Abstract
Neurofibrillary tangles comprised of the microtubule-associated protein tau are pathological features of Alzheimer's disease and several other neurodegenerative diseases, such as progressive supranuclear palsy. We previously overexpressed tau in the substantia nigra of rats and mimicked some of the neurodegenerative sequelae that occur in humans such as tangle formation, loss of dopamine neurons, and microgliosis. To study molecular changes involved in the tau-induced disease state, we used DNA microarrays at an early stage of the disease process. A range of adeno-associated virus (AAV9) vector doses for tau were injected in groups of rats with a survival interval of two weeks. Specific decreases in messages for dopamine related genes validated the technique with respect to the dopaminergic cell loss observed. Of the mRNAs upregulated, there was a dose-dependent effect on multiple genes involved in immune response such as chemokines, interferon-inducible genes and leukocyte markers, only in the tau vector groups and not in dose-matched controls of either transgene-less empty vector or control green fluorescent protein vector. Histological staining for dopamine neurons and microglia matched the loss of dopaminergic markers and upregulation of immune response mRNAs in the microarray data, respectively. RT-PCR for selected markers confirmed the microarray results, with similar changes found by either technique. The mRNA data correlate well with previous findings, and underscore microgliosis and immune response in the degenerative process following tau overexpression.
Keywords: adeno-associated virus, neurodegenerative diseases, microarray, microtubule-associated protein tau, gene transfer, immune response, gliosis, progressive supranuclear palsy
Introduction
A number of neurodegenerative diseases are characterized by microtubule-associated protein tau pathology, or tauopathy, such as Alzheimer's disease (AD), frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD). There are a range of clinical symptoms across the tauopathy diseases including dementia, changes in personality, parkinsonism, aphasia and speech apraxia, and signs of bulbar degeneration (Kumar-Singh & Van Broeckhoven, 2007; Ludolph et al., 2009). While neurofibrillary tangles are an end stage, post-mortem marker, the earlier stage neuropathology and dysfunction of tau may initially trigger the degenerative process. Disruption in tau's dynamic phosphorylation-dependent interaction with microtubules, leading to hyperphosphorylated tau and small oligomer formation, may destine the neuron to tangles and death later on (Buée et al., 2000; Golde, 2006; Gendron and Petrucelli, 2009 for review). We viewed mRNA changes at an early stage in tau-induced neurodegeneration in a rat model to make hypotheses about early events in tau's neurodegenerative mechanism, before all the neurons are lost, in hopes of speculating a preventative strategy.
Delivery of the human tau gene directly to the rat substantia nigra (SN) yields a range of neuronal loss that is time- and dose-dependent, and regulated by the animal's age and the efficiency of gene transfer (Klein et al., 2008; 2009). The SN is relevant for diseases involving tau pathology and neurofibrillary tangles where there is degeneration of the pigmented dopamine neurons and the potential for parkinsonism, such as PSP (Poorkaj et al., 2002), FTDP-17 (Mirra et al., 1999), and CBD (Wakabayashi et al., 1994; Di Maria et al., 2000). Tauopathy is also prevalent in the SN in AD (Schneider et al., 2002). The SN in the rat is small and therefore provides a facile index for loss of a specific neuronal population. The tau overexpression in rats with the adeno-associated virus (AAV) vector produces mature neurofibrillary tangles viewed by electron microscopy or Gallyas staining (Klein et al., 2005), although the number of confirmed neurons with tangles is small relative to the number of neurons expressing hyperphosphorylated tau, or the number of dopaminergic neurons that are lost. Since the hyperphosphorylated tau expression (i.e., pre-tangle pathology) matches better with degree of the neuronal loss, the rat model is a good way to study neuron loss induced by early stage tau pathology. With control over tau vector expression onset, a gradient of intensity of the tau disease state can be produced to mimic either early or later stages.
Here, we inspected an early time in the tau vector induced disease state for mRNA profiles. We tested for differences between a tau group and a control green fluorescent protein (GFP) group, and also compared changes at three tau vector doses. Other controls included sham vehicle and empty vector to attribute message changes specifically due to tau overexpression, and hopefully enrich our knowledge about the tau disease process. Histological analysis after tau gene transfer to the SN of either young or aged rats (Klein et al., 2009) suggested that microgliosis was an early and possibly causative event in the ensuing loss of dopaminergic neurons. Both microgliosis and neuron loss were augmented in aged rats, with the peak microgliosis preceding the peak cell loss. The microgliosis in the rat model could be relevant to microgliosis in tau diseases such as PSP (Ishizawa et al., 2000); its early occurrence in the degenerative sequence is consistent with tau transgenic mice where tangles formed after microgliosis (Yoshiyama et al., 2007). We therefore hypothesized that mRNAs for markers of microgliosis and inflammation, such as pro-inflammatory cytokines and their receptors, would be specifically elevated by tau at an early stage of the disease state, and that mRNAs for dopaminergic markers would be lost in a tau vector dose-dependent manner.
Materials and Methods
Vectors and injections
AAV9 vectors for human wild type tau including exons 2, 3 and 10 (the longest form of tau), GFP control, or empty control with no transgene, were prepared as described (Klein et al., 2009). The expression cassette included AAV2 terminal repeats, the cytomegalovirus/chicken beta-actin promoter, and a polyadenylation sequence. The AAV purification method involved transfecting 293-T cells, harvesting and lysing the cells 3 days later, running an iodixanol gradient, washing and concentrating on filter units, sterilizing through a syringe tip, aliquoting, and freezing (Klein et al., 2009). The AAV9s were dose matched by diluting with lactated ringer's buffer, the vehicle solution. Preps were titered for vector genomes (vg)/ml by a dot-blot method. Samples from each prep were directly loaded on protein gels and immunoblotted with an AAV capsid monoclonal antibody B1 from Meridian (Saco, ME; 1:750), to confirm the dot-blots and ensure equal dosing.
Male Sprague-Dawley rats (3 months of age, from Harlan, Indianapolis, IN) were anesthetized intramuscularly with a 1 ml/kg cocktail containing 3 ml xylazine (20 mg/ml, from Butler, Columbus, OH), 3 ml ketamine (100 mg/ml, from Fort Dodge Animal Health, Fort Dodge, IA), and 1 ml acepromazine (10 mg/ml, from Boehringer Ingelheim, St. Joseph, MO). Virus (3 μl) was injected with a 27-gauge cannula connected via 26-gauge polyethylene tubing to a 10 ul Hamilton syringe mounted to a microinjection pump (CMA/Microdialysis, North Chelmsford, MA) at a rate of 0.25 μl/min on one side of the brain. Stereotaxic coordinates for the SN were 5.3 P, 2.1 L, and 7.6 V (Paxinos and Watson, 1998). The needle remained there for an additional minute before being slowly withdrawn from the animal (over 2 min). The skin was then sutured and the animal placed on heating pads until conscious before being returned to its cage. All procedures were done in accordance with our institutional ACUC and the NIH Guide for Care and Use of Laboratory Animals.
Treatment groups, dosing, and comparisons
There were three vector groups, tau, GFP, empty, and a sham vehicle injection group, with five rats per groups. Tau or GFP were applied at two equal doses 2.4 × 109 and 1.2 × 1010 vg, and the empty vector was applied at the 1.2 ×1010 vg dose. The tau vector was also used at a higher dose, 3.6 × 1010 vg; this group had only four rats. We attempted to pinpoint an early stage in the disease process with low to mid range doses of the tau vectors and an interval of 2 weeks. We chose the lowest tau vector dose to produce a baseline of expression without lesion, based on previous doses (Klein et al., 2009). At the higher doses of the tau vector, we targeted stronger lesioning effects. Another study with AAV9 tau showed that losses in striatal dopamine content begin to occur by 2 weeks (Klein et al., 2008). By studying a 15-fold range of tau vector doses at 2 weeks, we aimed to generate mild to mid level disease states.
We were most interested in comparing tau versus GFP at the mid dose (1.2 × 1010 vg), with the tau, but not the GFP, vector causing a partial dopaminergic lesion. The low dose of either tau or GFP (2.4 × 109 vg) was not expected to cause as many changes either histologically or by microarray, and served as a baseline. While GFP controls for the purpose of expressing a foreign transgene other than tau, an empty vector could avoid GFP-specific effects, so we also analyzed tau versus empty at the mid dose. Next, we looked for dose-dependent effects, comparing the 3 doses of tau against the vehicle solution. Other comparisons were of interest, such as GFP versus empty (GFP effects), and empty versus vehicle (AAV effects).
The tau expression and effects on dopaminergic markers and microglia were viewed in sister animals (2 per group), using described immunohistochemistry methods (Klein et al., 2009). We monitored markers for dopaminergic neurons (tyrosine hydroxylase, TH; Pel-Freez, Rogers, AR, 1:2000), human tau (E1, provided by Leonard Petrucelli, Mayo Clinic, Jacksonville, FL, 1:2000), and hyperphosphorylated tau (CP13, provided by Peter Davies, Albert Einstein College of Medicine, New York, NY, 1:2000) in sister animals at 2 weeks, and for microglia (Cd11b, Chemicon, Temecula, CA, 1:1000) and astroglia (glial fibrillary acidic protein, GFAP, Chemicon, 1:1000) over a 14 day time-course (1, 3, 7, 14 days). Because microgliosis appears to be an early and therefore perhaps causative phenomenon in the tau vector induced disease state, which we predicted to be evidenced in mRNA profiles, we viewed immunoreactivity for microglia at intervals preceding the microarray in tau vector and control rats.
Microarray and statistics
Two weeks after gene transfer, the SN was dissected with the aid of a brain block and biopsy punch (both from World Precision Instruments, Sarasota, FL) and stored in 1 ml of RNAlater (Ambion, Austin, TX) overnight at 4°C. Tissue was homogenized in 1 ml RNA STAT-60 (Tel-Test Inc, Friendswood, TX). RNA was extracted with chloroform/isopropanol, washed with ethanol, and dissolved in RNase-free water (Ambion). RNA was then further purified using the RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA) and stored at -80°C.
RNA concentration was measured using a spectrophotometer while integrity was assessed by electrophoresis on the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Double-stranded cDNA was synthesized from approximately 7 μg total RNA using a Superscript cDNA Synthesis Kit (Invitrogen, Carlsbad, CA) in combination with a T7-(dT)24 primer. Biotinylated cRNA was transcribed in vitro using the GeneChip IVT Labeling Kit (Affymetrix, Santa Clara, CA) and purified using the GeneChip Sample Cleanup Module (Affymetrix). Purified cRNA (20 μg) was incubated in fragmentation buffer (200 mM Tris-acetate, pH 8.1, 500 mM potassium acetate, 150 mM magnesium acetate) at 94°C for 35 minutes and chilled on ice. Fragmented biotin-labeled cRNA (10 μg) was hybridized to the Rat Genome 230 2.0 Array (Affymetrix), interrogating 31,099 rat genes. Arrays were incubated for 16 hr at 45°C with constant rotation (60 rpm), washed and then stained for 10 min at 25°C with 10 μg/ml streptavidin-R phycoerythrin (Vector Laboratories, Burlingame, CA) followed by 3 μg/ml biotinylated goat anti-streptavidin antibody (Vector Laboratories) for 10 minutes at 25°C. Arrays were scanned using an Affymetrix GeneChip Scanner 3000 7G. Pixel intensities were measured, expression signals were analyzed and features extracted, mined, and exported using the manufacturer's software packages.
Arrays were globally scaled to a target intensity value of 2500 in order to compare individual experiments. Whether each mRNA was present in each sample, as well as the direction of change, and fold change of gene expressions between samples were determined by the software. Statistical analyses were performed with GeneSifter™ software (http://www.genesifter.net). Individual pairs of dose-matched groups were compared by student t-tests with Benjamini and Hochberg correction for multiple comparisons.
RT-PCR
RT-PCR was performed using the Taqman Universal PCR Master Mix with the 7900HT Real-Time PCR System (Applied Biosystems, Foster City, CA) to confirm microarray results. cDNA was transcribed from 1 ug of RNA using the iScript cDNA Synthesis Kit (Invitrogen, Carlsbad, CA) and stored at -20°C. Cycling parameters were 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds then 60°C for 1 minute. Reactions were run in triplicate in a 96-well fast optical plate (Applied Biosystems) and contained 20 ng of cDNA along with 1 μl of 20× primer/probe from the Taqman Gene Expression Assays (Applied Biosystems) for each gene probed. These included: interleukin 1-beta (IL1B; Catalog number Rn00580432_m1), tumor necrosis factor (TNF; Rn99999017_m1), RT1 class II locus Db1 (RT1-Db1; Rn01429350_m1), solute carrier family 6 (neurotransmitter transporter, dopamine) member 3 (SLC6A3; Rn00562224_m1), and TH (Rn00562500_m1). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Rn99999916_s1) was used as a housekeeping gene. Primer/probe pairs were purchased from Applied Biosystems. Ct (cycle threshold) values were generated by the software, and represent the cycle time it takes for the fluorescence to reach a designated threshold (up the linear portion of the sigmoidal PCR amplification); the more starting mRNA for that specific probe there is, the lower the Ct value. Data for each specific target is represented as ΔCt, the Ct value of the housekeeping gene GAPDH minus that of the specific target. Thus, while all ΔCt values are negative due to the relatively large amount of GAPDH mRNA (low Ct value) compared to the target mRNAs (higher Ct value), an increase in the ΔCt in treated groups signifies an increase in target mRNA amount.
Results
Quality control for viral protein
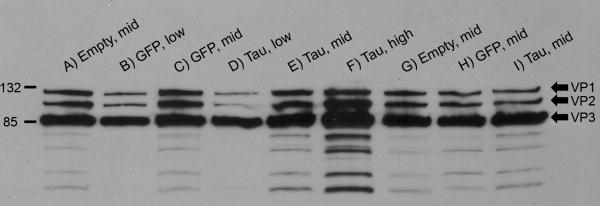
We compared amounts of viral capsid proteins in the preps to confirm equal dosing based on dot-blot titers of encapsidated DNA. Each AAV showed bands of consistent size (60-90 kDa) and stoichiometry (1:1:20) for VP1, VP2, and VP3 proteins. The B1 antibody detected the empty vector, AAV9 GFP, and AAV9 tau equally well, and different doses of each vector stained at different intensities accordingly (Fig. 1). The dot-blot titers for vector DNA matched well for levels of capsid proteins (equal vector genome comparisons in consecutive lanes on the right side of Fig. 1). There were lower molecular weight bands, which we assume to be degradation products, in all of the preps.
Fig 1.
AAV capsid protein western blot. Each viral dose used in the study was loaded and probed with an antibody against the AAV viral proteins VP1, VP2, and VP3 (indicated on right side of panel; size markers in kDa on left side), to ensure equal viral particles for the microarray study. Each matching dose of specific vectors had similar bands, and the intensity of the bands matched with the vector genome amounts loaded.
mRNA profiles after tau or GFP gene transfer
To identify differentially expressed messages in the SN of rats overexpressing tau, we used Affymetrix rat genome microarray chips to probe changes in 31,099 different mRNAs or expressed sequence tags. The groups (n=5) were set up as follows: lactated ringers vehicle, empty transgene-less AAV9 (1.2 × 1010 vector genomes), and 2 groups of dose-matched AAV9 GFP or AAV9 tau (2.4 × 109 or 1.2 × 1010 vector genomes). A third dose of AAV9 tau (3.6 × 1010 vector genomes) was also studied (n=4). A two week time-point was chosen to reflect changes occurring early in the neurodegenerative process, before maximal loss of neurons occur. Overexpression of tau or GFP did not change the overall number of messages detected in the rat SN relative to all of the probes present on the array, as approximately 55-60% of the total array were detected in each group (not shown). Direct comparisons between selected groups were made using the student's t-test with Benjamini and Hochberg correction. Differences in expression of at least 2-fold in either direction with P value < 0.05 were treated as significant.
We probed for potential effects specific to the virus or the GFP using several controls (vehicle injections, empty AAV9 and AAV9 GFP). Comparing vehicle to the empty AAV9 vector, only 24 messages were affected (10 mapped to known genes), with upregulation of 11 and downregulation of 13, the greatest effect a 3.35 fold decrease (osteoglycin, a bone associated glycoprotein). No increases in messages related to immune response were seen. AAV9 GFP, when compared to the empty vector at an equal dose of 1.2 × 1010 vg, produced more changes: 228 messages changed (142 mapped to identified genes), upregulating 80 and downregulating 148, with the greatest effect a 31.85 upregulation of forkhead box g1 (Foxg1), a transcription factor involved in brain development (Martynoga et al., 2005). The data suggest more changes due to GFP than the AAV9 under these conditions.
At the lowest dose of tau and GFP vectors (2.4 × 109 vg), tau produced few effects compared to GFP. Only 10 messages (5 mapped to identified genes) were altered when compared to AAV9 GFP, with 5 being upregulated and 5 downregulated and the largest change a 3.46 fold decrease. The identified messages and change in the tau group relative to GFP were as follows: killer cell lectin-like receptor, subfamily D, member 1, down 3.46-fold; discoidin domain receptor tyrosine kinase 2, down 2.57-fold; budding uninhibited by benzimidazoles 1 homolog, down 2.29-fold; forkhead box S1, up 2.44-fold; and spermatogenesis associated 2-like, up 2.22-fold. Message changes seen at this lowest dose were dissimilar from those at the higher doses, perhaps consistent with a lack of lesioning effect from the lowest dose of tau.
The middle dose of AAV9 tau was compared to AAV9 GFP (1.2 ×1010 vg). Compared to GFP, the middle dose of tau vector resulted in 299 message changes (166 mapped to known genes), with the majority of them (216) upregulated. The top 50 changes based on intensity are listed in Table 1, with the cutoff being a 2.73-fold change. The list is completed for all significant changes over 2-fold in Supplemental Table 1. Of the 166 message changes mapped to known genes, 47/166 (28.3%) were genes involved in immune response, including the most affected message, a 16.05-fold upregulation in chemokine (C-X-C motif) ligand 10 (Cxcl10), involved in T-cell generation and trafficking (Dufour et al., 2002). Forty four of the forty seven immune related messages were upregulated, signifying a pro-inflammatory response to tau. Other messages increased by tau were comprised of transcription factors (16/166, 9.6%) such as activating transcription factor 3 (Atf3) involved in neuronal stress response and survival (Song et al., 2008), cytoskeletal proteins (14/166, 8.4%) such as the microtubule subunits beta 4 and beta 6 tubulin (Tubb4, Tubb6), cell cycle regulation (8/166, 4.8%) such as the cell cycle inhibitor cyclin-dependent kinase inhibitor 1A (Cdkn1a, also known as p21; Delobel et al., 2006), and protein degradation (6/166, 3.6%) such as ubiquitin conjugating enzymes E2L 6 and E2N (Ube2l6, Ube2n) and proteasome subunit beta 9 (Psmb9; Oddo, 2008 for review). Decreased messages were comprised of synaptic proteins (5/166, 3%) such as alpha-synuclein (Snca) and synaptotagmin I (Syt1), receptors (3/166, 1.8%) such as cholinergic receptors, nicotinic, alpha 5&6 (Chrna5, Chrna6), and transporters (3/166, 1.8%) such as solute carrier family 6 (neurotransmitter transporter, dopamine) member 3 (Slc6a3, also known as dopamine transporter, or DAT). Markers of dopaminergic neurons, such as tyrosine hydroxylase (TH) and dopa decarboxylase (Ddc), were hypothesized to decrease due to tau, but did not show up in the comparison against GFP. AAV9 tau only decreased these mRNAs 1.51 and 1.77-fold, respectively when compared to AAV9 GFP. One of the messages for cytoskeletal proteins was for endogenous rat tau, which was increased in the tau group compared to GFP by 2.30-fold. Based on the hybridization probes used in the microarray, we are confident that the increase did not incorporate the recombinant human tau mRNA from the vector. We therefore re-examined the data and found a 1.89-fold decrease in rat tau in the GFP group relative to the empty vector (P < 0.05), which likely explains the lowered rat tau message in the GFP group compared to the tau group, and indicates a specific side effect of the GFP.
Table 1.
Messages changed by AAV9 tau relative to AAV9 GFP at the middle dose of 1.2 × 1010 vector genomes at two weeks.
| Function | Gene Identifier | Gene Name | Gene ID | Fold change | Direction |
|---|---|---|---|---|---|
| Immune Response | U22520 | Chemokine (C-X-C motif) ligand 10 | Cxcl10 | 16.05 | Up |
| NM_017143 | Coagulation factor X | F10 | 10.29 | Up | |
| AA819034 | Putative ISG12(b) protein | isg12(b) | 8.83 | Up | |
| AF068268 | 2-5 oligoadenylate synthetase 1B | Oas1b | 7.19 | Up | |
| AI409634 | Radical S-adenosyl methionine domain containing 2 | Rsad2 | 6.49 | Up | |
| AW531805 | Interferon-induced protein with tetratricopeptide repeats 3 | Ifit3 | 5.1 | Up | |
| BE096523 | Interferon, alpha-inducible protein (clone IFI-15K) | G1p2 | 5.04 | Up | |
| Z18877 | 2-5 oligoadenylate synthetase 1A | Oas1a | 4.52 | Up | |
| BI303379 | Tumor necrosis factor receptor superfamily, member 12a | Tnfrsf12a | 4.2 | Up | |
| BI279526 | RT1 class II, locus Db1 | RT1-Db1 | 3.59 | Up | |
| AA819629 | Histocompatibility 28 | H28 | 3.45 | Up | |
| BF411036 | Interferon regulatory factor 7 | Irf7 | 3.39 | Up | |
| NM_134350 | Myxovirus (influenza virus) resistance 2 | Mx2 | 3.38 | Up | |
| BG378630 | Placenta-specific 8 | Plac8 | 3.34 | Up | |
| AA892854 | Chemokine (C-X-C motif) ligand 13 | Cxcl13 | 3.27 | Up | |
| Y00480 | Histocompatibility 2, class II antigen E alpha | H2-Ea | 3.22 | Up | |
| NM_013069 | Cd74 molecule, major histocompatibility complex, class II invariant chain | Cd74 | 3.04 | Up | |
| BM390144 | Arachidonate 12-lipoxygenase | Alox12 | 2.92 | Up | |
| AI009765 | Interferon-induced protein with tetratricopeptide repeats 2 | Ifit2 | 2.86 | Up | |
| NM_133624 | Guanylate binding protein 2 | Gbp2 | 2.73 | Up | |
| BM382822 | Intercellular adhesion molecule 5, telencephalin | Icam5 | 4.47 | Down | |
| Lipid Regulation | AI180413 | Apolipoprotein H (beta-2-glycoprotein I) | Apoh | 3.29 | Up |
| AW435376 | Low density lipoprotein receptor-related protein 5 | Lrp5 | 2.79 | Up | |
| NM_012598 | Lipoprotein lipase | Lpl | 3.56 | Down | |
| NM_013126 | Diacylglycerol kinase, gamma | Dgkg | 2.79 | Down | |
| AI411493 | Acyl-CoA thioesterase 4 | Acot4 | 2.76 | Down | |
| Gene Regulation | NM_012912 | Activating transcription factor 3 | Atf3 | 5.21 | Up |
| NM_017103 | Transcription elongation factor B (SIII), polypeptide 3 | Tceb3 | 3.32 | Up | |
| BM391471 | Activating transcription factor 5 | Atf5 | 2.83 | Up | |
| BF402458 | Zinc finger protein 667 | Znf667 | 6.13 | Down | |
| Cell cycle/DNA repair | NM_024127 | Growth arrest and DNA-damage-inducible, alpha | Gadd45a | 3.06 | Up |
| U24174 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | Cdkn1a | 2.87 | Up | |
| AI010427 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | Cdkn1a | 2.74 | Up | |
| BF402732 | Stromal antigen 2 | Stag2 | 3 | Down | |
| Receptors | NM_024483 | Adrenergic, alpha-1D-, receptor | Adra1d | 5.4 | Down |
| NM_017078 | Cholinergic receptor, nicotinic, alpha 5 | Chrna5 | 3.36 | Down | |
| NM_057184 | Cholinergic receptor, nicotinic, alpha 6 | Chrna6 | 2.92 | Down | |
| Transporter | NM_130748 | Solute carrier family 38, member 4 | Slc38a4 | 4.95 | Down |
| M80233 | Solute carrier family 6 (neurotransmitter transporter, dopamine), member 3 | Slc6a3 | 3.76 | Down | |
| AF070475 | Solute carrier family 4, sodium bicarbonate cotransporter, member 7 | Slc4a7 | 3.34 | Down | |
| Other | BI286387 | Small proline-rich protein 1A-like | Sprr1al | 11.51 | Up |
| NM_133523 | Matrix metallopeptidase 3 | Mmp3 | 4.81 | Up | |
| AB023896 | Endothelin converting enzyme-like 1 | Ecel1 | 3.78 | Up | |
| AA892813 | Muscleblind-like 2 | Mbnl2 | 3.06 | Up | |
| NM_017180 | Pleckstrin homology-like domain, family A, member 1 | Phlda1 | 2.8 | Up | |
| AI317842 | Kinesin family member 22 | Kif22 | 2.76 | Up | |
| NM_053955 | Crystallin, mu | Crym | 5.86 | Down | |
| AF245172 | Guanine deaminase | Gda | 4.02 | Down | |
| NM_031045 | Inositol 1,4,5-trisphosphate 3-kinase A | Itpka | 3.56 | Down | |
| AW534032 | Copine VII | Cpne7 | 3.4 | Down |
The top 50 greatest message changes (out of 166) associated with known proteins affected by AAV9 tau classified by function. N = 5/group.
AAV9 tau was also compared to a dose-matched, transgene-less empty AAV9 vector. AAV9 tau produced fewer changes here than when compared to the AAV9 GFP group. AAV9 tau produced 137 changes (90 mapped to known genes), and similarly, the majority were upregulated (115). The top 50 changes based on intensity are listed in Table 2, with the cutoff being a 2.36-fold change. The list is completed for all significant changes over 2-fold in Supplemental Table 2. Of the 90 messages, again the majority were increases in messages involved in immune response, accounting for 54/90 (60%) of total changes, including the biggest change, a 16.3-fold upregulation in antigen presentation molecule RT1-Aw2 (Rolstad et al., 1997 for review). Other messages increased by tau were similar to changes seen when compared to GFP, and fell into cell cycle regulation (8/90, 8.9%) such as p21, protein degradation (4/90, 4.4%) such as ubiquitin-conjugating enzyme E2L 6 (Ube2l6) and proteasome subunits beta type 8 & 9 (Psmb8, Psmb9), and protein chaperones (3/90, 3.3%) such as heat shock protein 1 (Hspb1, also known as Hsp27). Messages decreased by tau were either transporters (6/90, 6.7%) such as DAT and solute carrier family 18 (vesicular monoamine), member 2 (Slc18a2, also known as vesicular monoamine transporter 2, or VMAT2), receptors (3/90, 3.3%) such as the alpha-5 nicotinic cholinergic receptor and dopamine receptor 2 (Drd2), or dopamine synthesis enzymes (2/90, 2.2%) such as TH and Ddc. These changes are likely due to loss of neurons in the SN due to tau, and validate the ability of AAV9 tau to produce a robust neuronal lesion. The overlap of tau's patterns relative to either GFP or empty vector support the argument that these are tau-specific changes. The significant changes in mRNAs for currently unnamed gene products are listed in Supplemental Table 3 for comparisons of the middle dose of AAV9 tau to either the middle dose AAV9 GFP or the AAV9 empty.
Table 2.
Messages changed by AAV9 tau relative to AAV9 empty at the middle dose of 1.2 × 1010 vector genomes at two weeks.
| Function | Gene Identifier | Gene Name | Gene ID | Fold change | Direction |
|---|---|---|---|---|---|
| Immune response | AJ243338 | RT1 class I, CE5 | RT1-Aw2 | 16.3 | Up |
| U22520 | Chemokine (C-X-C motif) ligand 10 | Cxcl10 | 9.17 | Up | |
| AA819034 | Putative ISG12(b) protein | isg12(b) | 8.86 | Up | |
| X52711 | Myxovirus (influenza virus) resistance 1 | Mx1 | 7.79 | Up | |
| AI409634 | Radical S-adenosyl methionine domain containing 2 | Rsad2 | 6.71 | Up | |
| AW531805 | Interferon-induced protein with tetratricopeptide repeats 3 | Ifit3 | 5.71 | Up | |
| BI303379 | Tumor necrosis factor receptor superfamily, member 12a | Tnfrsf12a | 5.5 | Up | |
| BE096523 | Interferon, alpha-inducible protein (clone IFI-15K) | G1p2 | 5.46 | Up | |
| BF411036 | Interferon regulatory factor 7 | Irf7 | 5.16 | Up | |
| NM_134350 | Myxovirus (influenza virus) resistance 2 | Mx2 | 4.88 | Up | |
| AF068268 | 2-5 oligoadenylate synthetase 1B | Oas1b | 4.75 | Up | |
| BE118697 | Interferon-induced protein with tetratricopeptide repeats 2 | Ifit2 | 4.73 | Up | |
| Z18877 | 2-5 oligoadenylate synthetase 1A | Oas1a | 3.99 | Up | |
| BG378630 | Placenta-specific 8 | Plac8 | 3.81 | Up | |
| NM_017143 | Coagulation factor X | F10 | 3.56 | Up | |
| AI009765 | Interferon-induced protein with tetratricopeptide repeats 2 | Ifit2 | 3.48 | Up | |
| NM_133624 | Guanylate nucleotide binding protein 2 | Gbp2 | 3.07 | Up | |
| BI296385 | Chemokine (C-X-C motif) ligand 16 | Cxcl16 | 2.95 | Up | |
| AF065438 | Lectin, galactoside-binding, soluble, 3 binding protein | Lgals3bp | 2.74 | Up | |
| BM391818 | 2 -5 oligoadenylate synthetase 1I | Oas1i | 2.73 | Up | |
| Y00480 | RT1 class II, locus Da | RT1-Da | 2.71 | Up | |
| AW915763 | Serine (or cysteine) peptidase inhibitor, clade G, member 1 | Serping1 | 2.62 | Up | |
| BG378249 | RT1 class II, locus Ba | RT1-Ba | 2.61 | Up | |
| BI292425 | Complement component 1, r subcomponent | C1r | 2.59 | Up | |
| AI011757 | Fc fragment of IgG, low affinity IIIa, receptor | Fcgr3a | 2.56 | Up | |
| AI011393 | Fc receptor-like S, scavenger receptor | Fcrls | 2.55 | Up | |
| Transporter | M80233 | Solute carrier family 6 (neurotransmitter transporter, dopamine), member 3 | Slc6a3 | 6.61 | down |
| NM_013031 | Solute carrier family 18 (vesicular monoamine), member 2 | Slc18a2 | 5.51 | down | |
| NM_012694 | Solute carrier family 6 (neurotransmitter transporter, dopamine), member 3 | Slc6a3 | 5.47 | down | |
| M97381 | Solute carrier family 18 (vesicular monoamine), member 2 | Slc18a2 | 3.93 | down | |
| AF070475 | Solute carrier family 4, sodium bicarbonate cotransporter, member 7 | Slc4a7 | 3.5 | down | |
| Cell cycle/DNA repair | AI010427 | Cyclin-dependent kinase inhibitor 1A | Cdkn1a | 3.84 | Up |
| U24174 | Cyclin-dependent kinase inhibitor 1A | Cdkn1a | 3.32 | Up | |
| NM_024127 | Growth arrest and DNA-damage-inducible 45 alpha | Gadd45a | 2.75 | Up | |
| BI285978 | Poly (ADP-ribose) polymerase family, member 12 | Parp12 | 2.6 | Up | |
| Dopamine | NM_012740 | Tyrosine hydroxylase | Th | 3.26 | down |
| U31884 | Dopa decarboxylase | Ddc | 2.36 | down | |
| Other | NM_012912 | Activating transcription factor 3 | Atf3 | 7.24 | up |
| AA819788 | Receptor transporter protein 4 | Rtp4 | 4.35 | Up | |
| AI502796 | Coiled-coil domain containing 109B | Ccdc109b | 3.57 | up | |
| AA943147 | Potential ubiquitin ligase | Herc6 | 3.3 | Up | |
| AB023896 | Endothelin converting enzyme-like 1 | Ecel1 | 3.14 | up | |
| AI180413 | Apolipoprotein H | Apoh | 2.67 | up | |
| AA945955 | Osteoglycin | Ogn | 2.61 | up | |
| NM_080767 | Proteasome (prosome, macropain) subunit, beta type 8 | Psmb8 | 2.55 | Up | |
| NM_017078 | Cholinergic receptor, nicotinic, alpha polypeptide 5 | Chrna5 | 3.56 | down | |
| AW434432 | Opsin 4 (melanopsin) | Opn4 | 2.79 | down | |
| BI286396 | PERP, TP53 apoptosis effector | Perp | 2.87 | down | |
| NM_053744 | Delta-like 1 homolog (Drosophila) | Dlk1 | 2.68 | down | |
| AW534032 | Copine VII | Cpne7 | 2.54 | down |
The top 50 greatest message changes (out of 90) associated with known proteins affected by AAV9 tau classified by function. N = 5/group.
Dose-effects of AAV9 tau were examined across three doses spanning a 15-fold range and compared to vehicle injections. The lowest dose of AAV9 tau (2.4 × 109 vector genomes) had a modest effect, producing changes in 48 messages (22 mapped to identified genes), with the largest change a 4.14 fold decrease. On the other end, the highest dose (3.6 × 1010 vector genomes) had large effects, producing changes in 1679 messages, with the largest being a 131.84-fold increase. The messages that were affected by both the mid dose and high dose of AAV9 tau are listed in Table 3. There was no overlap in messages affected by the low dose and either the middle or high dose, with 45 specific messages affected by both the middle and high doses of tau (Table 3). Of those affected at both doses, 27 (60%) were involved in an immune response, and all were increased by tau. Other messages dose-dependently increased by tau include the microtubule components Tubb6, kinesin family member 20B (Kif20b), and kinesin family member 22 (Kif22; Mandelkow et al., 1995 for review), and the ubiquitin system enzymes Ube2l6 and a potential ubiquitin ligase (Herc6; Hochrainer et al., 2005). Messages decreased by both tau vector doses compared to vehicle include dopamine synthesis enzymes TH and Ddc, transporters DAT and VMAT2, and the alpha-6 nicotinic cholinergic receptor.
Table 3.
Messages dose-dependently changed by AAV9 tau.
| Fold change | ||||||
|---|---|---|---|---|---|---|
| Function | Gene Identifier | Gene Name | Gene ID | Mid dose | High dose | Direction |
| Immune Response | AI409634 | Radical S-adenosyl methionine domain containing 2 | Rsad2 | 6.04 | 29.56 | Up |
| U22520 | Chemokine (C-X-C motif) ligand 10 | Cxcl10 | 5.98 | 78.36 | Up | |
| AA819034 | Putative ISG12(b) protein | isg12(b) | 5.46 | 60.24 | Up | |
| X52711 | Myxovirus (influenza virus) resistance 1 | Mx1 | 5.11 | 54.18 | Up | |
| BE096523 | Interferon, alpha-inducible protein (clone IFI-15K) | G1p2 | 5 | 38.42 | Up | |
| AF068268 | 2-5 oligoadenylate synthetase 1B | Oas1b | 4.83 | 14.01 | Up | |
| AW531805 | Interferon-induced protein with tetratricopeptide repeats 3 | Ifit3 | 4.16 | 38.5 | Up | |
| Z18877 | 2-5 oligoadenylate synthetase 1A | Oas1a | 3.85 | 19.91 | Up | |
| BF411036 | Interferon regulatory factor 7 | Irf7 | 3.37 | 34.23 | Up | |
| BM391818 | 2 -5 oligoadenylate synthetase 1I | Oas1i | 3.29 | 9.09 | Up | |
| NM_017143 | Coagulation factor X | F10 | 3.28 | 29.13 | Up | |
| AI009765 | Interferon-induced protein with tetratricopeptide repeats 2 | Ifit2 | 3.27 | 14.48 | Up | |
| NM_134350 | Myxovirus (influenza virus) resistance 2 | Mx2 | 3.18 | 23.12 | Up | |
| AJ243338 | RT1 class Ib, locus Aw2 | RT1-Aw2 | 3.06 | 62.81 | Up | |
| BI296097 | Interferon stimulated exonuclease 20 | Isg20 | 2.74 | 10.93 | Up | |
| AA819629 | Histocompatibility 28 | H28 | 2.73 | 19.54 | Up | |
| BF417541 | DEXH (Asp-Glu-X-His) box polypeptide 58 | Dhx58 | 2.65 | 21.19 | Up | |
| NM_133298 | Glycoprotein (transmembrane) nmb | Gpnmb | 2.57 | 8.84 | Up | |
| AI407339 | Immunity-related GTPase family, M | Irgm | 2.5 | 14.3 | Up | |
| AF065438 | Lectin, galactoside-binding, soluble, 3 binding protein | Lgals3bp | 2.38 | 18.41 | Up | |
| BG378630 | Placenta-specific 8 | Plac8 | 2.35 | 35.32 | Up | |
| NM_053736 | Caspase 4, apoptosis-related cysteine peptidase | Casp4 | 2.34 | 9.5 | Up | |
| L40362 | RT1 class Ib, locus Aw2 | RT1-Aw2 | 2.27 | 25.21 | Up | |
| M10072 | Protein tyrosine phosphatase, receptor type, C | Ptprc | 2.26 | 14 | Up | |
| NM_031832 | Lectin, galactoside-binding, soluble, 3 | Lgals3 | 2.23 | 14.92 | Up | |
| BI292425 | Complement component 1, r subcomponent | C1r | 2.09 | 9.55 | Up | |
| NM_032612 | Signal transducer and activator of transcription 1 | Stat1 | 2.06 | 11.93 | Up | |
| Cytoskeleton | BI274903 | Tubulin, beta 6 | Tubb6 | 2.54 | 7.02 | Up |
| AI317842 | Kinesin family member 22 | Kif22 | 2.15 | 4.51 | Up | |
| BE110723 | Kinesin family member 20B | Kif20b | 2.11 | 4.69 | Up | |
| Transporter | M80233 | Solute carrier family 6 (neurotransmitter transporter, dopamine), member 3 | Slc6a3 | 5.1 | 85.49 | Down |
| NM_012694 | Solute carrier family 6 (neurotransmitter transporter, dopamine), member 3 | Slc6a3 | 4.52 | 22.13 | Down | |
| BF545691 | Solute carrier family 18 (vesicular monoamine), member 2 | Slc18a2 | 4.24 | 15.63 | Down | |
| Dopamine | NM_012740 | Tyrosine hydroxylase | Th | 2.81 | 10.91 | Down |
| U31884 | Dopa decarboxylase (aromatic L-amino acid decarboxylase) | Ddc | 2.36 | 5.95 | Down | |
| Ubiquitin | BI279216 | Ubiquitin-conjugating enzyme E2L 6 | Ube2l6 | 2.63 | 8.78 | Up |
| AA943147 | Potential ubiquitin ligase | Herc6 | 2.49 | 5.82 | Up | |
| Other | NM_012912 | Activating transcription factor 3 | Atf3 | 8.04 | 17.47 | Up |
| AA819788 | Receptor (chemosensory) transporter protein 4 | Rtp4 | 2.87 | 16.67 | Up | |
| NM_053819 | TIMP metallopeptidase inhibitor 1 | Timp1 | 2.68 | 13.27 | Up | |
| BM389006 | Thymidine kinase 1, soluble | Tk1 | 2.18 | 4.8 | Up | |
| AA859235 | Cell division cycle associated 7 | Cdca7 | 2.01 | 4.39 | Up | |
| NM_030847 | Epithelial membrane protein 3 | Emp3 | 2.03 | 5.5 | Up | |
| AA819679 | Poly (ADP-ribose) polymerase family, member 9 | Parp9 | 2 | 10.24 | Up | |
| NM_057184 | Cholinergic receptor, nicotinic, alpha 6 | Chrna6 | 2.62 | 6.95 | Down | |
Messages associated with known proteins changed by both the middle and high doses of AAV9 tau (1.2 or 3.6 × 1010 vector genomes) relative to vehicle injections, and classified by function. N = 4-5/group.
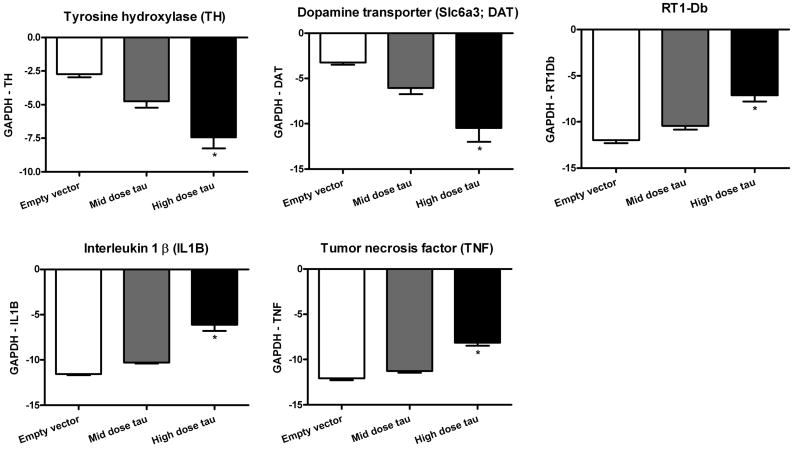
RT-PCR validated selected mRNAs from the microarray results. Primer/probe pairs were purchased for TH, DAT, and RT1-Db (antigen presentation molecule; Ettinger et al., 2004), as well as for pro-inflammatory cytokines interleukin 1 beta (IL-1β) and tumor necrosis factor-alpha (TNF), with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the housekeeping gene. The groups examined were the vehicle control, the middle and high dose of AAV9 tau, and empty vector. There were no changes seen between the vehicle and the empty vector for any of the genes tested (data not shown). Fig. 2 shows mRNA levels normalized to GAPDH for the empty vector and two doses of tau vector. The data were analyzed by ANOVA/Bonferroni multiple comparisons (empty vector versus dose matched tau or mid versus high dose tau). For each target mRNA analyzed by RT-PCR, there were tau vector dose-dependent changes (P < 0.05-0.001), similar to the microarray results, with the high dose tau vector decreasing levels of TH and DAT and increasing levels of RT1-Db, IL-1B, and TNF.
Fig 2.
RT-PCR validation of selected mRNAs from the microarray study: tyrosine hydroxylase (TH), dopamine transporter (DAT), RT1-Db (involved in antigen presentation), interleukin 1β (IL 1B), tumor necrosis factor (TNF). The amount of each target mRNA was normalized by subtracting the signal for a housekeeping mRNA (glyceraldehyde 3-phosphate dehydrogenase, GAPDH) from an aliquot of the same sample. Data are shown for the dose matching empty vector and tau groups (1.2 × 1010 vector genomes) and a higher dose of tau vector (3.6 × 1010 vector genomes) with 3-4 animals per group. In all cases, there were group differences by ANOVA with dose-dependent differences between the tau groups in Bonferroni post-tests (TH, DAT, P < 0.05; RT1-Db, P < 0.01; IL-1β, TNF, P < 0.001). High dose tau vector caused decreases in TH and DAT and increases in RT1-Db, IL-1B, and TNF, although all of the data are expressed as negative values as a result of the high levels of GAPDH.
Compared to mRNA changes reported to be caused by tau overexpression in tau transgenic mice (Chen et al., 2004; Ho et al., 2001), we found more changes, and of greater amplitude, in the course of two weeks of tau expression in rats. The genes reported from the tau transgenic mice did not appear on our lists, although differences in a number of factors, including the time-course, the host species, the genotype of tau, and the brain region collected could have contributed.
Concomitant dopaminergic neuronal loss and microgliosis
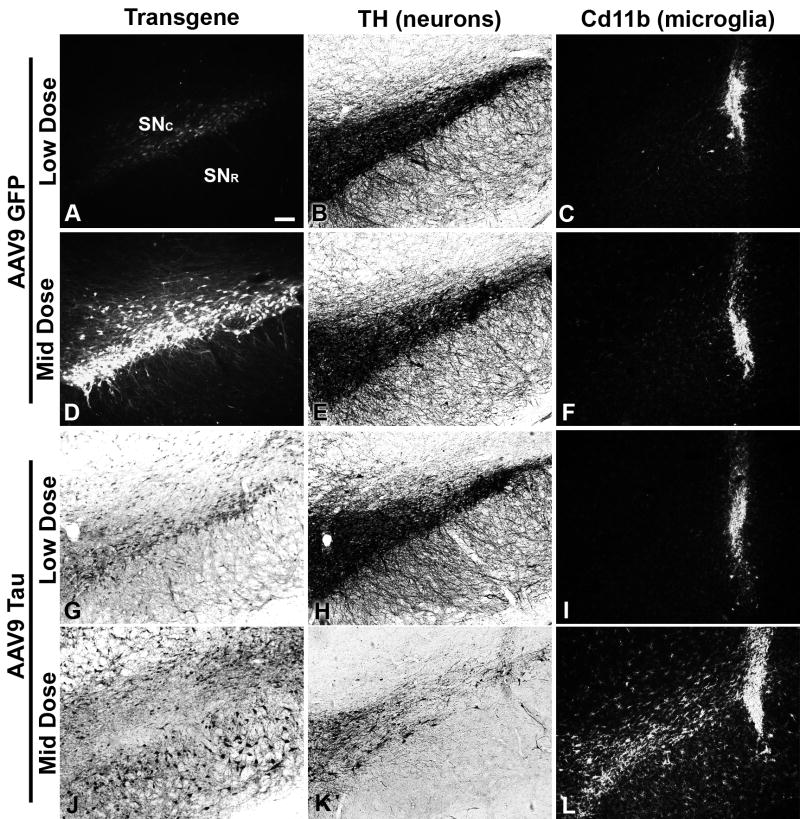
Histological analysis of transgene expression, and effects on dopamine neurons and glia were performed with two subjects per group to mirror the microarray results at 2 weeks. There was visible GFP expression from the lowest dose (2.4 × 109 vg) in the SN pars compacta (Fig. 3A). Staining for the dopamine neuron marker TH in the substania nigra (Fig. 3B) appeared normal relative to uninjected controls (not shown). Staining for the microglial marker CD11b was limited to the needle track (Fig. 3C). With the middle dose (1.2 × 1010 vg), there was more robust GFP expression in neurons and their processes (Fig. 3D) compared to the low dose (Fig. 3A). Similar to the low dose GFP, the middle dose did not appear to alter TH staining in the SN (Fig. 3E) and microglial staining was limited to the needle track (Fig. 3F).
Fig 3.
Histological analyses two weeks after injections of AAV9 GFP or AAV9 tau to the substantia nigra (SN). Results from the low and mid doses of the microarray study (2.4 × 109 or 1.2 × 1010 vector genomes) are shown for each vector. (A-C) GFP epifluorescence, TH immunoreactivity, and staining for the microglial marker Cd11b on adjacent sections after low dose AAV9 GFP injection. TH staining was similar to the uninjected contralateral side (not shown), and pronounced microglial staining was restricted to the needle track. Labels for the SN pars compacta and pars reticulata are provided in A. (D-F) There was an apparent dose-dependent increase in both the spread and intensity of GFP expression with the higher dose, although both TH and Cd11b staining were similar at both doses of AAV9 GFP. (G-I) Hyperphosphorylated tau immunoreactivity (CP13 antibody) after AAV9 tau injection was found mainly in the SN pars compacta, and both TH and Cd11b staining appeared similar to results with both doses of the GFP vector. (J-L) In contrast, the higher dose of tau resulted in spread of hyperphosphorylated tau into the pars reticulata, but the pars compacta was partially blank for tau staining, likely due to dopaminergic cell loss in that area, consistent with reduced TH immunoreactivity in the SN. The microglial staining pattern was distinct with the higher dose of tau, with clear microgliosis away from the needle track in the SN. A higher dose of AAV9 tau (3.6 × 1010 vector genomes) resulted in even more pronounced effects on TH and Cd11b (not shown). Camera settings for the fluorescent images in A, D or C, F, I, L were kept equal. Bar in A = 134 μm.
To monitor tau expression, we used the CP13 antibody specific for hyperphosphorylated tau, which provided a consistent subset of the staining for total human tau in this study and previously (Klein et al., 2009). At the lowest dose, hyperphosphorylated tau expression was detected in the SN (Fig. 3G), although TH immunoreactivity appeared to be unaffected (Fig. 3H) and microglial staining was limited to the needle track (Fig. 3I), as with the GFP vector injections. With the middle dose of tau vector, there was robust expression of hyperphosphorylated tau (Fig. 3J), with a blank area in the SN pars compacta, likely due to neuronal loss in that area, consistent with earlier findings (Klein et al., 2005; Klein et al., 2008; Klein et al., 2009). In terms of the transduction pattern, both the AAV9 GFP and AAV9 tau vectors produced transgene expression that was more limited to the pars compacta at the low dose and more widespread to the pars reticulata at the middle dose (pars reticulata GFP expression is not evident in Fig. 3D due to the camera exposure time), with no evidence of glial transduction, as found previously (Klein et al., 2008). The transduction pattern was therefore similar for either vector. The middle dose of tau vector resulted in loss of TH immunoreactivity in the SN (Fig 3K). The middle dose of tau vector induced microgliosis, with elevated CD11b immunoreactivity away from the needle track in the SN (Fig. 3L), in contrast to either dose of the GFP vector or the lower dose of tau vector. With the highest dose of tau vector (3.6 × 1010 vg), the loss of TH staining was more complete and the microgliosis even more pronounced (not shown). The dopaminergic cell loss and microgliosis mirrored each other, as only the middle dose of tau vector resulted in loss of TH immunoreactivity loss and increased microgliosis. Similar to the results with microglial staining, the middle dose of tau also led to an increase in staining for astroglia in the SN at 2 weeks (not shown). We performed time-course studies for the microgliosis comparing the middle dose of tau or GFP vectors with lactated ringer's vehicle injections at 1, 3, 7, and 14 days, and found pronounced microgliosis only at 14 days for the tau vector, with no signs of microgliosis away from the needle track with the controls at any of the intervals tested (Fig. S1). The microglial marker that we used, CD11b, is known as ITGAM (integrin alpha M) in the rat, but curiously, changes in ITGAM mRNA were not as consistently robust as observed with antibody detection of the protein. There was a greater level of ITGAM message with highest dose of tau vector relative to the mid dose (2.92 fold, P = 0.003), although when comparing the mid dose of tau to empty vector, there was only 1.60 fold more with the tau vector (not significant). The fact that the CD11b immunohistochemistry was noticeably elevated by the mid dose of tau vector at two weeks suggests either a greater sensitivity of the antibody technique or the possibility of post-transcriptional regulation.
Discussion
We studied gene transcript levels changed by human tau overexpression targeted to the rat SN to address processes by which the microtubule-associated protein tau can be toxic to neurons. Multiple groups, doses, and controls were studied. It was clear that the levels of transcripts for a number of pro-inflammatory markers were elevated specifically in response to tau, complementing the existing literature (Ishizawa et al., 2000; Yoshiyama et al., 2007; Klein et al., 2009) implicating inflammation as an important component of tau induced neurodegeneration. There were trends for tau vector dose-response in terms of tau expression, microgliosis, and dopaminergic cell loss, as well as for specific pro-inflammatory marker mRNAs in the microarray and RT-PCR (e.g., IL-1B and TNF). We hypothesize that the transcript profile largely reflects the gliosis induced by tau which peaked at two weeks when the RNA samples were collected, although both the tau expression and the neuronal loss also increase over this period. We correctly predicted specific message changes in dopaminergic neurons based on loss of neurons stained for TH after tau gene transfer (Klein et al., 2009). The lowest dose of AAV9 tau did not cause neuron loss or glial infiltration and served as a baseline, despite unequivocal tau expression. The highest dose of tau created a more complete lesion of the SN and the greatest reductions of dopaminergic mRNAs in the microarray. The tau vector dosing achieved baseline, partial, and nearly complete lesioning based on TH immunoreactivity. The middle tau vector dose decreased dopaminergic mRNAs, TH, Ddc, dopamine transporter (DAT), and the dopamine 2 receptor, and axon terminal loss was indicated by decreases for synaptic protein mRNAs such as synaptotagmin and alpha-synuclein. However, the study could neither determine changes that took place definitively in neurons versus glia, nor to directly attribute the inflammatory markers to the tau expression, or the gliosis or the neuronal loss, that was concomitantly caused by tau. More work would be needed to distinguish changes in neurons versus glia (i.e., laser capture microdissection) and to discern the sequence relative to injection, induction of expression, gliosis, and neuron loss.
With respect to the control vectors, the empty vector produced few changes compared to vehicle controls, affecting only 10 messages mildly, and did not increase any inflammatory markers. The AAV9 GFP produced more changes and of greater amplitude than the empty vector, and changes occurred in a variety of pathway categories. The trend of lowered endogenous rat tau mRNA could reflect neurotoxicity of the GFP, although TH immunohistochemistry appeared to be unaffected in the GFP group.
For the middle dose of tau vector, there was upregulation in many mRNAs involved in inflammation response relative to controls, including transcripts for leukocyte markers, chemokines, cytokine receptors, complement components, and interferon-inducible proteins. The control AAV9 injections of either the empty vector or the GFP vector to the SN of rats did not produce gliosis; there were a total of two immune related mRNA changes in the GFP group, and none in the empty vector group. We therefore believe that the changes in the middle and high dose tau vector groups are tau-specific, and not due to the AAV or the GFP. In terms of the potential relevance, gliosis and inflammation may occur at an early stage in the pathogenesis of PSP (Ishizawa et al., 2000). In tau transgenic mice, microgliosis precedes tangles and neuron loss (Yoshiyama et al., 2007), and in a tau transgenic rat model, inflammatory markers co-localize with degenerating axons (Zilka et al., 2009), suggesting that gliosis and inflammation may be underlying processes in tau neurofibrillary degeneration. Our previous study suggested that microgliosis was an early event in the progressing dopaminergic cell loss and motoric deficit, and was related to an augmented disease state in aged rats relative to young (Klein et al., 2009). The previous results along with the gliosis and upregulation of pro-inflammatory mRNAs in this study further implicate gliosis to be a key part of tau's neurotoxic mechanism. Our data are consistent with several models studying the relationship of pro-inflammatory cytokines and tau pathology. Treatment of cultured neurons with TNF, or co-culturing activated microglia, induced tau aggregation in neurites (Gorlovoy et al., 2009). AAV-mediated overexpression of TNF in transgenic AD mice resulted in greater neuronal loss relative to controls (Janelsins et al., 2008). The anti-inflammatory drug minocycline reduced tau phosphorylation and insoluble aggregates in tau transgenic mice (Noble et al., 2009).
Upregulation of several components of the ubiquitin-proteasome pathway (see Oddo, 2008 for review) occurred, possibly connected to a compensatory mechanism to clear excess tau. These included the E2 conjugating enzymes Ube2n and Ube2l6, a potential E3 ligase Herc6, and proteasome subunits Psmb8 and Psmb9 (Lmp2) in various comparisons of tau versus controls. The proteasomal subunits Psmb8 and Psmb9 belong to the immunoproteasome which replace catalytic subunits of the proteasome when cells are exposed to pro-inflammatory cytokines IFN-gamma or TNF, and can accelerate the degradation of tau (Cardozo and Michaud, 2002). The immunoproteasome (for which Lmp2 is a marker) was reported to be upregulated in Alzheimer's and aged brains (Mishto et al., 2006). Another change that could be related to clearing excess tau was increased heat shock protein 1 (Hsp27), which has potential relevance to tau function (Shimura et al., 2004) and pathology (Sahara et al., 2007).
Aberrant cell-cycle re-entry has been hypothesized to contribute to tau neurodegeneration (see Lee et al., 2009 for review). We observed upregulation of mRNA for p21, a cell cycle inhibitor. In tau transgenic mice, p21 was found in the cytoplasm of neurons containing inclusions made of hyperphosphorylated tau (Delobel et al., 2006). However, our study could not distinguish whether the cell cycle mRNA changes occurred in tau transduced neurons, or the glia.
The tau gene transfer lowered mRNAs for dopaminergic markers and increased mRNAs for pro-inflammatory markers, which were validated by immunohistochemistry for TH or microglia, respectively. We titrated expression levels and tau-induced lesioning of dopaminergic neurons at an early time point of two weeks, which was concomitant with specific dose-dependent message changes. The profiling efforts after tau gene transfer in rats found gene expression changes that could be relevant to tau neurodegenerative diseases (Wakabayashi et al., 1994; Mirra et al., 1999; Di Maria et al., 2000; Poorkaj et al., 2002; Schneider et al., 2002), and one day lead to a specific gene product that could be rationally targeted.
Supplementary Material
Messages changed by AAV9 tau relative to AAV9 GFP at the middle dose of 1.2 × 1010 vector genomes at two weeks (Supplemental to Table 1).
Message changes associated with known proteins affected by AAV9 tau classified by function. This list shows the lower amplitude changes in continuation from Table 1. N = 5/group.
Messages changed by AAV9 tau relative to AAV9 empty at the middle dose of 1.2 × 1010 vector genomes at two weeks (Supplemental to Table 2).
Message changes associated with known proteins affected by AAV9 tau classified by function. This list shows the lower amplitude changes in continuation from Table 2. N = 5/group.
ESTs and unidentified messages changed by AAV9 tau relative to either AAV9 GFP or AAV9 empty at the middle dose (1.2 × 1010 vector genomes) at two weeks.
Messages that are not associated with known proteins that were changed by AAV9 tau relative to controls are listed by GenBank identifier. N = 5/group.
Time-course of CD11b immunoreactivity in the rat SN after AAV9 tau, AAV9 GFP, or vehicle injections at 1, 3, 7, and 14 days, at the middle dose from the microarray study (1.2 × 1010 vector genomes) for the AAV vectors. (A-D) Lactated ringers vehicle; (E-H) AAV9 GFP; (I-L) AAV9 Tau. Pictures were taken at equal camera settings, showing the needle track into the SN. Tau gene transfer caused an increased microgliosis response in the SN away from the needle track at 14 days, whereas CD11b immunoreactivity at other time points after tau gene transfer, and at all intervals with the controls, was limited to the needle track. Bar in A = 134 μm.
Acknowledgments
Paula Polk performed the microarray procedures. NIH Grant NS048450 and Society for Progressive Supranuclear Palsy supported the research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Cardozo C, Michaud C. Proteasome-mediated degradation of tau proteins occurs independently of the chemotrypsin-like activity by a nonprocessive pathway. Arch Biochem Biophys. 2002;408:103–110. doi: 10.1016/s0003-9861(02)00493-9. [DOI] [PubMed] [Google Scholar]
- Chen F, Wollmer MA, Hoerndli F, Münch G, Kuhla B, Rogaev EI, Tsolaki M, Papassotiropoulos A, Götz J. Role of glyoxalase I in Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:7687–7692. doi: 10.1073/pnas.0402338101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobel P, Lavenir I, Ghetti B, Holzer M, Goedert M. Cell cycle markers in a transgenic mouse model of human tauopathy: increased levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1. Am J Pathol. 2006;168:878–887. doi: 10.2353/ajpath.2006.050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maria E, Tabaton M, Vigo T, Abbruzzese G, Bellone E, Donati C, Frasson E, Marchese R, Montagna P, Munoz DG, Pramstaller PP, Zanusso G, Ajmar F, Mandich P. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann Neurol. 2000;47:374–377. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Ettinger RA, Moustakas AK, Lobaton SD. Open reading frame sequencing and structure-based alignment of polypeptides encoded by RT1-Bb, RT1-Ba, RT1-Db, and RT1-Da alleles. Immunogenetics. 2004;56:585–596. doi: 10.1007/s00251-004-0725-7. [DOI] [PubMed] [Google Scholar]
- Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE. Disease modifying therapy for AD? J Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- Gorlovoy P, Larionov S, Pham TT, Neumann H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J. 2009;23:2502–2513. doi: 10.1096/fj.08-123877. [DOI] [PubMed] [Google Scholar]
- Ho L, Xiang Z, Mukherjee P, Zhang W, De Jesus N, Mirjany M, Yemul S, Pasinetti GM. Gene expression profiling of the tau mutant (P301L) transgenic mouse brain. Neurosci Lett. 2001;310:1–4. doi: 10.1016/s0304-3940(01)02044-4. [DOI] [PubMed] [Google Scholar]
- Hochrainer K, Mayer H, Baranyi U, Binder B, Lipp J, Kroismayr R. The human HERC family of ubiquitin ligases: novel members, genomic organization, expression profiling, and evolutionary aspects. Genomics. 2005;85:153–164. doi: 10.1016/j.ygeno.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Lin WL, Tiseo P, Honer WG, Davies P, Dickson DW. A qualitative and quantitative study of grumose degeneration in progressive supranuclear palsy. J Neuropathol Exp Neurol. 2000;59:513–524. doi: 10.1093/jnen/59.6.513. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, LaFerla FM, Callahan LM, Federoff HJ, Bowers WJ. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Lin WL, Dickson DW. Tau gene transfer, but not alpha-synuclein, induces both progressive dopamine neuron degeneration and rotational behavior in the rat. Neurobiol Dis. 2005;20:64–73. doi: 10.1016/j.nbd.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Tatom JB, Diaczynsky CG, Salvatore MF. Tau expression levels from various adeno-associated virus serotypes produce graded neurodegenerative disease states. Eur J Neurosci. 2008;27:1615–1625. doi: 10.1111/j.1460-9568.2008.06161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Diaczynsky CG, Wang DB. Pronounced microgliosis and neurodegeneration in aged rats after tau gene transfer. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2008.12.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S, Van Broeckhoven C. Frontotemporal lobar degeneration: current concepts in the light of recent advances. Brain Pathol. 2007;17:104–114. doi: 10.1111/j.1750-3639.2007.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Peterson RB, Bajic V, Smith MA. Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer's disease. Neurochem Int. 2009;54:84–88. doi: 10.1016/j.neuint.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph AC, Kassubek J, Landwehrmeyer BG, Mandelkow E, Mandelkow EM, Burn DJ, Caparros-Lefebvre D, Frey KA, de Yebenes JG, Gasser T, Heutink P, Höglinger G, Jamrozik Z, Jellinger KA, Kazantsev A, Kretzschmar H, Lang AE, Litvan I, Lucas JJ, McGeer PL, Melquist S, Oertel W, Otto M, Paviour D, Reum T, Saint-Raymond A, Steele JC, Tolnay M, Tumani H, van Swieten JC, Vanier MT, Vonsattel JP, Wagner S, Wszolek ZK, Reisensburg Working Group for Tauopathies With Parkinsonism Tauopathies with parkinsonism: clinical spectrum, neuropathologic basis, biological markers, and treatment options. Eur J Neurol. 2009;16:297–309. doi: 10.1111/j.1468-1331.2008.02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynoga B, Morrison H, Price DJ, Mason JO. Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis. Dev Biol. 2005;283:113–127. doi: 10.1016/j.ydbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Song YH, Schweers O, Marx A, Mandelkow EM. On the structure of microtubules, tau, and paired helical filaments. Neurobiol Aging. 1995;16:347–354. doi: 10.1016/0197-4580(95)00026-b. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Murrell JR, Gearing M, Spillantini MG, Goedert M, Crowther RA, Levey AI, Jones R, Green J, Shoffner JM, Wainer BH, Schmidt ML, Trojanowski JQ, Ghetti B. Tau pathology in a family with dementia and a P301L mutation in tau. J Neuropathol Exp Neurol. 1999;58:335–345. doi: 10.1097/00005072-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, Spazzafumo L, Chiappelli M, Licastro F, Sorbi S, Pession A, Ohm T, Grune T, Franceschi C. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer's disease brains. Neurobiol Aging. 2006;27:54–66. doi: 10.1016/j.neurobiolaging.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Noble W, Garwood C, Stephenson J, Kinsey AM, Hanger DP, Anderton BH. Minocycline reduces the development of abnormal tau species in models of Alzheimer's disease. FASEB J. 2009;23:739–750. doi: 10.1096/fj.08-113795. [DOI] [PubMed] [Google Scholar]
- Oddo S. The ubiquitin-proteasome system in Alzheimer's disease. J Cell Mol Med. 2008;12:363–373. doi: 10.1111/j.1582-4934.2008.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Poorkaj P, Muma NA, Zhukareva V, Cochran EJ, Shannon KM, Hurtig H, Koller WC, Bird TD, Trojanowski JQ, Lee VM, Schellenberg GD. An R5L tau mutation in a subject with a progressive supranuclear palsy phenotype. Ann Neurol. 2002;52:511–516. doi: 10.1002/ana.10340. [DOI] [PubMed] [Google Scholar]
- Rolstad B, Vaage JT, Naper C, Lambracht D, Wonigeit K, Joly E, Butcher GW. Positive and negative MHC class I recognition by rat NK cells. Immunol Rev. 1997;155:91–104. doi: 10.1111/j.1600-065x.1997.tb00942.x. [DOI] [PubMed] [Google Scholar]
- Sahara N, Maeda S, Yoshiike Y, Mizoroki T, Yamashita S, Murayama M, Park JM, Saito Y, Murayama S, Takashima A. Molecular chaperone-mediated tau protein metabolism counteracts the formation of granular tau oligomers in human brain. J Neurosci Res. 2007;85:3098–3108. doi: 10.1002/jnr.21417. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Bienias JL, Gilley DW, Kvarnberg DE, Mufson EJ, Bennett DA. Improved detection of substantia nigra pathology in Alzheimer's disease. J Histochem Cytochem. 2002;50:99–106. doi: 10.1177/002215540205000111. [DOI] [PubMed] [Google Scholar]
- Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J Biol Chem. 2004;279:17957–17962. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- Song DY, Yang YC, Shin DH, Sugama S, Kim YS, Lee BH, Joh TH, Cho BP. Axotomy-induced dopaminergic neurodegeneration is accompanied with c-Jun phosphorylation and activation transcription factor 3 expression. Exp Neurol. 2008;209:268–278. doi: 10.1016/j.expneurol.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Oyanagi K, Makifuchi T, Ikuta F, Homma A, Homma Y, Horikawa Y, Tokiguchi S. Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol. 1994;87:545–553. doi: 10.1007/BF00293314. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski TQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Zilka N, Stozicka Z, Kovac A, Pilipcinec E, Bugos O, Novak M. Human misfolded truncated tau protein promotes activation of microglia and leukocyte infiltration in the transgenic rat model of tauopathy. J Neuroimmunol. 2009;209:16–25. doi: 10.1016/j.jneuroim.2009.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Messages changed by AAV9 tau relative to AAV9 GFP at the middle dose of 1.2 × 1010 vector genomes at two weeks (Supplemental to Table 1).
Message changes associated with known proteins affected by AAV9 tau classified by function. This list shows the lower amplitude changes in continuation from Table 1. N = 5/group.
Messages changed by AAV9 tau relative to AAV9 empty at the middle dose of 1.2 × 1010 vector genomes at two weeks (Supplemental to Table 2).
Message changes associated with known proteins affected by AAV9 tau classified by function. This list shows the lower amplitude changes in continuation from Table 2. N = 5/group.
ESTs and unidentified messages changed by AAV9 tau relative to either AAV9 GFP or AAV9 empty at the middle dose (1.2 × 1010 vector genomes) at two weeks.
Messages that are not associated with known proteins that were changed by AAV9 tau relative to controls are listed by GenBank identifier. N = 5/group.
Time-course of CD11b immunoreactivity in the rat SN after AAV9 tau, AAV9 GFP, or vehicle injections at 1, 3, 7, and 14 days, at the middle dose from the microarray study (1.2 × 1010 vector genomes) for the AAV vectors. (A-D) Lactated ringers vehicle; (E-H) AAV9 GFP; (I-L) AAV9 Tau. Pictures were taken at equal camera settings, showing the needle track into the SN. Tau gene transfer caused an increased microgliosis response in the SN away from the needle track at 14 days, whereas CD11b immunoreactivity at other time points after tau gene transfer, and at all intervals with the controls, was limited to the needle track. Bar in A = 134 μm.