itself. Previously, we administered purified mouse CFH (mCFH) to Cfh−/− animals.19 This resulted in removal of C3 along the GBM with concomitant increase in plasma C3 levels over a relatively rapid time frame of 24 hours. Here, we have examined the efficacy of human CFH (hCFH) in the Cfh−/− mouse model. Our data show that administration of hCFH to Cfh−/− mice resulted in rapid normalization of plasma C3 levels and resolution of GBM C3 deposits. These data suggest that hCFH will be an effective therapy in individuals with DDD associated with CFH dysfunction. Long-term assessment of hCFH in mice was not possible due to the development of an immune response against hCFH.
Materials and methods
Animals
Cfh−/− mice were generated previously.10 All experiment animals were age and sex-matched and were bred on the C57BL/6 genetic background for at least 10 generations. All experimental procedures were done in accordance with institutional guidelines.
Human complement factor H (hCFH)
hCFH was provided by LFB (Laboratoires Français de Fractionnement et des Biotechnologies), a French pharmaceutical company specializing in plasma-derived medicinal products. The human plasma-derived factor H concentrate was purified by multi-step chromatography. The purification process included both viral inactivation by solvent detergent treatment (a process that inactivates lipid-enveloped viruses) and viral segregation using a sequence of nanofilters that remove small viruses and prions.
Administration of hCFH to Cfh−/− mice
Animals were injected intraperitoneally with 0.5 mg of hCFH or an identical volume of phosphate-buffered saline (PBS). Mice were sacrificed at the indicated time points following injection and plasma and renal tissue were collected for analysis.
Detection of hCFH
Plasma hCFH was detected by western blot using a polyclonal goat anti-human CFH antibody (Quidel, San Diego, CA, USA). hCFH was quantified by ELISA using a monoclonal anti-hCFH capture antibody (Quidel) and a polyclonal goat anti-hCFH detection antibody (Quidel).
Measurement of mouse C3
Mouse C3 levels were measured by ELISA using goat anti-mouse C3 antibody (MP Biomedicals, OH, USA) as previously described.10 Results were quantified by reference to a standard curve generated from acute phase sera containing a known quantity of C3 (Calbiochem, CA, USA).
Measurement of mouse urea and albuminuria
Serum urea was measured by using a UV method kit (R-Biopharm Rhone) according to manufacturer's instructions. Albuminuria was measured by radial immunodiffusion as previously described.10
Detection of murine anti-hCFH antibodies
Murine anti-hCFH antibodies were detected by ELISA. Nunc MaxiSorp ELISA plates (Nunc, Roskilde, Denmark) were coated with 0.3 μg of hCFH diluted in 0.1 M NaHCO3 pH 9.5. After washing and blocking free reactive sites with 1% BSA in PBS, the plasma to be tested was added at a dilution of 1:200 and incubated for 1 hour at room temperature. After washing, the plates were incubated for 1 h at room temperature with a goat anti-murine IgG antibody labelled with horseradish peroxidase (Sigma-Aldrich, Steinheim, Germany). After additional washing, enzymatic activity was revealed using a orthophenyldiamine substrate. Antibody titers were expressed as arbitrary ELISA units (AEU) calculated as optical density of the test sample x200. We also detected murine anti-hCFH antibodies using a far western blot technique. Murine sera was separated using SDS-acrylamide gel (8%) under non-reducing conditions. Following transfer to protein membrane, the membrane was incubated with a solution containing hCFH at a concentration of 10μg/ml for 2 hours. Following washing the membrane was then incubated with goat anti-human CFH antibody (Quidel, CA, USA) and bound antibody detected by incubation with HRP-conjugated rabbit anti-goat IgG (Sigma, Saint Louis, USA)
Histological studies
Kidneys were fixed in Bouin's solution (Sigma, Gillingham, UK) and paraffin sections stained with periodic acid-Schiff reagent. Glomerular histological analysis was performed in a blinded manner as previously described.10 Fifty glomeruli were analysed per section. For immunostaining snap-frozen renal tissue sections were used. Mouse C3 was detected using either FITC-conjugated goat anti-mouse C3 (MP Biomedicals, CA, USA) or an unconjugated polyclonal goat anti-mouse C3 antibody (Abcam, Cambridge, UK) followed by a secondary Texas red-labelled polyclonal rabbit anti-goat antibody (Abcam, Cambridge, UK). Mouse C3d staining was detected using a biotinylated-goat anti-mouse C3d (R&D system, MN, USA) and a secondary Streptavidin-PE conjugate (Euro Bioscience GmbH, Friesoythe, Germany). Previously we have shown that the anti-C3d antibody reacted with tissue associated C3d but not C3b or native C3. In contrast the anti-C3 antibody recognised native C3 and all C3 fragments except tissue associated C3d.19, 20 hCFH was detected using a FITC-conjugated polyclonal goat anti-human CFH antibody (Quidel, CA, USA).
Immunofluorescence staining was assessed in a blinded fashion based on its intensity in glomerular and interstitial structures and its extent along the glomerular capillary walls. Intensity was quantified using the following semi-quantitative scale: 0 (negative), 1 (mild), 2 (moderate) and 3 (intense). The extent of immunofluorescence staining along the glomerular capillary walls was quantified as the percentage of the entire capillary walls in each glomerulus with a positive immunofluorescence staining: 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%) and 4 (76-100%). Therefore, each glomerulus had an immunofluorescent intensity and an immunofluorescent extent score. For each kidney section, 50 glomeruli were examined and scored, and the average score recorded.
RESULTS
Administration of hCFH restored plasma AP regulation in Cfh−/− mice
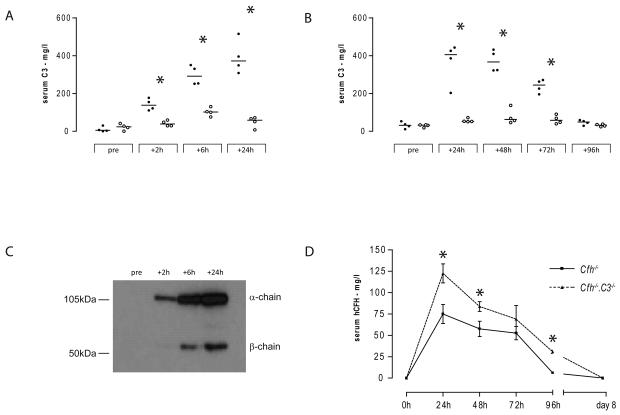
Cfh−/− mice spontaneously develop secondary C3 deficiency.10 Hence we first tested the ability of hCFH to restore AP regulation by measuring plasma C3 following hCFH administration. A single injection of 0.5mg hCFH intraperitoneally increased plasma C3 levels at 2 hours, peaking at 24 hours, at which time the plasma C3 level were comparable to normal wild-type C3 levels (figure 1a). In injected Cfh−/− mice plasma C3 levels remained normal at 48 hours falling to approximately 50% of normal levels by 72 hours and dropping to baseline values by 96 hours (figure 1b). We next determined the activation status of the plasma C3 by performing western blot for C3 under reducing conditions using serum from the hCFH-injected mice. This enabled us to differentiate between intact C3 and its activation products. Intact C3 α-chain and β-chain were detectable in hCFH-injected Cfh−/− mice (figure 1c). Intact C3 α-chain, absent in PBS-injected Cfh−/− mice, was detected as early as 2 hours following administration of hCFH (figure 1c). These data demonstrated that, in this heterologous in vivo system (human CFH, mouse C3 and mouse CFI), hCFH was able to restore plasma AP regulation in Cfh−/− mice.
Figure 1.
Plasma C3 levels in Cfh−/− mice injected with hCFH. (A) and (B) Mouse serum C3 levels at different time points following a single intraperitoneal injection of either 0.5mg hCFH (filled circles) or an equivalent volume of PBS (open circles). (A) and (B) represent two separate experiments. Horizontal bars denote median values. Asterisk (*) denotes p=0.0286, Mann Whitney test. In this ELISA pooled normal wild-type C3 level was 420 mg/l. (C) representative western blot of sera under reducing conditions for C3 from a Cfh−/− animal injected with hCFH. (D) Serum hCFH levels following single intraperitoneal injection of 0.5mg hCFH in to Cfh−/− (n=4, squares, solid line) and Cfh−/−.C3−/− (n=3, triangles, dotted line) mice. Data points represent mean +/− standard deviation. Asterisk (*) denotes p<0.05, Student t test.
The serum half-life of hCFH was influenced by the degree of C3 activation
We measured hCFH levels by ELISA before and at 24, 48, 72, 96 and 192 (day 8) hours following the intraperitoneal administration of 0.5mg hCFH (figure 1d). In Cfh−/− mice (n=4) serum hCFH levels were highest in the 24 hour samples (median 75.3mg/l, range 63.6 to 85.9) and fell to very low levels by 96 hours (median 5.6 mg/l, range 2.9 to 6.7). hCFH was undetectable by day 8. In parallel we administered an identical dose of hCFH to C3-deficient Cfh−/− mice (Cfh−/−.C3−/−, n=3). At each time point the median levels of hCFH were higher in the Cfh−/−.C3−/− compared to mice deficient in CFH alone. At 96 hours significant levels of hCFH were still detectable in the Cfh−/−.C3−/− mice (31.6mg/l, range 28.5 – 31.9, n=3) compared to very low in the Cfh−/− animals (median 5.6 mg/l, p<0.0001, Student t test). In both groups of mice, levels were undetectable at day 8. These data indicated that the serum half-life of hCFH was critically dependent on the degree of AP activation.
Administration of hCFH resulted in a rapid alteration in glomerular C3 staining in Cfh−/− mice
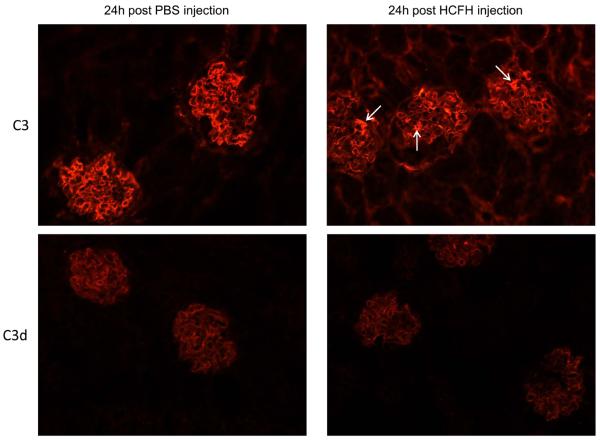
We next assessed renal C3 staining in Cfh−/− mice 24 hours after a single intraperitoneal injection of 0.5mg hCFH. Cfh−/− mice have marked C3 deposition along the GBM.10 This is evident on immunohistochemistry by a linear capillary wall glomerular staining distribution. In contrast, no staining for C3 is seen within the glomerular mesangium or tubulointerstitial areas. In vitro analysis of C3 isolated from laser-dissected Cfh−/− glomerular tissue demonstrated that the GBM-associated C3 included the C3 fragment iC3b, either in isolation or in addition to C3d.19 Using either anti-C3 or anti-C3d antibodies linear capillary wall staining was evident in untreated Cfh−/− animals (figure 2).19 24 hours after hCFH injection, there was marked alteration in glomerular and tubulointerstitial C3 staining patterns. There was a significant reduction in both the intensity and extent of glomerular capillary wall C3 reactivity using the anti-C3 antibody. Furthermore, mesangial C3 reactivity was now evident together with reactivity within the tubulointerstitium. In contrast, there was no change in the glomerular C3 reactivity pattern using the anti-C3d antibody. These changes (scored in table one and illustrated in figure 2) are similar to those that we have observed following a single injection of mouse CFH.19 Renal immunostaining for hCFH showed weak glomerular reactivity only (data not shown). Notably we did not detect any evidence of glomerular neutrophil accumulation in the Cfh−/− mice reconstituted with hCFH, a phenomenon that was observed when we administered mouse CFH to Cfh−/− animals.19 In Cfh−/− mice that had received a single injection of 0.5mg hCFH and then sacrificed 8 days later, renal C3 immunostaining (using either anti-C3 or anti-C3d antibodies) demonstrated linear capillary wall GBM reactivity identical to Cfh−/− mice that had received PBS (data not shown). This suggested that C3 had re-appeared along the GBM as the serum levels of the injected hCFH dropped (figure 1d). We next assessed the effects of repeated hCFH administration.
Figure 2.
Representative images of C3 immunostaining in Cfh−/− mice 24 hours after the injection of hCFH (right panel) or PBS (left panel). In PBS-injected animals capillary wall staining using both anti-C3 and anti-C3d antibodies is evident from the linear staining pattern outlining the glomerular capillary walls. No staining of the tubulointerstitium is seen with either of these antibodies. In hCFH-injected animals, the pattern of reactivity with the anti-C3 antibody demonstrated that the capillary wall staining had markedly reduced with concomitant appearance of reactivity within mesangial (examples indicated by arrows) and tubulointerstitial areas. In contrast, the administration of hCFH has not altered the staining pattern seen with the anti-C3d antibody. Original magnification x20.
Table One.
Serological and renal parameters following administration of hCFH to Cfh−/− mice
| Time of sacrifice | 24hours | 5 days | 10 days | |||
|---|---|---|---|---|---|---|
| hCFH dosing1 | single 0.5mg hCFH | daily 0.5mg hCFH | daily 0.5mg hCFH | |||
| hCFH (n=4) | control (n=4) | hCFH (n=5) | control (n=3) | hCFH (n=4) | control (n=6) | |
| Serological analysis | ||||||
| Urea –mmol/l | NA | NA | 9.2 (6.1-13.2) | 14 (9.4-15) | 44.4 (43.3-44.6)2 | 9.5 (6.4-15.3) |
| Serum C3 levels – mg/l | 370 (308-515)2 | 60 (7-71) | 402 381-423)2 | 16 (12-19) | 105 (76-140)2 | 25 (11-48) |
| Anti-hCFH antibodies - AEU | NA | NA | negative | negative | 11.5 (11-12.8) | negative |
| Renal C3 immunostaining | ||||||
| Capillary wall intensity (0-3) | 0.9 (0.7-1)2 | 2.6 (2.3-2.6) | negative | 3 (2.5-3) | 0.8 (0.8-0.9)2 | 2.8 (2.7-3) |
| Capillary wall extent (0-4) | 1.4 (0.8-1.9)2 | 3.8 (3.1-4) | negative | 4 (4-4) | 1.1 (0.9-1.6)2 | 3.8 (3.7-4) |
| Mesangium(0-4) | 1.8 (1.5-2.1) | negative | 0.1 (0-0.3) | negative | 1.2 (1-1.4) | negative |
| Tubulointerstitium (0-3) | 1.5 (1.5-2) | negative | 2 (1.5-2.5) | negative | 2 (1.5-2) | negative |
| Renal C3d immunostaining | ||||||
| Capillary wall intensity (0-3) | 2.5 (2.3-2.5) | 2.3 (2.2-2.6) | 0.7 (0.6-1)2 | 2.5 (1.4-2.6) | 1.2 (0.6-1.4)2 | 2.6 (2.4-2.9) |
| Capillary wall extent (0-4) | 3.9 (3.3-4) | 3.8 (3.4-4) | 1 (0.9-1.3)2 | 3.4 (1.8-3.5) | 1.7 (1.1-2.1)2 | 3.4 (3.3-3.8) |
| Mesangium (0-4) | negative | negative | negative | negative | 0.2 (0-0.4) | 0 (0-0.2) |
| Tubulointerstitium (0-3) | negative | negative | 1 (0.5-1.5) | negative | 2 (1-3)3 | negative |
| Renal IgG immunostaining | ||||||
| Glomerular (0-3) | negative | negative | negative | negative | 2.1 (1.8-2.4) | negative |
| Tubulointerstitium (0-3) | negative | negative | negative | negative | negative | negative |
Values represent median with range of values in parentheses.
hCFH administered by intraperitoneally injection,
p<0.05 versus PBS control group, Mann Whitney test,
p<0.05 versus value at 5 days, Bonferroni test, NA – not assessed, AEU – arbitrary ELISA units
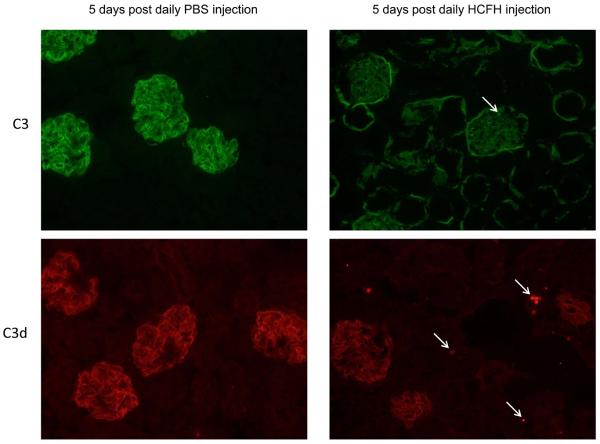
Short-term administration of hCFH resulted in normalization of glomerular C3 reactivity and a significant reduction in glomerular C3d reactivity
We administered 0.5mg hCFH intraperitoneally daily for 5 days to Cfh−/− mice to determine if repeated dosing would normalise renal C3 immunostaining. Control animals received intraperitoneal injections of an equivalent volume of PBS. Serum C3 levels at the time of sacrifice (24 hours after the final hCFH dose) were comparable to those seen in normal C57BL/6 mice (table one). Serum urea remained normal (table one) and no glomerular neutrophil accumulation was seen. Striking changes were noted when we examined glomerular C3 reactivity. Glomerular reactivity with anti-C3 antibodies was now negative along capillary walls and mesangial staining barely detectable (table one, figure 3). Furthermore, for the first time we were now able to detect a significant reduction in glomerular C3d reactivity (table one, figure 3). In hCFH-injected animals, some areas within the tubulointerstitium reacted with the anti-C3d antibody (figure 3, arrows). We next assessed if longer term administration of hCFH could completely remove glomerular C3d deposits by increasing the treatment period to 10 days.
Figure 3.
Representative images of C3 immunostaining in Cfh−/− mice 5 days after daily injection of 0.5mg of hCFH (right panel) or PBS (left panel). In PBS-injected animals capillary wall staining using both anti-C3 and anti-C3d antibodies is evident by the linear staining pattern outlining the glomerular capillary walls. No staining of the tubulointerstitium is seen with either of these antibodies. In hCFH-injected animals, the pattern of reactivity with the anti-C3 antibody demonstrated that the capillary wall staining had completely resolved. Furthermore, only minor and small areas of mesangial staining (example indicated by arrow) remained compared to that seen after 24 hours (figure 2). Normal tubulointerstitial C3 reactivity is also now present. The glomerular reactivity with the anti-C3d antibody has significantly reduced although is still detectable. Furthermore, in hCFH injected animals, some areas within the tubulointerstitium react with the anti-C3d antibody (examples indicated by arrows). Original magnification x20.
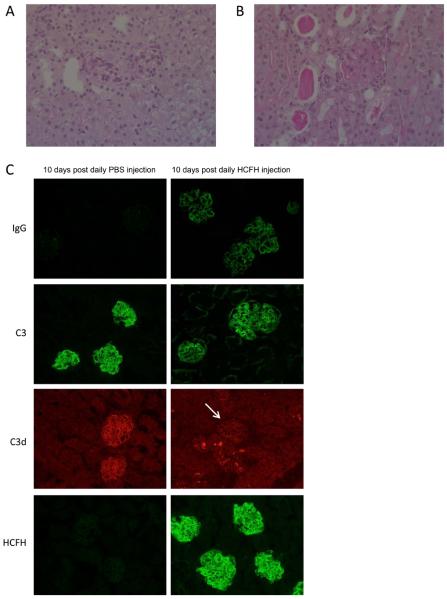
Long-term administration of hCFH to Cfh−/− mice results in the generation of murine anti-hCFH antibodies triggering immune complex glomerulonephritis with renal failure
Animals were injected intraperitoneally with either 0.5mg hCFH (n=4) or an equivalent volume of PBS (n=6) daily for 10 days. Unexpectedly, albuminuria developed in all of the hCFH-treated animals (median at day 10 = 26.9mg/ml, range 12-39.9) whilst it was undetectable in the PBS-injected animals. At sacrifice all hCFH-treated animals were uremic (median 44.4 mmol/l, table one) whilst urea levels were normal in the control mice (median 9.5, p<0.05, Mann Whitney test). Renal histology showed florid glomerular hypercellularity consistent with proliferative glomerulonephritis (figure 4b). There was marked deposition of IgG within the glomeruli of all the hCFH-injected animals (table one, figure 4C). Glomerular reactivity with anti-C3 antibodies, which had been negative along capillary walls in the Cfh−/− mice treated with hCFH for 5 days(figure 3), was now strongly positive in a staining pattern identical to IgG (table one, figure 4C). In contrast, the glomerular C3d immunostaining showed significant reduction compared to the control animals (table one). When glomerular C3d staining was compared to that seen in the Cfh−/− mice treated with hCFH for 5 days there was no significant difference in either the intensity or extent of C3d staining (table one). However, tubulointerstitial staining for C3d was significantly increased (figure 4C and table one) leading us to speculate that GBM-associated C3d was being removed via the urinary space. However, we were unable to reliably demonstrate the presence of C3 in the urine hCFH-treated animals.
Figure 4.
Representative light microscopic images of Cfh−/− mice 10 days after daily injection of PBS (A) or 0.5mg hCFH (B). Glomerular inflammation and protein casts are evident in the animals treated with hCFH (B) in contrast to the absence of these changes in the mice treated with PBS (A), Original magnification x20. (C) Representative images of IgG, C3, C3d and hCFH immunostaining in Cfh−/− mice 10 days after daily injection of 0.5mg of hCFH (right panel) or PBS (left panel). In PBS-injected animals capillary wall staining using both anti-C3 and anti-C3d antibodies shows a linear staining pattern outlining the glomerular capillary walls. No staining for mouse IgG is evident and only slight staining for hCFH is seen, most likely reflecting cross-reactivity with mouse CFH-related proteins 19. In contrast florid glomerular staining for mouse IgG, C3 and hCFH is seen in the animals treated with hCFH. Glomerular C3d immunostaining remains reduced compared to control animals (glomeruli indicated by arrow) and comparable to that seen after 5 days of hCFH treatment (figure 3). In contrast, tubulointerstitial C3d staining in hCFH-treated mice is more marked. Original magnification x20.
The presence of IgG within the kidney of the hCFH-treated mice suggested that the Cfh−/− animals had generated a mouse anti-hCFH antibody response. This was further supported by the observation that serum C3 levels at the time of sacrifice (24 hours after the final hCFH injection) had not normalised but were approximately 25% of pooled wild-type C3 levels (median level 105 mg/l, table one). Using an ELISA method, analogous to that used to detect anti-CFH IgG auto-antibodies in humans 21, mouse anti-hCFH antibodies were detected in all four of the hCFH-treated Cfh−/− mice (table one). These antibodies were also detected using a far western blot assay (data not shown). Antibodies were not detected in Cfh−/− mice that had received hCFH over 5 days (table one). Serum hCFH could not be detected using western blot at the time of sacrifice (24 hours after the final injection of 0.5mg hCFH). Our earlier experiments had shown that hCFH was easily detectable at this time point following a single injection (figure 1d). In contrast, renal immunostaining in the hCFH-treated mice, revealed strong glomerular hCFH staining in a pattern equivalent to that seen for IgG (figure 4C). These data confirmed the presence of mouse anti-hCFH antibodies and suggested that antibodies result in rapid clearance of injected hCFH from the circulation and deposition of mouse IgG-hCFH immune complexes within the kidney.
Discussion
Administration of hCFH to Cfh−/− mice resulted in restoration of plasma C3 levels together with the appearance of intact C3 in plasma. These observations are similar to those that we reported when we administered mCFH to the Cfh−/− mice.19 Furthermore, the rapid reduction in GBM C3 staining was also comparable to that which we observed in Cfh−/− mice treated with mCFH.19 These data demonstrated that hCFH was capable of interacting efficiently with the mouse factor I and mouse C3 in vivo. Our present findings using hCFH and previous observations with mCFH 19 demonstrated that a single injection of CFH markedly reduced GBM C3 reactivity but not reactivity to C3d in Cfh−/− mice within 24 hours. The reduction in GBM C3 reactivity was accompanied by the appearance of mesangial C3 but not C3d reactivity suggesting that the mesangial C3 fragment was C3c. When we administered repeated doses of hCFH the GBM C3d reactivity progressively fell so that after 10 days of hCFH treatment GBM C3d reactivity was markedly reduced compared to untreated mice. Furthermore, after 10 days of treatment with hCFH C3d reactivity was seen within tubular areas. These data lead us to conclude that the administration of hCFH restored plasma AP regulation resulting in the cessation of C3 deposition along the GBM in the Cfh−/− mice. This enabled the clearance of GBM-associated C3, which we have previously shown to be iC3b,19 to begin. We speculate that GBM-associated iC3b is initially cleaved to C3d releasing C3c to the mesangium. The remaining C3d is removed several days after the onset of plasma AP regulation, most likely via the urinary space. The removal of C3d from the GBM within 10 days is rapid in comparison to the estimated half-life of C3d associated with glomerular immune complexes.22 In these studies, whilst glomerular C3c was cleared within 24 hours, glomerular C3d reactivity remained unchanged 23 days after cessation of complement activation. Finally, when we administered mCFH to Cfh−/− mice we noted accumulation of glomerular neutrophils which we considered a consequence of low levels of endotoxin in our mCFH preparation.19 This interpretation is consistent with the lack of glomerular neutrophil accumulation in the present study where we used endotoxin-free hCFH.
Our data demonstrated that the administration of hCFH over a short time frame resulted in clearance of C3 from the GBM of Cfh−/− mice. The appearance of C3 along the GBM is the earliest pathological renal abnormality in both Cfh−/− mice 10 and Cfh−/− pigs.16 Therefore it might be expected that clearance of GBM C3 would result in amelioration or prevention of glomerular inflammation and membranoproliferative changes. The potent and rapid development of murine anti-hCFH antibodies prevented us from testing the effects of long-term administration of hCFH on either established renal inflammation or preventing the development of such inflammation. However, despite the development of an immune-complex glomerulonephritis, administration of HCFH daily for 10 days resulted in significant loss of glomerular C3d reactivity. To our knowledge, this is the first demonstration of a significant reduction of C3d deposits in a DDD model following the administration of an exogenous compound.
Experimental data from the Cfh−/− mice has shown that restoring AP regulation can reverse GBM C3 staining: transplantation of kidneys from Cfh−/− mice into wild-type recipients resulted in complete normalization of glomerular C3 staining.23 In the Cfh−/− pigs a single 30ml/kg plasma or 5mg/kg porcine CFH infusion increased plasma C3 levels and reduced soluble plasma terminal complement complex levels for approximately 3 days.14 Weekly plasma infusions to Cfh−/− pigs (from the age of 2 weeks) resulted in improved survival (median survival was 37 and 82 days, treated versus non-treated respectively).14 Renal histology in the treated Cfh−/− pigs showed the persistence of membranoproliferative changes although the severity of the lesions was less when compared to the untreated animals.14
Reports of plasma therapy in individuals with complete CFH deficiency and DDD are rare. Two patients with DDD in association with C3 nephritic factor and homozygous CFH mutation were reported to have stable renal function when treated with fortnightly plasma infusions (10-15ml/kg) for a total of 36 months.24, 25 One patient developed haematuria following upper airways infection which responded to additional plasma infusions.24 aHUS is empirically treated with plasma therapy (plasma exchange or plasma infusion).26 For individuals with aHUS associated with complete CFH deficiency plasma infusions have been beneficial in maintaining remission and treating relapses in some cases27-29 whilst in others the response was variable 30 or poor.31, 32 In one individual with aHUS and CFH deficiency, successfully treated with weekly plasma infusions over a four year period, a plasma-resistant relapse developed.33 The reason for this was not clear but importantly anti-CFH antibodies were not present. It seems reasonable to conclude that, whilst the development of mouse anti-hCFH antibodies in the absence of adjuvant was rapid, the observed immunogenicity of the hCFH in the CFH−/− animals in our study was most likely the consequence of the human origin of the injected CFH.
Serial CFH measurements in one CFH-deficient individual with aHUS successfully treated with repeated infusions of plasma, suggested a serum CFH half-life of 6 days.28 After infusion of 20ml/kg plasma, serum CFH increased from undetectable levels to 110 μg/ml, falling by 50% six days after the infusion.28 Our data suggest that the serum half-life of hCFH is influenced by the degree of C3 activation. hCFH was detectable in plasma for a greater duration in mice with combined deficiency of CFH and C3 than mice deficient in CFH alone. The half-life of CFH seems likely to vary according to the extent of C3 activation. This may be important for dosing studies in humans with DDD who may have distinct causes of AP dysregulation associated with quantitatively different degrees of AP dysregulation.
In conclusion, the impressive results obtained over the short term administration of hCFH in this murine model of DDD suggest that hCFH is a promising therapy for human DDD associated with CFH deficiency. The advantage of purified CFH concentrate is that it should be possible to administer the amount of CFH required to normalise AP regulation without concerns of excessive volume and protein load that limit plasma therapy. Further studies will be required to address efficacy over the longer term and to investigate if administration of exogenous hCFH is of benefit to individuals with DDD driven by causes other than CFH deficiency.
Acknowledgements
We thank the staff of the Biological Services Unit at Imperial College, London, United Kingdom for the care of the animals involved in this study. MCP is a Wellcome Trust Senior Fellow in Clinical Science (WT082291MA) and EGdJ is funded by this fellowship. FF was a recipient of a European Molecular Biology Organisation (EMBO) fellowship (ASTF 437-2008). Additional funding was provided by LFB (Laboratoires Français de Fractionnement et des Biotechnologies).
References
- 1.Smith RJ, Alexander J, Barlow PN, et al. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol. 2007;18:2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pickering MC, Cook HT. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol. 2008;151:210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Larrea C, Dieguez MA, Enguix A, et al. A familial deficiency of complement factor H. Biochem Soc Trans. 1987;15:648–649. [Google Scholar]
- 4.Jokiranta TS, Solomon A, Pangburn MK, et al. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol. 1999;163:4590–4596. [PubMed] [Google Scholar]
- 5.Meri S, Koistinen V, Miettinen A, et al. Activation of the alternative pathway of complement by monoclonal lambda light chains in membranoproliferative glomerulonephritis. J Exp Med. 1992;175:939–950. doi: 10.1084/jem.175.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linshaw MA, Stapleton FB, Cuppage FE, et al. Hypocomplementemic glomerulonephritis in an infant and mother. Evidence for an abnormal form of C3. Am J Nephrol. 1987;7:470–477. doi: 10.1159/000167525. [DOI] [PubMed] [Google Scholar]
- 7.Marder HK, Coleman TH, Forristal J, et al. An inherited defect in the C3 convertase, C3b,Bb, associated with glomerulonephritis. Kidney Int. 1983;23:749–758. doi: 10.1038/ki.1983.89. [DOI] [PubMed] [Google Scholar]
- 8.Daha MR, Fearon DT, Austen KF. C3 nephritic factor (C3NeF): stabilization of fluid phase and cell-bound alternative pathway convertase. J Immunol. 1976;116:1–7. [PubMed] [Google Scholar]
- 9.Spitzer RE, Vallota EH, Forristal J, et al. Serum C'3 lytic system in patients with glomerulonephritis. Science. 1969;164:436–437. doi: 10.1126/science.164.3878.436. [DOI] [PubMed] [Google Scholar]
- 10.Pickering MC, Cook HT, Warren J, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 11.Pickering MC, Warren J, Rose KL, et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci U S A. 2006;103:9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose KL, Paixao-Cavalcante D, Fish J, et al. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608–618. doi: 10.1172/JCI32525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogasen K, Jansen JH, Harboe M. Eradication of porcine factor H deficiency in Norway. Vet Rec. 1997;140:392–395. doi: 10.1136/vr.140.15.392. [DOI] [PubMed] [Google Scholar]
- 14.Hogasen K, Jansen JH, Mollnes TE, et al. Hereditary porcine membranoproliferative glomerulonephritis type II is caused by factor H deficiency. J Clin Invest. 1995;95:1054–1061. doi: 10.1172/JCI117751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen JH, Hogasen K, Grondahl AM. Porcine membranoproliferative glomerulonephritis type II: an autosomal recessive deficiency of factor H. Vet Rec. 1995;137:240–244. doi: 10.1136/vr.137.10.240. [DOI] [PubMed] [Google Scholar]
- 16.Jansen JH, Hogasen K, Harboe M, et al. In situ complement activation in porcine membranoproliferative glomerulonephritis type II. Kidney Int. 1998;53:331–349. doi: 10.1046/j.1523-1755.1998.00765.x. [DOI] [PubMed] [Google Scholar]
- 17.Jansen JH, Hogasen K, Mollnes TE. Extensive complement activation in hereditary porcine membranoproliferative glomerulonephritis type II (porcine dense deposit disease) Am J Pathol. 1993;143:1356–1365. [PMC free article] [PubMed] [Google Scholar]
- 18.Appel GB, Cook HT, Hageman G, et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol. 2005;16:1392–1403. doi: 10.1681/ASN.2005010078. [DOI] [PubMed] [Google Scholar]
- 19.Paixao-Cavalcante D, Hanson S, Botto M, et al. Factor H facilitates the clearance of GBM bound iC3b by controlling C3 activation in fluid phase. Mol Immunol. 2009;46:1942–1950. doi: 10.1016/j.molimm.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung VW, Yun S, Botto M, et al. Decay-accelerating factor suppresses complement C3 activation and retards atherosclerosis in low-density lipoprotein receptor-deficient mice. Am J Pathol. 2009;175:1757–1767. doi: 10.2353/ajpath.2009.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dragon-Durey MA, Loirat C, Cloarec S, et al. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2005;16:555–563. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- 22.Schulze M, Pruchno CJ, Burns M, et al. Glomerular C3c localization indicates ongoing immune deposit formation and complement activation in experimental glomerulonephritis. Am J Pathol. 1993;142:179–187. [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander JJ, Wang Y, Chang A, et al. Mouse podocyte complement factor H: the functional analog to human complement receptor 1. J Am Soc Nephrol. 2007;18:1157–1166. doi: 10.1681/ASN.2006101125. [DOI] [PubMed] [Google Scholar]
- 24.Habbig S, Mihatsch MJ, Heinen S, et al. C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int. 2009;75:1230–1234. doi: 10.1038/ki.2008.354. [DOI] [PubMed] [Google Scholar]
- 25.Licht C, Heinen S, Jozsi M, et al. Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II) Kidney Int. 2006;70:42–50. doi: 10.1038/sj.ki.5000269. [DOI] [PubMed] [Google Scholar]
- 26.Taylor CM, Machin S, Wigmore SJ, et al. Clinical Practice Guidelines for the management of atypical Haemolytic Uraemic Syndrome in the United Kingdom. Br J Haematol. 2009 doi: 10.1111/j.1365-2141.2009.07916.x. [DOI] [PubMed] [Google Scholar]
- 27.Cho HY, Lee BS, Moon KC, et al. Complete factor H deficiency-associated atypical hemolytic uremic syndrome in a neonate. Pediatr Nephrol. 2007;22:874–880. doi: 10.1007/s00467-007-0438-x. [DOI] [PubMed] [Google Scholar]
- 28.Licht C, Weyersberg A, Heinen S, et al. Successful plasma therapy for atypical hemolytic uremic syndrome caused by factor H deficiency owing to a novel mutation in the complement cofactor protein domain 15. Am J Kidney Dis. 2005;45:415–421. doi: 10.1053/j.ajkd.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Nathanson S, Fremeaux-Bacchi V, Deschenes G. Successful plasma therapy in hemolytic uremic syndrome with factor H deficiency. Pediatr Nephrol. 2001;16:554–556. doi: 10.1007/s004670100609. [DOI] [PubMed] [Google Scholar]
- 30.Boyer O, Noel LH, Balzamo E, et al. Complement factor H deficiency and posttransplantation glomerulonephritis with isolated C3 deposits. Am J Kidney Dis. 2008;51:671–677. doi: 10.1053/j.ajkd.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 31.Landau D, Shalev H, Levy-Finer G, et al. Familial hemolytic uremic syndrome associated with complement factor H deficiency. J Pediatr. 2001;138:412–417. doi: 10.1067/mpd.2001.112649. [DOI] [PubMed] [Google Scholar]
- 32.Ohali M, Shalev H, Schlesinger M, et al. Hypocomplementemic autosomal recessive hemolytic uremic syndrome with decreased factor H. Pediatr Nephrol. 1998;12:619–624. doi: 10.1007/s004670050515. [DOI] [PubMed] [Google Scholar]
- 33.Nathanson S, Ulinski T, Fremeaux-Bacchi V, et al. Secondary failure of plasma therapy in factor H deficiency. Pediatr Nephrol. 2006;21:1769–1771. doi: 10.1007/s00467-006-0237-9. [DOI] [PubMed] [Google Scholar]