Abstract
The mitogen-activated protein kinase (MAPK) kinase 4 (MKK4) is a non-redundant component of stress activated MAPK signaling modules. Its function in tumorigenesis remains highly controversial with some studies indicating that MKK4 is a tumor suppressor, while others have reported a pro-oncogenic role. To clarify the role of MKK4 in cancer we have created a novel mouse model to test the effect of the specific loss of MKK4 in the epidermis on the formation of papillomas caused by activated ras mutation. We have discovered that skin-specific MKK4-deficient mice are resistant to carcinogen-induced tumorigenesis. One mechanism by which MKK4 promotes cell proliferation and the formation of tumors is by increasing EGFR expression via the c-Jun N-terminal protein kinase (JNK)/c-Jun signaling pathway. Together our results provide the first genetic demonstration that MKK4 is essential to mediate the oncogenic effect of Ras in vivo, thereby validating MKK4 as a potential drug target for cancer therapy.
Keywords: MAPK, MKK4, JNK, c-Jun, skin
Introduction
Mitogen-activated protein kinase (MAPK) kinase 4 (MKK4) is a component of stress activated MAPK signaling modules (1). It can activate by phosphorylation the c-Jun N-terminal protein kinase (JNK) and p38 families of MAPKs in response to various stimuli. However, our previous studies have provided genetic evidence that MKK4 is mostly required for JNK activation in vivo (2), although MKK4 was shown to contribute to p38 stimulation in cells where both MKK3 and MKK6 have been removed (3).
JNK regulates numerous physiological processes by controlling gene expression via the phosphorylation of components of the AP-1 family of transcription factors, including c-Jun (4). c-Jun is a critical contributor to transformation and tumorigenesis (5). The demonstration that mice carrying a mutated allele of c-jun that cannot be phosphorylated by JNK are resistant to tumor development caused by constitutive activation of Ras, provided the first genetic link between JNK, c-Jun and cancer (6). However, more recently, jnk gene disruption has been shown to enhance Ras-stimulated transformation (7). In this model, JNK may restrict tumor burden by promoting apoptosis (7). These conflicting findings may be explained by the distinct function of JNK isoforms with evidence that JNK1 acts as a tumor suppressor while JNK2 is a tumor promoter (8, 9). Moreover, the observation that JNK1 contributes to B cell lymphoma caused by Bcr-Abl (10) indicates that JNKs may play different roles depending on tumor type.
Like JNK, the role of MKK4 in cancer appears complex with some data suggesting that MKK4 acts as a tumor and metastasis suppressor, while other studies point to a pro-oncogenic role for MKK4 (11-14). Furthermore, understanding the requirement of MKK4 in mediating JNK signaling downstream of oncogenes has been complicated by the existence of a second activator of JNK, MKK7 (1). Like MKK4, MKK7 activates JNK by dual phosphorylation at Thr and Tyr residues within the Thr-Pro-Tyr motif. However, the early embryonic death of mice caused by the targeted deletion of either the mkk4 or mkk7 genes indicates that MKK4 and MKK7 have non-redundant functions in vivo (1). The inability of MKK4 and MKK7 to compensate for each others functions may be due to their selective regulation by extracellular stimuli and their distinct tissue distribution (1). Overall, conflicting findings together with the lack of understanding of the molecular function of MKK4 downstream of oncogenes have been an obstacle for considering MKK4 as a valuable drug target for cancer therapy.
To clarify the role of MKK4 in cancer, we have examined the effect of mkk4 gene deletion in the basal cells of the epidermis on the development of skin tumors using the two stage carcinogenesis protocol (15). This simple, versatile and highly reproducible protocol gives rise to benign papillomas with a high incidence of ras mutation. Furthermore, in contrast to previous studies (14), this system recapitulates the complex interactions between tumors and host as well as the cellular heterogeneity of tumors. This is critical considering that the environment greatly influences the spatial and temporal regulation of signals activated by oncogenes, and consequently determines the biological outcome of the response (16). Our results show that skin-specific MKK4-deficient mice are resistant to tumorigenesis associated with oncogenic activation of the ras gene. These data provide the first genetic demonstration that signaling downstream of MKK4 is essential for tumor formation in the skin.
Material and Methods
Genotype determination of mice and tissues
Offspring carrying the mkk4-flox allele and the K14-CreERT2 transgene were identified by PCR on tail and on keratinocyte DNA, as previously described (2, 17).
Two-stage carcinogenesis
Mice were backcrossed into an FVB background. 4 weeks old littermates expressing or not the K14-CreERT2 transgene were injected intraperitoneally every day for 5 days with a non-toxic amount of tamoxifen (200 μg) to induce Cre activation. Two weeks later, the shaved backs of the mice received a single application of 25 μg of 7, 12-dimethyl-benzanthracene (DMBA) in 150 μl of acetone. One week after DMBA, the skin of the mice was treated bi-weekly with 6.25 μg of 12-O-tetradecanoylphorbol 13-acetate (TPA) in 150 μl of acetone for a total of 20 to 30 weeks. The tumor incidence and burden was recorded every 2 weeks for the duration of the experiment. Careful clinical examination of the mice was carried out to allow detection of deterioration of their physical condition associated with progression of the tumors. Animals showing signs of distress, including ulceration of the skin, weight loss, reduced activity, and lack of response to stimuli, were sacrificed before any further deterioration in condition occurred.
Immunoblot analysis
Proteins were extracted from tissues in Triton lysis buffer (TLB: 20 mM Tris pH 7.4, 137 mM NaCl, 2 mM EDTA, 1% Triton X100, 25 mM β glycerophosphate, 10% glycerol, 1 mM orthovanadate, 1 mM phenylsulphonyl fluoride (PMSF), 10 μg/ml leupeptin, 10 μg/ml aprotinin). Extracts were clarified by centrifugation (14,000 g for 10 min at 4°C). The concentration of soluble proteins in the supernatants was quantified by the Bradford method (Bio-Rad). Extracts (20 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE, 10% or 8% polyacrylamide gel) and electrophoretically transferred to an Immobilon-P membrane (Millipore, Inc.). The membranes were incubated with 3% nonfat dry milk at 4°C for 30 min and probed overnight with antibodies (1:1000) to MKK4 (BD Pharmingen) or Tubulin (Sigma). Immunecomplexes were detected by enhanced chemiluminescence with anti-mouse immunoglobulin G coupled to horseradish peroxidase as the secondary antibody (Amersham-Pharmacia).
Histological and immunohistochemical analyses
Freshly isolated skin biopsies and tumors were fixed at room temperature in 4% paraformaldehyde for 6 hours, dehydrated, and embedded in paraffin. 5 μm thick sections were cut. For histological analysis, sections were stained with heamatoxylin and eosin (H&E) (18). For immunohistochemistry, sections were deparaffinized, rehydrated, and treated in boiling sodium citrate buffer (10 mM pH 6.0) for 10 minutes to unmask the antigen. Endogenous peroxidase activity was quenched by treating the slides with 0.3% hydrogen peroxide for 10 minutes. Sections were blocked in PBS containing 5-10% goat serum and 0.1% Triton X100 for 1 hour at room temperature prior to being incubated overnight at 4°C with primary antibodies (1:100) to MKK4 (Cell Signaling), MKK7 (Cell Signaling), JNK1 (BD Pharmingen), JNK2 (Santa Cruz), phospho-JNK (Cell Signaling), keratin 6 (Covance), c-Jun (Cell Signaling), phospho-c-Jun (Ser 73) (Cell Signaling), EGFR (Santa Cruz), and p53 (Calbiochem). The following day the slides were rinsed in PBS and incubated at room temperature for 1 hour with secondary biotinylated anti-mouse, anti-rabbit, anti-goat or anti-sheep antibodies. The slides were processed using the ABC detection kit (Vector Laboratories). The presence of the antigens were revealed using the Vector® SG (gray) or diaminobenzidine (DAB, brown) peroxidase substrate kit (Vector Laboratories) and counter stained with nuclear red or with heamatoxylin (blue). For bromodeoxyuridine (BrdU) staining, the mice were injected with 500 mg/kg BrdU and sacrificed 2 hours later. Immunohistochemistry was performed using the above method, with an additional HCl treatment for 8 min at 60°C prior to the blocking step. Mouse monoclonal antibody to BrdU was obtained from Dako. For TUNEL staining, sections were processed using the In situ cell death detection kit, POD (Roche), according to the manufacturer's instructions. All slides were viewed using the Axioplan2 microscope.
Real-time quantitative PCR
Total RNA was isolated from the epidermis using the Trizol™ reagent and cDNA synthesis was carried out as previously described (19). Real-time quantitative PCRs were performed using the SYBR Green I Core Kit (Eurogentec). Sequences of the forward and reverse primers are indicated in the Supplemental Data available with this article online (Fig. S1). PCR products were detected in the ABI-PRISM 7700 sequence detection systems (Applied Biosystems). Results were analyzed using the 2−ΔΔG methods. The level of expression of mRNA was normalized to actin mRNA.
Results
Inactivation of MKK4 in the skin
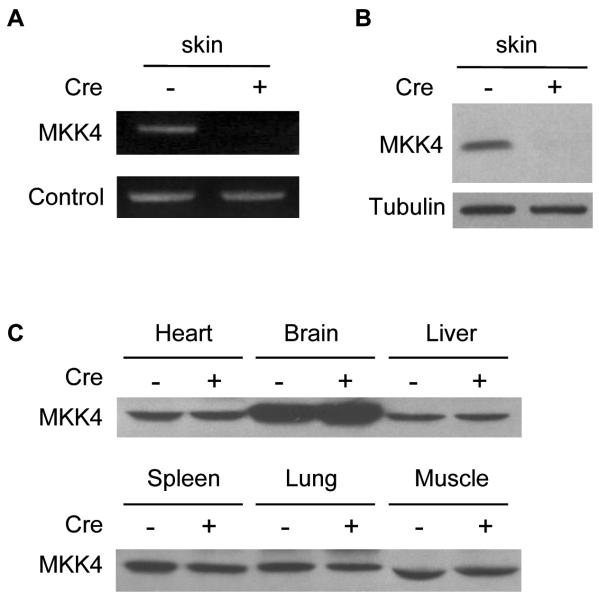
The embryonic lethality caused by the targeted deletion of the mkk4 gene in mice (1) led us to use the Cre-loxP system (20) to disrupt the mkk4 gene in skin epidermis to elucidate the function of MKK4 in cancer. Mice carrying the mkk4 gene flanked with LoxP sites (referred to as the flox (fl) allele) (2) were mated with a transgenic line expressing Cre fused to a mutated form of the ligand binding domain of the estrogen receptor (CreERT2) under the control of the keratin 14 (K14) promoter (17). Expression of K14 is restricted to proliferating keratinocytes in the basal layer of the epidermis where tumor induction is initiated, and is down-regulated as the basal layer differentiates. The ERT2 moiety ensures the cytoplasmic sequestration of Cre. Cre translocates to the nucleus where it specifically recombines the fl allele in the epidermis upon injection of the mice with tamoxifen. Multiple litters of mkk4loxP mice were crossed with K14-CreERT2 animals. Amplification by PCR with primers specific for the mkk4 gene on genomic DNA isolated from the skin epidermis extracted from (K14-CreERT2)mkk4fl/fl adult mice 2 weeks after tamoxifen injection, showed the specific recombination of the mkk4 gene (Fig. 1A). Immunoblot analysis of epidermal extracts of littermates homozygous for the mkk4-flox allele confirmed that the inactivation of the mkk4 gene in the skin only occurred in mice injected with tamoxifen and expressing Cre (Fig. 1B). The selective ablation of mkk4 in the skin was demonstrated by similar expression of MKK4 in heart, brain, liver, spleen, lung and muscle of both mkk4fl/fl and (K14-CreERT2)mkk4fl/fl mice injected with tamoxifen (Fig. 1C). In subsequent experiments, mkk4fl/fl and (K14-CreERT2)mkk4fl/fl mice injected with tamoxifen will be referred to as mkk4skin+/+ and mkk4skin−/− animals, respectively.
Figure 1.
Disruption of the mkk4 gene in skin epidermis. Adult mkk4fl/fl mice carrying (+) or not (−) the K14-CreERT2 transgene were injected intraperitoneally every day for 5 days with a non-toxic amount of tamoxifen (200 μg) to induce Cre activation. The mice were sacrificed 2 weeks later. The epidermises of dorsal skins were separated from dermal tissues. A, Genomic DNA was isolated and amplified by PCR with primers specific for the mkk4 gene. B and C, The specific inactivation of the mkk4 gene in the epidermis of mice expressing Cre was confirmed by immunoblot analyses of protein extracts obtained from various tissues using a specific antibody to MKK4. The detection of tubulin expression was performed to monitor protein loading. Similar results were obtained in two independent experiments.
Phenotypic analysis of the MKK4 mutant mice
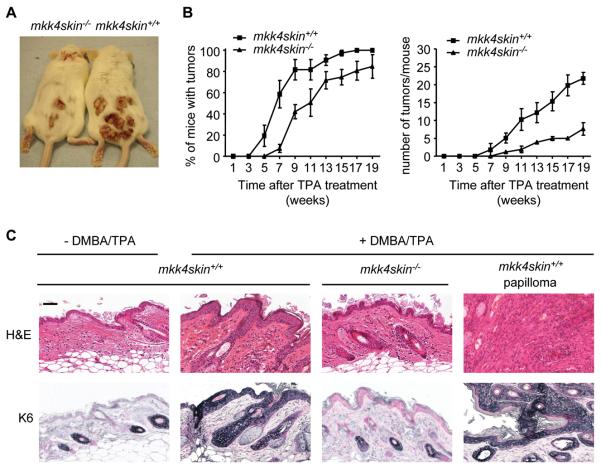
To elucidate the function of MKK4 in skin cancer, mice were subjected to a two-stage carcinogenesis protocol (15). Briefly, a single dose of the carcinogen DMBA was applied on the skin of the mice 2 weeks after the first tamoxifen injection. One week after DMBA, the skin were treated bi-weekly with the tumor promoter TPA. This protocol recapitulates the fundamental concept that tumor development is a multi-step process that includes: initiation of benign papilloma (mutation of c-Ha-ras following DMBA treatment), promotion (a clonal expansion of the initiated cell triggered by the repeated treatment with TPA to form a benign tumor), and progression to malignant carcinoma (an additional evolutionary step) (15). Using this model, we discovered that skin-specific MKK4-deficient mice were resistant to tumorigenesis (Fig. 2A). Control experiment indicated that oncogenic mutation of the c-H-ras gene (21) was detected in the epidermis of both mkk4skin+/+ and mkk4skin−/− animals treated with DMBA (data not shown). Strikingly, both tumor incidence (percentage of mice bearing one or more papillomas) and tumor burden (number of tumors per mouse) were greatly decreased in mice lacking MKK4 (Fig. 2B). In fact, papillomas that did develop on the back skin of the mkk4skin−/− mice were wild type (Fig. S2). This was expected since the deletion of floxed genes following tamoxifen-induced Cre does not occur with 100% efficiency.
Figure 2.
MKK4 is required for skin tumor formation. mkk4skin+/+ and mkk4skin−/− animals were treated with DMBA/TPA. Controls correspond to age matched untreated mkk4skin+/+ mice. A, Representative picture of mice bearing papillomas 20 weeks after TPA treatment. B, Papillomas on the back skin of the mice were counted. The data, expressed as the mean +/− standard error (SE), were generated from three independent experiments (n = 10). C, H&E staining of sections of skin biopsies 20 weeks after TPA treatment demonstrates that DMBA/TPA treatment significantly increases the thickness of the epidermis of mkk4skin+/+, but not that of mkk4skin−/−, animals. Concomitantly, the loss of MKK4 prevents the expansion of a population of epithelial cells expressing the progenitor cell marker K6 as demonstrated by immunostaining with a specific antibody to K6. Scale bar represents 50 μM.
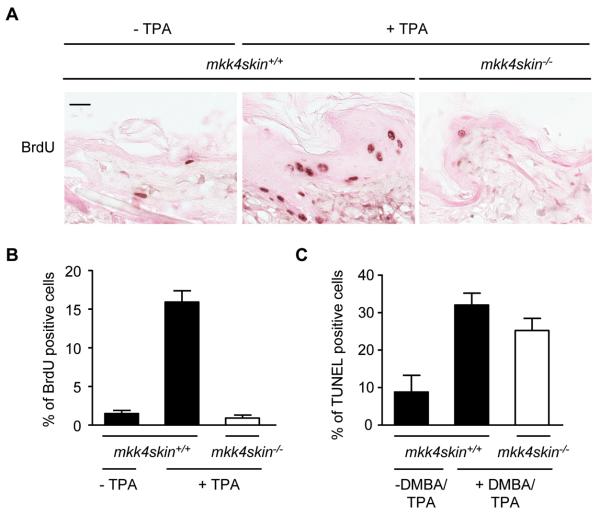
The loss of MKK4 in the epidermis of adult mice did not cause any gross morphological defect suggesting that MKK4 is not required for skin homeostasis (data not shown). In contrast, DMBA/TPA treatment significantly increased the thickness of the epidermis of mkk4skin+/+, but not mkk4skin−/−, animals reflecting an increased number of dividing wild type keratinocytes (Fig. 2C). We also analyzed the expression of keratin 6 (K6) as a marker of hyperproliferative skin and found positive staining throughout all epidermal layers of wild type mice treated with DMBA/TPA (Fig. 2C). In contrast, K6 was found in the outer root sheath of hair follicles in mkk4skin−/− animals treated with DMBA/TPA, as in untreated mkk4skin+/+ animals (Fig. 2C). Consistently, epidermal cell proliferation was significantly induced by a single application of TPA on the dorsal skin of the wild type mice, as demonstrated by BrdU incorporation (Fig. 3A). Proliferating keratinocytes which are normally confined to the basal layer had expanded to the suprabasal compartment (Fig. 3A). The percentage of BrdU-labeled wild type cells was 2% before and 16% ± 2% 24 hours after TPA treatment (Fig. 3B). In contrast, the proliferation rate of MKK4-deficient keratinocytes was unchanged upon TPA stimulation (Fig. 3A and B). Furthermore proliferating mutant keratinocytes were largely localized to the basal layer as in the epidermis of untreated wild type mice (Fig. 3A).
Figure 3.
Lack of MKK4 prevents TPA-induced cell proliferation. A, Dorsal skins of mkk4skin+/+ and mkk4skin−/− mice received a single application of TPA. Controls correspond to age matched untreated mkk4skin+/+ mice. The mice were injected intraperitoneally with BrdU 24 h after TPA treatment. The dorsal skins were isolated 2 h later and processed for BrdU immunoreactivity. Positive cells are stained in brown. B, Quantitative analysis of BrdU-positive cells demonstrates that the number of proliferating cells is higher in the epidermis of mkk4skin+/+ compared to that of mkk4skin−/− animals treated with TPA. C, mkk4skin+/+ and mkk4skin−/− animals were treated with DMBA/TPA for 20 weeks. Controls correspond to age matched untreated mkk4skin+/+ mice. Skin sections were stained by TUNEL. Quantification of TUNEL positive cells indicates no marked difference in the number of apoptotic cells in the epidermis of treated mkk4skin+/+ and mkk4skin−/− mice. The data expressed as percent +/− standard error (SE) were generated from three animals/genotype. 1000 cells per section were counted. Scale bar represents 20 μM.
Next, we determined whether the resistance of MKK4-deficient mice to tumorigenesis could also be attributed to an altered rate of cell death, expected after DNA damage such as DMBA treatment. We found no marked difference in the number of apoptotic cells in the basal or suprabasal layers of the epidermis of mkk4skin+/+ and mkk4skin−/− mice after DMBA/TPA treatment with TUNEL staining (Fig. 3C). Together these results indicate that epidermal hyperplasia exhibited by the wild type skin following DMBA/TPA treatment involved the expansion of a population of epithelial cells expressing the progenitor cell marker keratin 6. Inhibition of keratinocyte proliferation caused by the loss of MKK4 could explain why MKK4-deficient tumors do not form on the back of the mkk4skin−/− mice.
Mice that lack MKK4 in the skin display a defect in JNK signaling
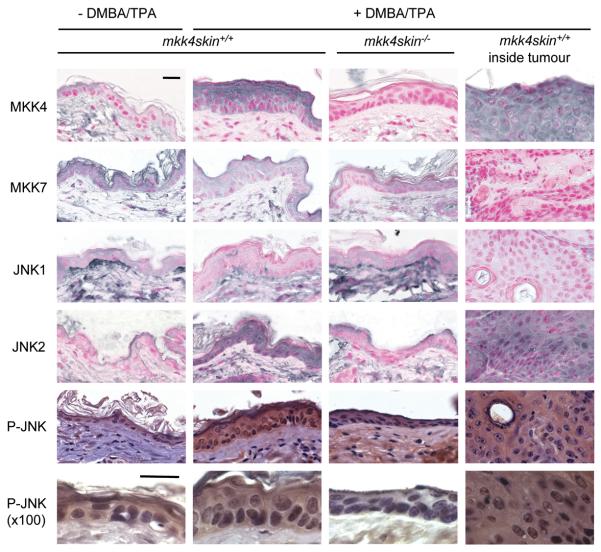
The role of MKK4 in regulating JNK signaling was determined by comparing sections of wild type and MKK4-deficient skin 20 weeks after DMBA/TPA treatment (Fig. 4). Immunostaining of skin sections with an antibody to MKK4 ascertained the absence of MKK4 in the mutant epidermis (Fig. 4). Furthermore, it demonstrated that MKK4 expression increased in the wild type skin exposed to DMBA/TPA. Increased MKK4 staining was localized in both the nuclear and cytoplasmic compartment of keratinocytes present in the upper layer of the epidermis. In contrast, the level of the other activator of JNK, MKK7, slightly decreased in the skin of both wild type and mutant mice treated DMBA/TPA (Fig. 4). Consistently, papillomas expressed a high level of MKK4 but a low level of MKK7 (Fig. 4). It is interesting to note that MKK7 is expressed in both the nucleus and the cytoplasm of keratinocytes of untreated wild type mice, while MKK4 is mostly excluded from the nuclei.
Figure 4.
MKK4 is required for mediating the phosphorylation of JNK in the epidermis of the skins treated with DMBA/TPA. mkk4skin+/+ and mkk4skin−/− animals were treated with DMBA/TPA. Controls correspond to age matched untreated mkk4skin+/+ mice. Skin biopsies and tumors were isolated 20 weeks later. Sections were immunostained with the antibodies indicated. Immunostaining with a specific antibody to MKK4 confirms the loss of MKK4 in the epidermis of mkk4fl/fl mice expressing the K14-CreERT2 transgene and treated with tamoxifen. The absence of MKK4 prevents increased JNK2 expression following DMBA/TPA treatment. In contrast, the level of JNK1 is reduced in the wild type skin treated with DMBA/TPA. Immunostaining with a specific antibody to phospho (P)-JNK demonstrates that DMBA/TPA induces the phosphorylation of JNK and that this is prevented by the loss of MKK4. Unless indicated otherwise, the pictures were taken at x63 magnification. Scale bar represents 20 μM.
JNK1 and JNK2 have been shown to exert opposite effect with regards to skin tumorigenesis with evidence that JNK1 acts as a tumor suppressor while JNK2 is a tumor promoter (8, 9). Immunostaining of skin sections with antibodies specific to JNK1 or JNK2 demonstrated that the level of JNK1 decreased, while that of JNK2 increased, in wild type skin treated with DMBA/TPA (Fig. 4). Increased accumulation of nuclear and cytosolic JNK2 was observed throughout the wild type epidermis. These changes were dependent on the presence of MKK4 since the level of expression of JNK1 and JNK2 was similar in mkk4skin−/− animals treated with DMBA/TPA (Fig. 4). Sections of skin biopsies were immunostained with a specific antibody to phospho (P)-JNK to determine the requirement of MKK4 in regulating JNK activity (Fig. 4). We found that DMBA/TPA treatment increased the phosphorylation of JNK. Similar to JNK2, P-JNK accumulated in both the nuclear and cytosolic compartment of keratinocytes throughout the treated wild type epidermis. This was prevented by the loss of MKK4 (Fig. 4).
Consistent with the requirement of JNK to stimulate the transcriptional activity of c-Jun (4), the loss of MKK4 resulted in impaired phosphorylation of c-Jun at Ser73 (Fig. 5). c-Jun increases the transcription of numerous genes, one of which is the c-jun gene itself (22). This auto-regulation of the c-jun promoter by c-Jun explains why increased c-Jun protein level caused by DMBA/TPA treatment of wild type skin was prevented in the epidermis of mice lacking MKK4 (Fig. 5). Both the phosphorylation and expression of c-Jun accumulated in cells localized to the edge of the upper layer of the wild type epidermis (Fig. 5). Increased phosphorylation of c-Jun was also detected in proliferating cells surrounding the hair follicle. Taken together these results led us to conclude that MKK4 is a critical activator of JNK signaling in the skin.
Figure 5.
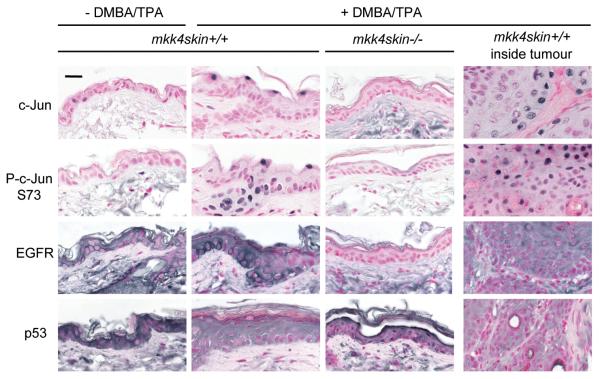
The loss of MKK4 prevents the phosphorylation of c-Jun in the skin treated with DMBA/TPA. mkk4skin+/+ and mkk4skin−/− animals were treated with DMBA/TPA. Controls correspond to age matched untreated mkk4skin+/+ mice. Sections of skin biopsies and tumors 20 weeks after TPA treatment were immunostained with antibodies to c-Jun, phospho (P)-c-Jun at Ser 73, EGFR, and p53. The results show that MKK4 is required for increased expression and phosphorylation of c-Jun, and increased expression of the c-Jun target EGFR. Elevated expression of p53 in MKK4-deficient epidermis of animals treated with DMBA/TPA is consistent with evidence that p53 is a tumor suppressor whose transcription can be repressed by c-Jun. Scale bar represents 20 μM.
Identification of target genes downstream of MKK4
To shed light into the molecular mechanism by which MKK4/JNK/c-Jun promote tumorigenesis, we examined the effect of the loss of MKK4 on epidermal growth factor receptor (EGFR), a known target of c-Jun and an essential contributor of skin tumorigenesis (23). We found that MKK4 was required for increased EGFR expression in the skin of animals treated with DMBA/TPA (Fig. 5). Increased level of EGFR was observed in the cytoplasmic membrane of keratinocytes present in the wild type epidermis of treated mice. Consequently, the requirement of MKK4 to increase EGFR expression via c-Jun constitutes one mechanism by which MKK4 initiates the formation of tumors downstream of Ras. Additionally, skin biopsies were immunostained with a specific antibody to p53, since p53 is a tumor suppressor whose transcription can be repressed by JNK signaling (24, 25) (Fig. 5). We find that the treatment of wild type mice with DMBA/TPA reduced the expression of p53 in the epidermis. In contrast, the level of p53 in MKK4-deficient keratinocytes remained high, particularly in cells localized at the edge of the upper layer of the epidermis, indicating that MKK4 is required to prevent increased p53 expression following oncogenic Ras activation (Fig. 5).
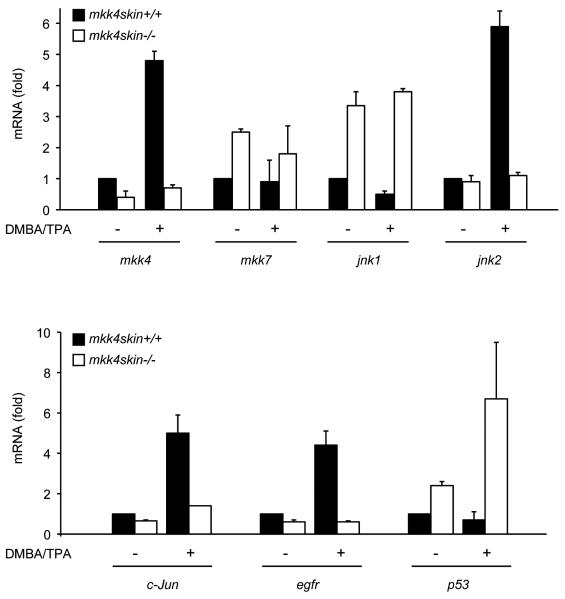
The changes observed using immunohistochemistry were confirmed by real-time PCR experiments (Fig. 6). The result showed a significant increase of mkk4 which correlated with elevated level of jnk2, c-Jun and egfr transcripts in treated compared with untreated wild type skin. In contrast, DMBA/TPA treatment decreased the amount of jnk1 and p53 mRNA. These regulations were abolished in the absence of MKK4. Furthermore, the loss of MKK4 caused a relatively moderate but statistically significant (P < 0.05, paired t test) decrease in the normal level of c-jun and egfr (Fig. 6), suggesting that MKK4 is also required for c-Jun and EGFR protein expression under basal conditions. This is in agreement with the low level of c-Jun and EGFR detected in mkk4−/− skin treated with DMBA/TPA (Fig. 5). In addition, mkk4−/− skin displayed a high level of p53 which was greatly increased upon stimulation (Fig. 6). This result supports the immunohistochemical evidence that MKK4 is required to downregulate p53 expression under both basal and stimulated conditions (Fig. 5). The amount of mkk7 and jnk1 was also significantly increased following the loss of MKK4 (Fig. 6). However this was not reflected in protein levels (Fig. 4).
Figure 6.
Identification of downstream targets of MKK4. Total RNA was extracted from both DMBA/TPA treated and untreated wild type and mutant epidermises and the amounts of various transcripts were measured by quantitative real-time PCR. The data expressed as fold relative to the mRNA extracted from wild type untreated skin correspond to the mean ± SD of two independent experiments performed in duplicate.
Discussion
Our results show that MKK4 has a pro-oncogenic function in the skin. This contrasts with previous studies that have supported a role for MKK4 as a tumor suppressor (14). These previous conclusions were based on the identification of the functional loss of MKK4 in a small percentage (~3 to 5%) of human cancer cell lines derived from tumors of various origin including, pancreas, lung, breast, colon, prostate, ovary and testis (14, 26). In addition, a reduction in the level of expression of MKK4 was observed in prostate and ovarian metastatic tissues, compared to normal prostate and ovarian epithelial cells, thereby suggesting that MKK4 has a metastatic suppressive role (27-29). This was further supported by evidence that prostate and ovarian cancer cell lines displaying a low level of MKK4 exhibited increased metastatic potential (27, 29). Consistently, a link between the loss of MKK4 and the more advanced stages of cancer progression was demonstrated by the clinical finding that the lack of expression of MKK4 in pancreatic cancer correlated with shorter survival times (30). However, the demonstration that mice injected with the pancreatic cancer cell line PL5 featuring the targeted disruption of the mkk4 gene had very few lung metastases compared to those injected with the parental or mkk4+/− cells provided a contrasting view (13). It was also reported that the ectopic expression of MKK4 in breast and pancreatic cancer cell lines lacking endogenous MKK4 stimulated cell proliferation and invasion (12). A pro-oncogenic function of MKK4 has been further supported by the analysis of gastric cancers and laryngeal squamous cell carcinomas. Patients with MKK4 present in tumor tissues had a significantly shorter survival compared to patients with reduced MKK4 expression (31, 32). Overall, the inconsistent findings of these studies may reflect the context-dependent complexity of MKK4 signaling and illustrate the difficulty to draw conclusions from the analysis of human cancers considering their heterogeneity, even within a given tissue, in terms of genetic background and histological subtype. A more precise understanding of the role of MKK4 in cancer will be acquired from studies like ours, based on cancer model that occurs in situ in mice where these variables can be controlled. This knowledge will lead to novel ideas and hypothesis that can be further tested in the human population for logical consistency.
The mechanism by which MKK4 promotes tumorigenesis in the skin appears to be mediated via JNK. This is based on evidence that the targeted deletion of the mkk4 gene in the skin prevents increased JNK phosphorylation following DMBA/TPA treatment. This defect does not affect the rate of cell death in contrast to expectations as one major function of the JNK signaling pathway is to promote apoptosis (33). The physiological significance of this result in cancer biology was provided by a study showing that JNK deficiency decreases the rate of apoptosis associated with Ras-stimulated transformation, thereby promoting the formation of lung tumors in a model of tumor metastasis (7). However, other studies have suggested an essential role for JNK in tumor development (34). In particular, JNK has been shown to potentiate B-cell lymphomas caused by Bcr-Abl and to accelerate tumorigenesis in a model of colorectal carcinogenesis (10, 35). The requirement of JNK for the survival of transformed B lymphoblasts is likely to contribute to BCR-Abl-induced cell transformation (10), while tumor cell proliferation was significantly increased by augmented JNK signaling in the gut (35). Overall, the ability of JNK to promote apoptosis, survival or proliferation depending on the tissue origin of the tumor provides an explanation into the context-dependent complexity of the function of MKK4 signaling in cancers.
Consistent with the demonstration that JNK1 acts as a suppressor, while JNK2 is a promoter of skin tumor (8, 9), we found that expression of JNK2 is induced, while that of JNK1 is decreased, in wild type skin treated with DMBA/TPA. Gene expression arrays using jnk1−/− and jnk2−/− mouse embryonic cells were performed in an attempt to uncover the molecular events that distinguish the functions of JNK isoforms in vivo (36). In agreement with their opposing functions in skin tumor development, the loss of JNK1 enhanced the expression of anti-apoptotic genes, while cells lacking JNK2 display increased expression of genes related to tumor suppression and induction of cell differentiation, apoptosis, or cell growth. However, the mechanism underlying the specificity of the transcriptional regulation networks downstream of JNK1 and JNK2 is not understood because so far there is no evidence that JNK1 and JNK2 can regulate by phosphorylation different sets of transcription factors. Indeed, one study showed that, in contrast to JNK1, JNK2 is a negative regulator of c-Jun function (37). But this conclusion has since been challenged by evidence that chemical inhibition of JNK2 in cells lacking JNK1 potently inhibited c-Jun phosphorylation (38). Jaeschke et al. proposed that increased expression of c-Jun in jnk2−/− cells which has led to the conclusion that JNK2 is a negative regulator of c-Jun (37), may be a consequence of compensatory adaptations in jnk2−/− cells that include increased JNK1 expression (38). In our system, we discovered that reduced JNK2 expression caused by the loss of MKK4 correlated with decreased expression and phosphorylation of c-Jun. The inability of JNK1 to compensate for a defective JNK2 in mkk4skin−/− animals can be explained by the observation that DMBA/TPA treatment inhibits JNK1 expression.
The significance of impaired c-Jun phosphorylation in the epidermis lacking MKK4 is demonstrated by altered expression of EGFR, a known target of c-Jun (39). Genetic studies have provided evidence that a functional c-Jun/EGFR pathway is required for skin tumor formation induced by expression of the K5-SOS-F transgene (23). The ability of EGFR to prevent cell differentiation, thereby maintaining keratinocytes in a proliferative state, is one mechanism by which c-Jun contributes to tumor formation upon Ras activation (23). This is consistent with the demonstration that c-Jun is not essential in vivo for the proliferation of keratinocytes under both normal and stress conditions (23). This contrasts with the proliferative defect displayed by the mkk4−/− epidermis treated with TPA. Therefore, although increased expression of EGFR expression via c-Jun is important, it is unlikely to be the only mechanism by which MKK4 promotes cell proliferation and initiates the formation of tumors downstream of Ras. This is further supported by the evidence that the phenotypic defect displayed by the loss of MKK4 in skin tumor development is more severe than that caused by the deletion of c-jun (23). Decreased p53 stability via JNK (25) may be the other important regulatory mechanism implicated in mediating the pro-oncogenic effect of MKK4 independently of c-Jun. This is demonstrated by our finding that both treated and untreated MKK4-deficient epidermises display a high level of p53. In addition, this result led to the hypothesis that MKK4 may be involved in malignancy by down-regulating p53. This model is supported by evidence that the loss of p53 enhances malignant progression of chemically induced skin tumors (40). Together, these observations highlight the need for future studies aimed at determining the role of MKK4 in the malignant progression of skin tumors. This work will be crucial for further validating MKK4 as a valuable drug target for cancer therapy.
Supplementary Material
Acknowledgments
We are indebted to D. Metzger (Institut de Génétique et de Biologie Moléculaire et Cellulaire, CNRS/INSERM/Université Louis Pasteur, Illkirch, France) for kindly providing us with the K14-CreERT2 transgenic mice. We thank D James and W Breitwieser (The Paterson Institute for Cancer Research, University of Manchester, UK) for helpful advice regarding tamoxifen injection and two-stage carcinogenesis protocol, A. Whitmarsh (University of Manchester, UK) for critically reviewing the manuscript, and the technicians at the animal facility for looking after the mice.
This work was supported by a grant from the Wellcome Trust
References
- 1.Wang X, Destrument A, Tournier C. Physiological roles of MKK4 and MKK7: insights from animal models. Biochim Biophys Acta. 2006;1773:1349–57. doi: 10.1016/j.bbamcr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Nadarajah B, Robinson AC, et al. Mitogen-activated protein kinase kinase 4 is an essential activator of the c-Jun N-terminal protein kinase during brain development. Mol Cell Biol. 2007;27:7935–46. doi: 10.1128/MCB.00226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branco D, Tanaka N, Jaeschke A, et al. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 2003;17:1969–78. doi: 10.1101/gad.1107303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- 5.Vogt PK. Jun, the oncoprotein. Oncogene. 2001;20:2365–77. doi: 10.1038/sj.onc.1204443. [DOI] [PubMed] [Google Scholar]
- 6.Behrens A, Jochum W, Sibilia M, Wagner EF. Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene. 2000;19:2657–63. doi: 10.1038/sj.onc.1203603. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy NJ, Sluss HK, Jones SN, Bar-Sagi D, Flavell RA, Davis RJ. Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev. 2003;17:629–37. doi: 10.1101/gad.1062903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N, Nomura M, She QB, et al. Suppression of skin tumorigenesis in c-Jun NH(2)-terminal kinase-2-deficient mice. Cancer Res. 2001;61:3908–12. [PubMed] [Google Scholar]
- 9.She QB, Chen N, Bode AM, Flavell RA. Dong Z Deficiency of c-Jun-NH(2)-terminal kinase-1 in mice enhances skin tumor development by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 2002;62:1343–48. [PubMed] [Google Scholar]
- 10.Hess P, Pihan G, Sawyers CL, Flavell RA, Davis RJ. Survival signaling mediated by c-Jun NH(2)-terminal kinase in transformed B lymphoblasts. Nat Genet. 2002;32:201–5. doi: 10.1038/ng946. [DOI] [PubMed] [Google Scholar]
- 11.Su GH, Hilgers W, Shekher MC, et al. Alterations in pancreatic, biliary, and breast carcinomas support MKK4 as a genetically targeted tumor suppressor gene. Cancer Res. 1998;58:2339–42. [PubMed] [Google Scholar]
- 12.Wang L, Pan Y, Dai JL. Evidence of MKK4 pro-oncogenic activity in breast and pancreatic tumors. Oncogene. 2004;23:5978–85. doi: 10.1038/sj.onc.1207802. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham SC, Gallmeier E, Hucl T, et al. Targeted deletion of MKK4 in cancer cells: a detrimental phenotype manifests as decreased experimental metastasis and suggests a counterweight to the evolution of tumor-suppressor loss. Cancer Res. 2006;66:5560–4. doi: 10.1158/0008-5472.CAN-06-0555. [DOI] [PubMed] [Google Scholar]
- 14.Whitmarsh AJ, Davis RJ. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26:3172–84. doi: 10.1038/sj.onc.1210410. 1. [DOI] [PubMed] [Google Scholar]
- 15.Frame S, Balmain A. Integration of positive and negative growth signals during ras pathway activation in vivo. Curr Opin Genet Dev. 2000;10:106–13. doi: 10.1016/s0959-437x(99)00052-0. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SV, Gajowniczek P, Way IP, et al. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10:425–35. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indra AK, Li M, Brocard J, et al. Targeted somatic mutagenesis in mouse epidermis. Horm Res. 2000;54:296–300. doi: 10.1159/000053275. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman MH. Morphological stages of postimplantation embryonic development. In: Copp AJ, Cockroft DL, editors. Postimplantation mammalian embryos: A Practical Approach. IRL Press; Oxford: 1990. pp. 81–91. [Google Scholar]
- 19.Kayahara M, Wang X, Tournier C. Selective regulation of c-jun gene expression by the mitogen-activated protein kinases via the TPA-responsive element and the myocyte enhancer factor 2 binding sites. Mol Cell Biol. 2005;25:3784–92. doi: 10.1128/MCB.25.9.3784-3792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–92. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- 21.Chakravarti D, Pelling JC, Cavalieri EL, Rogan EG. Relating aromatic hydrocarbon-induced DNA adducts and c-H-ras mutations in mouse skin papillomas: the role of apurinic sites. Proc Natl Acad Sci U S A. 1995;92:10422–6. doi: 10.1073/pnas.92.22.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angel P, Hattori K, Smeal T, Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988;55:875–85. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 23.Zenz R, Scheuch H, Martin P, et al. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell. 2003;4:879–89. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber M, Kolbus A, Piu F, et al. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–19. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs SY, Adler V, Thomas B, et al. JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 1998;12:2658–63. doi: 10.1101/gad.12.17.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng DH, Perry WL, III, Hogan JK, et al. Human mitogen-activated protein kinase 4 as a candidate tumor suppressor. Cancer Res. 1997;57:4177–82. [PubMed] [Google Scholar]
- 27.Yoshida BA, Dubauskas Z, Chekmareva MA, Christiano TR, Stadler WM, Rinker-Schaeffer CW. Mitogen-activated protein kinase kinase 4/stress-activated protein/ERK kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res. 1999;59:5483–87. [PubMed] [Google Scholar]
- 28.Kim HL, Vander Griend DJ, Yang X, et al. Mitogen-activated protein kinase kinase 4 metastasis suppressor gene expression is inversely related to histological pattern in advancing human prostatic cancers. Cancer Res. 2001;61:2833–37. [PubMed] [Google Scholar]
- 29.Yamada SD, Hickson JA, Hrobowski Y, et al. Mitogen-activated protein kinase kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res. 2002;62:6717–23. [PubMed] [Google Scholar]
- 30.Xin W, Yun KJ, Ricci F, et al. MAP2K4/MKK4 expression in pancreatic cancer: genetic validation of immunohistochemistry and relationship to disease course. Clin Cancer Res. 2004;10:8516–20. doi: 10.1158/1078-0432.CCR-04-0885. [DOI] [PubMed] [Google Scholar]
- 31.Wu CW, Li AF, Chi CW, et al. Human gastric cancer kinase profile and prognostic significance of MKK4 kinase. Am J Pathol. 2000;156:2007–15. doi: 10.1016/s0002-9440(10)65073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Huang K, Wang C, et al. Overexpression of mitogen-activated protein kinase kinase 4 and nuclear factor-kappaB in laryngeal squamous cell carcinoma: a potential indicator for poor prognosis. Oncol Rep. 2009;22:89–95. [PubMed] [Google Scholar]
- 33.Tournier C, Hess P, Yang DD, et al. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–4. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy NJ, Davis RJ. Role of JNK in tumor development. Cell Cycle. 2003;2:199–201. [PubMed] [Google Scholar]
- 35.Sancho R, Nateri AS, de Vinuesa AG, et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009;28:1843–54. doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen N, She QB, Bode AM, Dong Z. Differential gene expression profiles of Jnk1- and Jnk2-deficient murine fibroblast cells. Cancer Res. 2002;62:1300–4. [PubMed] [Google Scholar]
- 37.Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol Cell. 2004;15:713–25. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 38.Jaeschke A, Karasarides M, Ventura JJ, et al. JNK2 is a positive regulator of the cJun transcription factor. Mol Cell. 2006;23:899–911. doi: 10.1016/j.molcel.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Zenz R, Wagner EF. Jun signalling in the epidermis: From developmental defects to psoriasis and skin tumors. Int J Biochem Cell Biol. 2006;38:1043–9. doi: 10.1016/j.biocel.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Kemp CJ, Donehower LA, Bradley A, Balmain A. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell. 1993;74:813–22. doi: 10.1016/0092-8674(93)90461-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.