Abstract
Background:
To evaluate whether a fermented dairy drink containing the probiotic strain Lactobacillus casei DN-114 001 could reduce the incidence of common infectious diseases (CIDs) and the change of behavior because of illness in children.
Subjects/Methods:
We conducted a double-blinded, randomized, placebo-controlled allocation concealment clinical trial in the Washington, DC metropolitan area. Participants were 638 children 3–6 years old in daycare/schools. The intervention was a fermented dairy drink containing a specific probiotic strain or matching placebo with no live cultures for 90 consecutive days. Two primary outcomes were assessed: incidence of CIDs and change of behavior because of illness (both assessed by parental report).
Results:
The rate of change of behavior because of illness was similar among active and control groups. However, the incidence rate for CIDs in the active group (0.0782) is 19% lower than that of the control group (0.0986) (incidence rate ratio=0.81, 95% CI: 0.65, 099) P=0.046.
Conclusions:
Daily intake of a fermented dairy drink containing the probiotic strain L. casei DN-114 001 showed some promise in reducing overall incidence of illness, but was primarily driven by gastrointestinal infections and there were no differences in change of behavior.
Keywords: probiotics, clinical trial, infections, functional foods
Introduction
Common infectious diseases (CIDs) cause discomfort to individuals and result in economic losses because of missed days from work, seeking of medical care, and medication costs (Feeney et al., 1998; Greenberg, 2002). Daycare centers and schools are ideal places for the transmission of respiratory infections as well as childhood diarrhea, often resulting in many missed days of both daycare and parental work (Fleming et al., 1987; Cordell et al., 1997; Dales et al., 2004). Illnesses related to daycare centers have been estimated to cost $1.8 billion per year in the United States (Haskins, 1989). Children in daycare centers have shown to have more outpatient doctor visits, emergency room visits, and increased usage of prescription medicines than children not in daycare (Silverstein et al., 2003).
Probiotics are live microorganisms, which when administered in adequate amounts confer a health benefit (FAO/WHO, 2001). In the past few years, scientific and commercial interest in probiotics has grown rapidly as these microorganisms have shown potential benefits, primarily in prevention, in health conditions such as diarrhea, necrotizing enterocolitis, and allergies (Vanderhoof et al., 1999; Kalliomaki et al., 2001; Rosenfeldt et al., 2002; Mastrandrea et al., 2004; Lin et al., 2005, 2009).
However, there is need for controlled clinical studies evaluating the health benefits of probiotic foods containing well-defined probiotic strains. The effects of one product are not extrapolative of another, and may also depend on the amount ingested and the pattern of consumption. In addition, the outcomes measured in probiotic trials need to properly reflect the outcomes of interested individuals consuming the probiotics, if the results are going to impact public health (Tunis et al., 2003; Glasgow et al., 2005).
Our overall aim was to study health benefits of a well-characterized probiotic food that is readily available to consumers—DanActive (also referred to as Actimel), a probiotic dairy drink, available in grocery stores in many countries worldwide. Earlier published clinical trials have found this product to decrease incidence and duration of diarrhea and allergic rhinitis in infants and children (Pedone et al., 1999, 2000; Agarwal and Bhasin, 2002; Giovannini et al., 2007). Furthermore, clinical studies conducted on DanActive to assess the survival of the Lactobacillus (L.) casei through the digestive tract have shown high survivable numbers from the stools of subjects consuming the product (Guerin-Danan et al., 1998; Oozeer et al., 2002, 2006; Rochet et al., 2008). Survival of probiotic strains through intestinal transit is considered an important biomarker for potential functionality in the intestinal tract.
The objective of this study was to investigate the beneficial effects of this probiotic fermented product on common infections by conducting a large-scale study on children attending daycare/schools. The outcomes of this clinical study are patient-oriented, not surrogate, end points (Fleming and DeMets, 1996; Shaughnessy and Slawson, 1997, 2003). We hypothesized that, because of high levels of the probiotic in the dairy drink, children in daycare/school who received the active drink would have reduced overall illness and thus reduced changes in activity because of illness as assessed by their parents.
Materials and methods
Study design
A double-blinded, randomized, placebo-controlled, patient-oriented trial was conducted. Participants consumed one active or control drink for 90 consecutive days and were followed weekly during consumption. The Georgetown University IRB, in Washington, DC, approved all aspects of the trial and participants' parents signed informed consent. An independent Data and Safety Monitoring Board met and reviewed data at 25, 50, and 75% completion and reviewed all adverse events (AEs).
In addition to weekly assessments conducted by phone interviews between clinical coordinators and parents, parents were provided with daily calendars (diary). Diary data provided secondary assessment. All data were double entered into a Microsoft Access database developed by the independent Data Management Coordinating Center.
Participants
Healthy children between the age of 3 and 6 years attending daycare center/school 5 days a week in Washington, DC area were recruited into the study. Exclusion criteria were taking any regular medicines at initiation of study, lactose intolerance, allergy to strawberry, inability of a parent to speak English or Spanish, active respiratory or gastrointestinal infection, or chronic disease. Participants were also excluded for consuming other probiotic foods or supplements.
Randomization
The randomization scheme was generated using SAS software by data managers, who had no participant contact. Households were randomized in a 1:1 ratio to receive either the active or control drink, using a block size of 12. Children in the same household were assigned to the same drink group. Once eligibility criteria were met, the participant was randomized to one of the two groups, study identification was generated and a number from 0 to 9 was assigned. Participants were enrolled by research assistants.
Interventions
The active drink was strawberry-flavored DanActive, a fermented probiotic dairy drink, which has been commercially available since 1994 in Europe, under the commercial name ‘Actimel' and is currently available in the US market. The drink contains the probiotic strain L. casei DN-114 001/CNCM I-1518 (also named Lactobacillus paracasei subsp. paracasei after the current nomenclature) combined with two cultures commonly used in yogurt, Streptococcus thermophilus and Lactobacillus bulgaricus. As the purpose of this study was to evaluate the effects of this specific product as a whole, the control product used was a sweetened, flavored non-fermented acidified dairy drink. Subjects were allocated one bottle per day of either drink. Microbiological content was verified by an independent laboratory, The National Food Laboratory, Inc. (Dublin, CA, USA). The microbiological composition of the active drink at the end of shelf life met targets of 1 × 108 cfu/g of L. casei DN-114 001. The yogurt starter, symbiotic cultures, S. thermophilus, and L. bulgaricus were also present in the final product at levels >107 cfu/g. The Placebo was a sweetened, flavored non-fermented acidified dairy drink without the active components of the tested product. See Table 1 for nutritional content.
Table 1. Nutritional facts for both drinks.
| Cholesterol (mg) | 10.18 |
| Calcium (mg) | 200 |
| Potassium (mg) | 266 |
| Sodium (mg) | 85 |
| Total fat (g) | 3 |
| Protein (g) | 6 |
| Total carbohydrates (g) | 27 |
| Total added sugars (g) | 19 |
| Calories kcals/200 g | 161 |
Participants consumed 200 ml/day.
Nutritional facts are per 200 ml.
Blinding
Through masking and use of 10 different numbers, 0 through 9, it was impossible for research personnel to adjust randomization or deduce what group participants were assigned. In addition, parents were told that the trial was investigating a probiotic drink, but they were never alerted to the actual product. The appearance, taste, nutritional composition (proteins, carbohydrates, lipids, and energy), and packaging (200 g bottles) of the active and control products were identical to ensure that neither subjects, their parents, nor researchers knew the identity of the study samples. All of these measures led to successful true allocation concealment and proper blinding.
Outcome measures
The study was designed for two primary outcomes: (1) the change of behavior because of illness as assessed by parents and (2) the rate of CIDs. To assess behavior changes, parents were specifically asked, ‘In the past week, has your child had an illness that resulted in change in activity, such as missed school, birthday party, soccer game, etc.? ‘Change in activity' refers to all activities, not just structured ones.' All assessments were performed by parents to be consistent with a community trial of a commercial product.
CID was categorized based on the reported health-related symptoms that parents relayed on a weekly basis to the research personnel. CID was separated into three categories of infections, a priori to review of data: upper respiratory tract infections, which included ear infections, sinusitis, streptococcal pharyngitis, non-strep pharyngitis, nasal discharge, and laryngitis; lower respiratory tract infections, which included pneumonia, influenza, coughs, and breathing problems; and gastrointestinal tract infections (GITI), which included gastroenteritis, diarrhea, nausea, and vomiting. Diarrhea was not clinically defined, but parent reported. The overall CID at each follow-up visit (with period covering 1 week) could be ⩽3.
Secondary outcomes included absences from daycare or school because of illness and missed parental work because of child being ill, days with diarrhea, vomiting, stomach pain, constipation, runny nose, cough, decreasing appetite, fever, medication usage, or rash. AEs were collected on the weekly follow-up or parents had a 24-h phone number to call and report any AEs. All AEs were also included in illness reports. AEs were determined by the parent or their personal physician if they believed that any event was potentially related to the drink. Serious AEs were defined as any incidences of death, life-threatening event, hospitalization, prolongation of hospital stay, or event resulting in permanent disability.
Statistical analysis
The estimates used to conduct the sample size calculations were obtained from two earlier probiotic studies and a 90-day trial was selected based on rate of earlier reported infections. In addition, we choose 3 months over the colder period of the year because of an increase in respiratory illness (Hatakka et al., 2001; Weizman et al., 2005). The sample size calculations considered in this study are based on a cluster sampling approach that accounts for multiple children enrolled into the study from the same household. Children in the same household are not independent; hence, the power calculations and the analysis were adjusted for the loss of power because of randomizing by these clusters. To calculate the adjusted sample size, we used an estimate of 0.1 as the intra-class correlation between household, the design effect was 1.05, and the adjusted sample size required was 638 accounting for 20% effect size and a 20% dropout. The number of households required to provide this sample was around 426 with the assumption that average household size is 1.5. This sample size was based on setting statistical significance at 0.05 and 80% power. Missing data were replaced by the Last Value Carried Forward method using the last post-baseline value for one subject at the earlier time when appropriate.
Statisticians masked to the group allocation conducted statistical analyses. Furthermore, all research personnel were masked while examining initial data. Baseline demographics were compared between the groups using independent t-test for continuous variables and χ2 test for categorical variables. All analyses of primary and secondary analyses of outcomes were conducted on an intention-to-treat basis. Incidence rate ratios were calculated using the number of events divided by number of days in study. The mixed model was used to adjust for clustered observations from the same household. For binary outcomes, the generalized non-linear mixed model was used; and for continuous outcomes, the generalized linear mixed model was used. Adjustments were made based on age and number of drinks consumed. A P-value of <0.05 was considered to indicate statistical significance. All P-values were two sided. SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and Intercooled Stata 9.2 for Windows (StataCorp LP, College Station, TX, USA) were used to run the analyses.
Results
Recruitment, enrollment, and participant flow
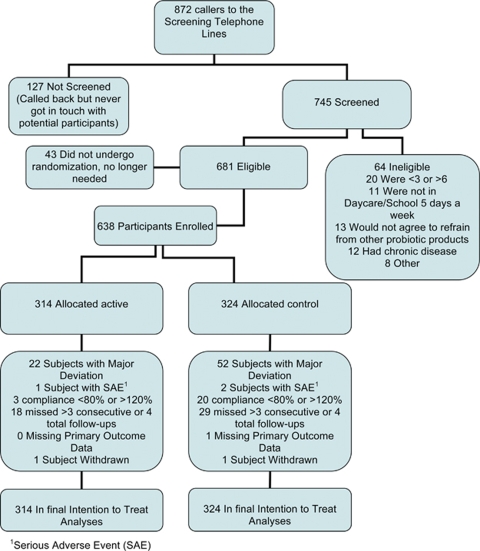
During the 5 months of enrollment from 27 September 2006 until 22 February 2007, 872 messages were left on the recruitment line (Figure 1). Eligibility could not be assessed for 127 families because of unsuccessful attempts to contact families. Thus, 745 families were assessed for eligibility, with 64 not meeting inclusion criteria. Six hundred thirty-eight participants were enrolled from these callers, or 73% of the original families who left messages on the recruitment line, 314 were allocated into the active participant group, and 324 into the control group with 250 families in both groups (Figure 1).
Figure 1.
Participant flow chart.
Baseline demographics
There were no major differences with respect to any of the baseline characteristics between the two groups (Table 2). Both groups had 250 families; the active group had 193 families with 1 child, 51 with 2 children, and 6 with 3 or more children; the control group had 184 families with 1 child, 59 with 2 children, and 7 with 3 or more children. The majority of the children spent at least 30 h per week in daycare/school at baseline. On a 10-point Likert scale ranking overall health, both groups were reported as 9.2 (10=extremely healthy, rated by the parents). As in most clinical trials, the majority of participants were white with high family incomes, but the study population also consisted of 18% Hispanics (participant's parents classified race and ethnicity), and 20% of families had annual incomes <$30 000. In addition, 17% of the interviews were conducted in Spanish (data not shown).
Table 2. Participant demographics.
| Active N (%) | Control N (%) | |
|---|---|---|
| Number of subjects | 314 | 324 |
| Gender | ||
| Male | 157 (50.0%) | 172 (53.1%) |
| Female | 157 (50.0%) | 152 (46.9%) |
| Age (years) | ||
| Mean (s.d.) | 4.86 (+1.12) | 4.94 (+1.13) |
| Hours/week in school | ||
| <15 h | 6 (1.9%) | 5 (1.5%) |
| 15–20 h | 33 (10.5%) | 44 (13.6%) |
| 21–30 h | 62 (19.7%) | 47 (14.5%) |
| 31–40 h | 143 (45.5%) | 159 (49.1%) |
| >40 h | 70 (22.3%) | 69 (21.3%) |
| Race (biological mother) | ||
| Asian (M) | 26 (8.3%) | 18 (5.6%) |
| American Indian or Alaska Native (M) | 8 (2.5%) | 15 (4.6%) |
| Black or African American (M) | 70 (22.3%) | 71 (21.9%) |
| Native Hawaiian or other Pacific Islander (M) | 1 (0.3%) | 2 (0.6%) |
| White (M) | 192 (61.1%) | 195 (60.2%) |
| Other (M) | 16 (5.1%) | 20 (6.2%) |
| Race (biological father) | ||
| Asian (F) | 26 (8.3%) | 11 (3.4%) |
| American Indian or Alaska Native (F) | 6 (1.9%) | 18 (5.6%) |
| Black or African American (F) | 67 (21.3%) | 70 (21.6%) |
| Native Hawaiian or other Pacific Islander (F) | 3 (1.0%) | 2 (0.6%) |
| White (F) | 174 (55.4%) | 194 (59.9%) |
| Other (F) | 30 (9.6%) | 27 (8.3%) |
| Ethnicity (biological mother) | ||
| Hispanic or Latino (M) | 52 (17.1%) | 51 (15.8%) |
| Not hispanic or Latino (M) | 252 (82.9%) | 272 (84.2%) |
| Ethnicity (biological father) | ||
| Hispanic or Latino (F) | 55 (18.3%) | 59 (18.3%) |
| Not hispanic or Latino F) | 245 (81.7%) | 263 (81.7%) |
| Overall | 300 | 322 |
| Health insurance | ||
| No | 8 (2.5%) | 12 (3.7%) |
| Yes | 306 (97.5%) | 312 (96.3%) |
| Income | ||
| <$15 000 | 9 (3.8%) | 22 (9.0%) |
| $15 000–$30 000 | 34 (14.2%) | 29 (11.9%) |
| $30 001–$50 000 | 26 (10.9%) | 23 (9.4%) |
| $50 001–$75 000 | 29 (12.1%) | 27 (11.1%) |
| $75 001–$100 000 | 46 (19.2%) | 36 (14.8%) |
| >$100 000 | 95 (39.7%) | 107 (43.9%) |
| Overall healtha | ||
| Mean (s.d.) | 9.2 (+0.9) | 9.2 (+0.9) |
Abbreviation: s.d., standard deviation.
On a scale of 1–10, how would you rate your child's overall health in the past week? 1=very unhealthy, 10=extremely healthy.
Compliance
The number of drinks consumed per week differed between the active (6.5 drinks per week) and control (6.1 drinks per week) groups (P=0.004). However, the difference in compliance between those who predicted correctly what drink their child had consumed and those who predicted incorrectly in the active group was not significant (P=0.321). Similarly, the difference in compliance between those who predicted correctly and those who predicted incorrectly in the control group was not significant (P=0.967).
Primary outcomes
As discussed in Materials and methods section, the study was a priori arranged for two primary outcomes (Table 3). The primary outcome, rate of days with change in activity because of illness per 100 person day, was similar in the active and control groups (active=2.30, control=2.27, P=0.91). However, the incidence rate for CIDs in the active group (0.0782) was 19% lower than in the control group (0.0986), representing a statistically significant difference in the active group (incidence rate ratio=0.81, 95% CI: 0.65, 0.99 P=0.046). Further subdivision by the three components of CID (upper respiratory tract infections, lower respiratory tract infections, GITI) showed the primary cause of lower overall incidence of GITI infections followed by upper respiratory tract infections. The incidence rate for GITI in the active group (0.012) was 24% lower than in the control group (0.016), (incidence rate ratio=0.76, 95% CI: 0.58, 0.99, P=0.042). The incidence rate for upper respiratory tract infections in the active group was 0.027, which was 18% lower than in the control group (0.033) (incidence rate ratio=0.82, 95% CI: 0.68, 0.99, P=0.036). The incidence rate for lower respiratory tract infections in the active group was 0.027, which is 2% lower than in the control group (0.028) (incidence rate ratio=0.98, 95% CI: 0.82, 1.18, P=0.829). Although we cluster randomized by households, we did not randomize per daycare/school as participants were from 358 different daycare/schools. However, analysis showed no differences in primary outcomes per daycare (data not shown).
Table 3. Primary and secondary outcomes.
| Active Least squares mean (s.e.) | Control Least squares mean (s.e.) | P-value | |
|---|---|---|---|
| Primary outcomes | |||
| Incidence rate of CID per 100 person daya | 7.8 (0.4) | 9.8 (0.7) | 0.046 |
| Rate of days with change in activity because of illness per 100 person dayb | 2.30 (0.21) | 2.27 (0.21) | 0.91 |
| Secondary outcomes | |||
| Incidence rate of GITI per 100 person daya | 1.2 (0.10) | 1.6 (0.15) | 0.042 |
| Incidence rate of URTI per 100 person daya | 2.7 (0.18) | 3.3 (0.20) | 0.036 |
| Incidence rate of LRTI per 100 person daya | 2.7 (0.16) | 2.8 (0.18) | 0.829 |
| Rate of vomiting per 100 person day | 0.55 (0.08) | 0.73 (0.08) | 0.10 |
| Rate of stomach pain per 100 person day | 1.23 (0.21) | 1.50 (0.21) | 0.36 |
| Rate of constipation per 100 person day | 0.81 (0.18) | 0.71 (0.18) | 0.68 |
| Rate of runny nose per 100 person day | 7.88 (0.63) | 8.65 (0.63) | 0.39 |
| Rate of cough per 100 person day | 9.18 (0.63) | 8.37 (0.63) | 0.36 |
| Rate of decreasing appetite per 100 person day | 2.58 (0.31) | 2.31 (0.31) | 0.54 |
| Rate of fever per 100 person day | 1.34 (0.14) | 1.34 (0.14) | 0.99 |
| Rate of rash per 100 person day | 1.17 (0.23) | 0.76 (0.23) | 0.21 |
Abbreviations: CID, common infectious disease; GITI, gastrointestinal tract infections; LRTI, lower respiratory tract infections; URTI, upper respiratory tract infections.
Incidence rate per 100 person day=incidence rate per day × 100.
Rate of days with change in activity because of illness=number of days with change in activity/number of days in the study.
Secondary outcomes
There were no statistically significant differences in any of the secondary outcomes shown in Table 3, such as participant missed daycare/school or parental missed work between the groups. However, some differences were observed with medication use. In the active group, the mean number of days a medicine used was 3.02 days versus 3.32 days in the control group (P<0.0001). Furthermore, there was also a significant statistical difference in antibiotics (N=58 in intervention and N=69 for control, P=0.002) and anti-inflammatory (N=77 in intervention and N=97 in control, P=0.03) drug usage in the control group compared with the active group, when used as a covariate in the primary analysis model. However, the absolute numbers are not large and we believe not clinically significant.
Additional analysis
In general, parents were more compliant with weekly follow-up phone calls than with maintaining the diary. In the active group, at days 30, 60, and 90, 84, 76, and 66% of parents completed diaries compared with 82, 71, and 57% in the control group, respectively. As expected, the parents who were more compliant with the diary were slightly more compliant ensuring that their children consumed the drinks. The consumption rate in the active group was a mean of 6.6 drinks per week among those who completed the diary, compared with 6.5 in the overall group, whereas control consumption rates were 6.3 among those who completed the diary compared with 6.1 overall. In addition, missed participants' days because of illness showed non-significant 24% difference in the active group (P=0.08) and 33% less parental missed work in the active group compared with control (P=0.22).
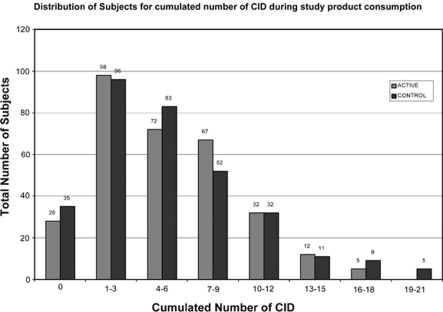
Figure 2 shows CIDs are differently distributed between groups, with more outliers in the control group. Per protocol analysis is not shown, but is similar for all outcomes. The difference in CID subcategories is still significant, when adjusting for multiple comparisons using Bonferroni's adjusted P-value of 0.025.
Figure 2.
Distribution of subjects for cumulated number of CID during the study product consumption.
Adverse events
Global safety in the study was excellent as expected for a study on a commercially available food using healthy subjects. In the active group, 18 subjects had at least one AE compared with 22 in the control group (Table 4). In addition, one subject in the active group had a serious AEs compared with two in the control group. The SAEs were all hospitalizations that resolved spontaneously and believed not related to study product. All participants recovered within days without any subsequent sequelae.
Table 4. Adverse events.
| Outcomes | Active N (%) | Control N (%) |
|---|---|---|
| 314 (100%) | 324 (100%) | |
| Number of subjects with at least one adverse event (AEs) | 18 (5.73%) | 22 (6.81%) |
| Diarrhea | 6 | 3 |
| Gas | 1 | 0 |
| Vomiting | 0 | 3 |
| Lack of appetite | 0 | 3 |
| Constipation | 2 | 2 |
| Hives | 1 | 0 |
| Rash | 7 | 10 |
| Other | 5 | 9 |
| Number of subjects with at least one serious adverse event (SAEs)a | 1 | 2 |
Active group SAE versus gastro-intestinal virus, participant evaluated in emergency room and discharged, and control included an asthma attack induced from pneumonia, and a viral infection requiring hospitalization because of high fever.
Discussion
Yogurt and yogurt-like fermented milks are well established and popular with children and parents. These products provide live cultures to the diet and nutrition in the form of protein, vitamins, and minerals. In addition, these products are relatively inexpensive, widely available, and easy to ingest. However, the value of the microbiological components of yogurts containing only traditional live cultures (S. thermophilus and L. bulgaricus) is generally limited to improving lactose digestion in lactose malabsorbers. These two active starter cultures do not survive intestinal transit in significant quantities and thus, have limited ability to positively impact intestinal health. This is the rationale behind many newer dairy products, which contain supplemental probiotic bacteria believed to survive the gastrointestinal tract. Unfortunately, validation of health effects of these products with meaningful patient-oriented, rather than surrogate, end points is often lacking.
Our aims in this study were to examine whether children who received a fermented milk containing the probiotic, L. casei strain DN-114 001, and two traditional yogurt starters would have reduced overall illness and have less change in activity because of illness, as reported by their parents, than the children receiving control product. Although, we have mixed results, to our knowledge this is the largest probiotic clinical trial conducted in the United States and provides much needed data. One of the primary and most of the secondary outcome measures were negative, and although some of our positive findings are driven by a few individuals, the reduction of GITI (24% lower than control) is a robust outcome consistent with earlier probiotic research on this product. There are many potential hypotheses as to why we had mixed results in our study. Perhaps, it was easier for parents to determine whether a child had a runny nose, ear pain, or other symptom, but less clear if a young child's activity level was changed. Possibly, symptoms were sufficiently obvious to report, but insufficient in severity to result in a canceled activity. In addition, most of the positive earlier research on this product has been with gastrointestinal symptoms (Pedone et al., 1999, 2000; Agarwal and Bhasin, 2002; Giovannini et al., 2007; Hickson et al., 2007). Our results are similar in that the drink has its greatest impact in reducing GITI; however, this may impact overall health, but not activity levels of young children. A recently published manuscript examining the same intervention found a significantly decreased duration of CID (P=0.009) in comparison with the control group in an elderly population (Guillemard et al., 2010). Earlier conducted preclinical studies provide hypotheses on mechanisms of action and relevant information about the biological plausibility of the observed clinical effects on CIDs in human studies conducted with our intervention (Djouzi et al., 1997; Guerin-Danan et al., 2001; Freitas et al., 2003; Ingrassia et al., 2005; Medici et al., 2005; Parassol et al., 2005; Tien et al., 2006; de Moreno de LeBlanc et al., 2008; Baba et al., 2009).
Similar to primary outcomes, secondary outcomes showed mixed results. The active group used statistically less medicine, but this was of questionable clinical significance as the absolute numbers were similar. In addition, there were no differences among groups in outcomes such as missed work or daycare/school. A study by Hatakka et al. examined the effects of the probiotic Lactobacillus rhamnosus GG on children in daycare centers. Children in the L. rhamnosus GG group showed a 16% decrease in absences from daycare compared with control, and a significantly lower incidence of respiratory infection (relative reduction 17%, absolute reduction 9%) (Hatakka et al., 2001). Weizman et al. also studied probiotics in daycare centers. They fed infants either Bifidobacterium lactis Bb-12, Lactobacillus reuteri ATCC55730, or control formula containing no added probiotic. The control group had more days of febrile illness, increased episodes of diarrhea, and increased absences from daycare than the groups on the probiotic-fortified formulas (Weizman et al., 2005). Our diary data did show participants in the active group had a statistically insignificant 24% decrease in daycare/school absences during the study period and parents had a statistically insignificant 33% less missed work in the active group compared with control during the 90-day trial. It is likely that the reason these major differences are not significant is due to the low number of completed diaries and a larger study may have found differences in activity levels. In addition, our study population was much more diverse than these two studies and many other more disease-oriented probiotic trials.
Our study has several limitations that need to be noted. We intentionally did not include independent examinations of children by physicians and instead relied on parental report. We studied a functional food, not a medicinal product; parents will thus feed their children without any physician input and we felt it was best to assess it under similar conditions. A limitation of this method is that some of these assessments are subjective and vary by evaluator. However, the large sample size and strict methodology should result in equal assessments per group. In addition, we enrolled generally healthy children from daycare/school settings. It is possible if our source population was not as healthy, we would have had different findings. Our overall illness rate was less than we anticipated from earlier literature and it was reported as a ‘mild' winter for illness in our recruitment area. Finally, compliance was measured by self-report. However, we analyzed through intention to treat, which is especially appropriate for a food product, which is unlikely to be consumed in real life everyday without missed servings.
Our randomized clinical trial shows that a fermented dairy drink with a characterized probiotic strain holds promise, but has limitations in promoting the health of children aged 3–6 years. The results of our clinical trial support the effectiveness of this product with an important patient-oriented outcome, CID, most specifically in gastrointestinal illness. It is important to recognize that this trial studied a specific probiotic strain, dose, and age group, and our findings cannot be extrapolated for other strains or outcomes. It is important that commercial products continue to be independently studied, important patient-oriented outcomes assessed, and subjected to high quality research techniques.
Acknowledgments
We thank the families who participated in this study; Jennifer Foley Zaccagino, the administrator of this grant; Greg Foster for creating and implementing the Data Management Program and his biostatistical assistance; Dr Helaine Resnick for her statistical input and early protocol development; Shaunaugh Browning and the GCRC staff; Colleen Varga, senior research assistant, and the research assistants, A Degbo, M Ellestad, J Foster, J Gonzalez, K Herbin-Smith, C Jackson, A McEwan, L Paster, R Sandler, D Simmons, I Williams; Li Lu for her statistical support; the Data Safety Monitoring Board, with Chair Dr Marie Diener-West and members Drs Beth Barnet, Charles Mouton, and Nicole Ugel; Cargo Transport, Inc. (Dulles, VA, USA), which provided shipping logistics and delivery of study product; and Bartson's Child's Play (Washington, DC, USA), which provided age-appropriate compensation for the participating children. This study was an investigator-initiated industry funded study by The Dannon Company, Inc. The non-industry authors developed the initial protocol, gathered, supervised double data entry, and analyzed the data, and vouch for the completeness and accuracy of the data and analysis. All research personnel and statisticians were blinded throughout the study, including initial review of all data. The industry authors provided technical expertise related to the protocol, clinical supplies, and logistics, made observations throughout the study, helped review blinded data, and participated in manuscript preparation. PI and non-sponsor authors have full legal ability to publish any findings.
Drs Davis, Niborski, and Tondu were employees of the Dannon Company during this trial. Dr Mary Ellen Sanders consults for numerous probiotic companies including the Dannon Company.
References
- Agarwal KN, Bhasin SK. Feasibility studies to control acute diarrhoea in children by feeding fermented milk preparations Actimel and Indian Dahi. Eur J Clin Nutr. 2002;56 (Suppl 4:S56–S59. doi: 10.1038/sj.ejcn.1601664. [DOI] [PubMed] [Google Scholar]
- Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. Selected commensal-related bacteria and toll-like receptor 3 agonist combinatorial codes synergistically induce interleukin-12 production by dendritic cells to trigger a T helper type 1 polarizing programme. Immunology. 2009;128:e523–e531. doi: 10.1111/j.1365-2567.2008.03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell RL, MacDonald JK, Solomon SL, Jackson LA, Boase J. Illnesses and absence due to illness among children attending child care facilities in Seattle-King County, Washington. Pediatrics. 1997;100:850–855. doi: 10.1542/peds.100.5.850. [DOI] [PubMed] [Google Scholar]
- Dales R, Cakmak S, Brand K, Judek S. Respiratory illness in children attending daycare. Pediatric Pulmonology. 2004;38:6409. doi: 10.1002/ppul.20034. [DOI] [PubMed] [Google Scholar]
- de Moreno de LeBlanc A, Chaves S, Carmuega E, Weill R, Antoine J, Perdigon G. Effect of long-term continuous consumption of fermented milk containing probiotic bacteria on mucosal immunity and the activity of peritoneal macrophages. Immunobiology. 2008;213:97–108. doi: 10.1016/j.imbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Djouzi Z, Andrieux C, Degivry MC, Bouley C, Szylit O. The association of yogurt starters with Lactobacillus casei DN 114.001 in fermented milk alters the composition and metabolism of intestinal microflora in germ-free rats and in human flora-associated rats. J Nutr. 1997;127:2260–2266. doi: 10.1093/jn/127.11.2260. [DOI] [PubMed] [Google Scholar]
- Feeney A, North F, Head J, Canner R, Marmot M. Socioeconomic and sex differentials in reason for sickness absence from the Whitehall II Study. Occup Environ Med. 1998;55:91–98. doi: 10.1136/oem.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming DW, Cochi SL, Hightower AW, Broome CV. Childhood upper respiratory tract infections: to what degree is incidence affected by day-care attendance. Pediatrics. 1987;79:55–60. [PubMed] [Google Scholar]
- Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled. Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations and World Health Organization 2001Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteriaReport of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Córdoba, Argentina, 1–4 October 2001.
- Freitas M, Tavan E, Cayuela C, Diop L, Sapin C, Trugnan G. Host-pathogens cross-talk. Indigenous bacteria and probiotics also play the game. Biol Cell. 2003;95:503–506. doi: 10.1016/j.biolcel.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, et al. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res. 2007;62:215–220. doi: 10.1203/PDR.0b013e3180a76d94. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Magid DJ, Beck A, Ritzwoller D, Estabrooks PA. Practical clinical trials for translating research to practice: design and measurement recommendations. Med Care. 2005;43:551–557. doi: 10.1097/01.mlr.0000163645.41407.09. [DOI] [PubMed] [Google Scholar]
- Greenberg SB. Respiratory viral infections in adults. Curr Opin Pulm Med. 2002;8:201–208. doi: 10.1097/00063198-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Guerin-Danan C, Chabanet C, Pedone C, Popot F, Vaissade P, Bouley C, et al. Milk fermented with yogurt cultures and Lactobacillus casei compared with yogurt and gelled milk: influence on intestinal microflora in healthy infants. Am J Clin Nutr. 1998;67:111–117. doi: 10.1093/ajcn/67.1.111. [DOI] [PubMed] [Google Scholar]
- Guerin-Danan C, Meslin JC, Chambard A, Charpilienne A, Relano P, Bouley C, et al. Food supplementation with milk fermented by Lactobacillus casei DN-114 001 protects suckling rats from rotavirus-associated diarrhea. J Nutr. 2001;131:111–117. doi: 10.1093/jn/131.1.111. [DOI] [PubMed] [Google Scholar]
- Guillemard E, Tondu F, Lacoin F, Schrezenmeir J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br J Nutr. 2010;103:58–68. doi: 10.1017/S0007114509991395. [DOI] [PubMed] [Google Scholar]
- Haskins R. Acute illness in day care: how much does it cost. Bull N Y Acad Med. 1989;65:319–343. [PMC free article] [PubMed] [Google Scholar]
- Hatakka K, Savilahti E, Ponka A, Meurman JH, Poussa T, Nase L, et al. Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322:1327. doi: 10.1136/bmj.322.7298.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson M, D'Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80. doi: 10.1136/bmj.39231.599815.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrassia I, Leplingard A, Darfeuille-Michaud A. Lactobacillus casei DN-114 001 inhibits the ability of adherent-invasive Escherichia coli isolated from Crohn's disease patients to adhere to and to invade intestinal epithelial cells. Appl Environ Microbiol. 2005;71:2880–2887. doi: 10.1128/AEM.71.6.2880-2887.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- Lin JS, Chiu YH, Lin NT, Chu CH, Huang KC, Liao KW, et al. Different effects of probiotic species/strains on infections in preschool children: a double-blind, randomized, controlled study. Vaccine. 2009;27:1073–1079. doi: 10.1016/j.vaccine.2008.11.114. [DOI] [PubMed] [Google Scholar]
- Mastrandrea F, Coradduzza G, Serio G, Minardi A, Manelli M, Ardito S, et al. Probiotics reduce the CD34+ hemopoietic precursor cell increased traffic in allergic subjects. Allerg Immunol (Paris) 2004;36:118–122. [PubMed] [Google Scholar]
- Medici M, Vinderola CG, Weill R, Perdigon G. Effect of fermented milk containing probiotic bacteria in the prevention of an enteroinvasive Escherichia coli infection in mice. J Dairy Res. 2005;72:243–249. doi: 10.1017/s0022029905000750. [DOI] [PubMed] [Google Scholar]
- Oozeer R, Goupil-Feuillerat N, Alpert CA, van de Guchte M, Anba J, Mengaud J, et al. Lactobacillus casei is able to survive and initiate protein synthesis during its transit in the digestive tract of human flora-associated mice. Appl Environ Microbiol. 2002;68:3570–3574. doi: 10.1128/AEM.68.7.3570-3574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oozeer R, Leplingard A, Mater DD, Mogenet A, Michelin R, Seksek I, et al. Survival of Lactobacillus casei in the human digestive tract after consumption of fermented milk. Appl Environ Microbiol. 2006;72:5615–5617. doi: 10.1128/AEM.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parassol N, Freitas M, Thoreux K, Dalmasso G, Bourdet-Sicard R, Rampal P. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res Microbiol. 2005;156:256–262. doi: 10.1016/j.resmic.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Pedone CA, Bernabeu AO, Postaire ER, Bouley CF, Reinert P. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int J Clin Pract. 1999;53:179–184. [PubMed] [Google Scholar]
- Pedone CA, Arnaud CC, Postaire ER, Bouley CF, Reinert P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int J Clin Pract. 2000;54:568–571. [PubMed] [Google Scholar]
- Rochet V, Rigottier-Gois L, Levenez F, Cadiou J, Marteau P, Bresson JL, et al. Modulation of Lactobacillus casei in ileal and fecal samples from healthy volunteers after consumption of a fermented milk containing Lactobacillus casei DN-114 001Rif. Can J Microbiol. 2008;54:660–667. doi: 10.1139/w08-050. [DOI] [PubMed] [Google Scholar]
- Rosenfeldt V, Michaelsen KF, Jakobsen M, Larsen CN, Moller PL, Tvede M, et al. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort of nonhospitalized children attending day-care centers. Pediatr Infect Dis J. 2002;21:417–419. doi: 10.1097/00006454-200205000-00013. [DOI] [PubMed] [Google Scholar]
- Shaughnessy AF, Slawson DC. POEMs: patient-oriented evidence that matters. Ann Intern Med. 1997;126:667. doi: 10.7326/0003-4819-126-8-199704150-00032. [DOI] [PubMed] [Google Scholar]
- Shaughnessy AF, Slawson DC. What happened to the valid POEMs? A survey of review articles on the treatment of type 2 diabetes. BMJ. 2003;327:266. doi: 10.1136/bmj.327.7409.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein M, Sales AE, Koepsell TD. Health care utilization and expenditures associated with child care attendance: a nationally representative sample. Pediatrics. 2003;111:e371–e375. doi: 10.1542/peds.111.4.e371. [DOI] [PubMed] [Google Scholar]
- Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppee JY, et al. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J Immunol. 2006;176:1228–1237. doi: 10.4049/jimmunol.176.2.1228. [DOI] [PubMed] [Google Scholar]
- Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]
- Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564–568. doi: 10.1016/s0022-3476(99)70053-3. [DOI] [PubMed] [Google Scholar]
- Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. 2005;115:5–9. doi: 10.1542/peds.2004-1815. [DOI] [PubMed] [Google Scholar]