Abstract
Dental pulp stem cells (DPSCs) are a unique precursor population isolated from post-natal human dental pulp and have the ability to regenerate a reparative dentin-like complex. Canonical Wnt signaling plays a critical role in tooth development and stem cell self-renewal through β-catenin. In this study, the regulation of odontoblast-like differentiation of DPSCs by canonical Wnt signaling was examined. DPSCs were stably transduced with canonical Wnt-1 or the active form of β-catenin, with retrovirus-mediated infection. Northern blot analysis found that Wnt-1 strongly induced the expression of matricellular protein osteopontin, and modestly enhanced the expression of type I collagen in DPSCs. Unexpectedly, Wnt-1 inhibited alkaline phosphatase (ALP) activity and the formation of mineralized nodules in DPSCs. Moreover, over-expression of β-catenin was also sufficient to suppress the differentiation and mineralization of DPSCs. In conclusion, our results suggest that canonical Wnt signaling negatively regulates the odontoblast-like differentiation of DPSCs.
Keywords: Wnt, mineralization, stem cell, dental pulp, osteopontin
INTRODUCTION
Dental pulp stem cells (DPSCs) are a unique mesenchymal stem cell (MSC) population that is present in the cell-rich zone and core of the pulp (Gronthos et al., 2002). These cells have the ability to differentiate into odontoblast-like cells, pulpal fibroblasts, adipocytes, and neural-like cells (Gronthos et al., 2002). Primary human DPSCs maintain their stem cell properties and continue to express STRO-1, even after cryopreservation and 20 rounds of passaging (Zhang et al., 2006). In the presence of dexamethasone, β-glycerophosphate (β-GP), and inorganic phosphate, DPSCs are capable of differentiating into an odontoblast-like lineage and expressing alkaline phosphatase. This transition is accompanied by deposition and mineralization of collagenous matrix (Liu et al., 2005). While DPSCs have great potential for dentin regeneration and tooth repair, molecular regulation of differentiation of DPSCs is currently poorly understood.
Growing evidence demonstrates that Wnt signaling plays a critical role in development and stem cell self-renewal (Reya and Clevers, 2005). Wnts are secreted glycoproteins that can exert their effects on neighboring cells in a paracrine manner. There are currently 19 identified Wnt family proteins, divided into two main categories, canonical and non-canonical, based on their role in cytosolic β-catenin stabilization (Reya and Clevers, 2005). Canonical Wnts transduce their signals through intracellular β-catenin. In the absence of Wnt proteins, β-catenin is associated with a cytoplasmic complex containing adenomatous polyposis coli (APC), glycogen synthase kinase-3β (GSK-3β), axin, and casein kinase 1. In this complex, GSK-3β constitutively phosphorylates β-catenin, resulting in its ubiquitination and degradation by the 26S proteasome. In the presence of Wnt stimulation, a frizzled receptor and the Wnt co-receptor LDL receptor-related protein 5 (LRP5) or LRP6 transduce signals to inhibit Axin/APC/GSK-3β activity. This inhibition leads to the accumulation of free cytosolic β-catenin. The elevated cytosolic β-catenin can translocate to the nucleus, form a complex with members of the T-cell factor (Tcf)/lymphoid enhancer factor (LEF) family of transcription factors, and activate the expression of Wnt target genes. In contrast, non-canonical Wnts transduce their signal independent of β-catenin (Reya and Clevers, 2005). Genetic studies have found that Wnt/β-catenin signaling plays an essential role in mesenchymal tissue development, including skeletal maturation and tooth formation (Kratochwil et al., 2002; Sasaki et al., 2005; Jarvinen et al., 2006). However, the effects of canonical Wnt signaling on osteogenic differentiation of MSCs and their ability to form bone remain controversial (Boland et al., 2004; de Boer et al., 2004; Gaur et al., 2005; Kang et al., 2007). In this study, we sought to determine whether canonical Wnt/β-catenin signaling regulates DPSC differentiation and mineralization.
MATERIALS & METHODS
Cell Culture and Retroviral Infection
DPSCs isolated from human adult third molars were kindly provided by Dr. Songtao Shi at The University of Southern California (Gronthos et al., 2000). DPSCs were cultured in α-modified Eagle’s medium (α-MEM) (Invitrogen, Carlsbad, CA, USA) supplemented with 15% fetal calf serum (FCS; Invitrogen), 10 mM L-ascorbic acid 2-phosphate (AA), 2 mM L-glutamate, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37° C and 5% CO2, as described previously (Gronthos et al., 2000). To generate DPSCs stably expressing Wnt-1 or β-catenin, we generated vector-containing retroviruses in 293T cells (Chen et al., 2001; You et al., 2002). DPSCs were infected with the retroviruses expressing hemagglutinin (HA) HA.11 epitope tagged Wnt-1, HA-β-catenin, or vector control in the presence of 6 μg/mL polybrene (Sigma-Aldrich, St. Louis, MO, USA). Thirty-six hrs after infection, cells were selected with neomycin (600 μg/mL) for 10 days. The resistant clones were pooled, and cells expressing Wnt-1 or β-catenin were confirmed by Western blot analysis. For growth rate analysis, 100,000 cells were plated onto a 15 × 60 mm plate. Plates were then trypsinized at 32, 56, 84, 122, and 172 hrs, and triplicate cell counts were performed visually with the use of a hemacytometer, with trypan blue staining for viable cells.
Alkaline Phosphatase Activity
DPSCs were induced to differentiate in α-MEM supplemented with 10 mM β-glycerophosphate, 10 nM dexamethasone, and 1.8 mM KH2PO4. Induced cells were rinsed with phosphate-buffered saline (PBS) and fixed in 70% ethanol. One mL per well of substrate solution was added; the plate was covered with foil and incubated at 37° C for 30 min. Substrate solution contained the following in 15 mL of Tris-HCl (pH 9.6): 12 mg Fast Blue BB Salt, 4 mg naphthol AS-TR phosphate, and 0.15 mL DMF. The naphthol was first dissolved in the DMF before being mixed with the Tris-HCl. The mix was filtered, and a 50-μL quantity of MgCl2 was added. Quantification of the staining density was performed with Scion Image software (Scion Corp., Frederick, MD, USA).
Alizarin Red Staining
Induced cells were rinsed with PBS and fixed in 70% ethanol for 1 hr at 4° C. Cells were stained with 40 mM Alizarin red, pH 4.2, at room temperature for 10 min on a shaker. Cells were rinsed 5x with water to remove unbound Alizarin red. PBS was added for an additional 15 min for further reduction of non-specific staining. Quantification of the staining intensity was done with Scion Image software.
Western Blot Analysis
DPSCs were harvested and underwent lysis in RIPA buffer (10 mM Tris-HCl, 1 mM EDTA, 1% SDS, 1% Nonidet P-40, 1:100 proteinase inhibitor cocktail, 50 mM β-GP, 50 mM sodium fluoride). We determined extracted lysate concentrations by measuring absorbance at 595 nm using a protein assay solution (Bio-Rad, Hercules, CA, USA). From 20- to 50-μg aliquots of cell lysates per sample were run on a 7.5% SDS-PAGE gel and then transferred to a PVDF membrane (Bio-Rad). Western blot analysis was performed as described previously (Yang et al., 2004). Primary antibodies used were as follows: monoclonal anti-HA.11 (Covance, Berkeley, CA, USA), monoclonal anti-Flag (Sigma, St. Louis, MO, USA), monoclonal anti-human β-catenin (BD Biosciences, San Jose, CA, USA), monoclonal anti-α-tubulin (Sigma), and polyclonal anti-TFIIB (Santa Cruz Biotech, Santa Cruz, CA, USA). Cytoplasmic and nuclear extracts were prepared as described previously (Chen et al., 2001; You et al., 2002).
Northern Blot Analysis
RNA was harvested with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). A 10-μg quantity of each sample was loaded onto a 1.4% MOPS gel and transferred to a nylon membrane (Bio-Rad). The membrane was cross-linked with UV. The human full-length cDNA probes for OPN, type I collagen, and osteonectin (ON) were obtained from Dr. L. Fisher at the National Institute of Dental and Craniofacial Research (Bethesda, MD, USA). A random-primed labeling kit was used to generate cDNA probes (Amersham Biosciences, Piscataway, NJ, USA), which were then labeled with [α-32P]dCTP (MP Biomedicals, Irvine, CA, USA) and purified on a micro-G-50 Sephadex column (Amersham Biosciences). Hybridization was performed as described previously (You et al., 2001).
Quantitative Polymerase Chain-reaction (qPCR) Analysis
Total RNA was extracted from cells at specific time-points with the TRIzol reagent (Invitrogen). From 2 to 5 μg of total RNA was converted to cDNA by means of the SuperScript First Strand Synthesis kit (Invitrogen). The resulting cDNA was diluted 1:20 and used for 20-μL reactions containing CYBR green master mix, dNTPs, primers, and Platinum Taq DNA Polymerase (Invitrogen). As an internal control, 18S cDNA was simultaneously quantified. All experiments were run in triplicate and repeated at least two times to confirm results. Primers for human bone sialoprotein (BSP) were designed based on sequence NM_004967 from the NCBI database and were as follows: 5′-CAGGCCACGATATTATCTT TACA-3′ (forward) and 5′-CTCCTCTTCTTCCT CCTCCTC-3′ (reverse).
RESULTS
Wnt-1 Increases β-catenin in DPSCs
To investigate whether DPSCs respond to Wnt stimulation, we stably transduced DPSCs with retroviruses expressing HA-Wnt-1 or control neomycin vector (Fig. 1A). Western blot analysis revealed that β-catenin in the cytosolic and nuclear compartments was increased in Wnt-1-expressing DPSCs (DPSC/Wnt-1) compared with control cells (DPSC/V), suggesting that Wnt-1 stabilizes β-catenin in DPSCs (Fig. 1B). Growth rate analysis revealed that there was no significant difference in the proliferation rates between DPSC/Wnt-1 and DPSC/V cells (Fig. 1C).
Figure 1.
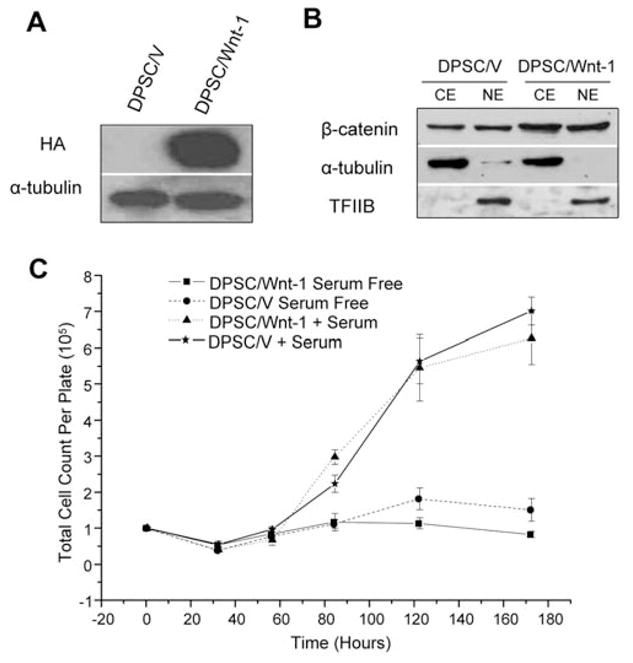
Wnt signaling induced β-catenin in DPSCs. (A) DPSCs stably expressing hemagglutinin (HA)-tagged Wnt-1 were established. DPSCs were stably transduced with retroviruses expressing Wnt-1 or control vector. Wnt-1 expression was determined by Western blot analysis. (B) Wnt-1 stabilized β-catenin in DPSCs. The cytosolic and nuclear extracts from both DPSC/V and DPSC/Wnt-1 cells were probed with anti-β-catenin monoclonal antibodies. For loading control, the cytosolic extracts (CE) were probed with anti-α-tubulin monoclonal antibodies, and nuclear extracts (NE) were probed with anti-TFIIB polyclonal antibodies. (C) Wnt-1 did not affect the proliferation of DPSCs. Both DPSC/Wnt-1 and DPSC/V cells were grown with or without serum. Cell numbers were counted in triplicate at the indicated times. CE, cytosolic extract; NE, nuclear extract; HA, hemagglutinin; TFIIB, transcription factor IIB.
To induce cell differentiation, we treated both DPSC/Wnt-1 and DPSC/V cells with dexamethasone and β-glycerophosphate. Type I collagen is the most abundant dentin matrix protein. Thus, we examined whether Wnt-1 regulated the expression of type I collagen. Northern blot analysis found that Wnt-1 slightly enhanced the gene expression of type I collagen (Fig. 2A). Interestingly, while OPN was barely detected in DPSC/V cells, OPN was significantly induced in DPSC/Wnt-1 cells. Upon the induction of differentiation, the level of OPN was slightly reduced in DPSC/Wnt-1 cells (Fig. 2B). ON was expressed at similar levels in DPSC/Wnt1 cells relative to DPSC/V cells (Fig. 2C). Unlike OPN and ON, BSP expression was strongly induced after differentiation of DPSC/V cells for 7 to 14 days, with only a transient induction noted in DPSC/Wnt-1 cells, as determined by qPCR (Fig. 2D).
Figure 2.
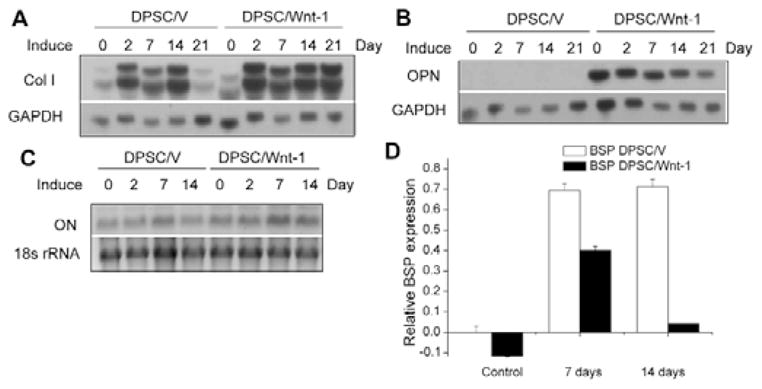
Wnt-1 strongly induced OPN expression and maintained extracellular matrix proteins. (A) Wnt-1 slightly enhanced type I alpha collagen expression by DPSCs during differentiation. (B) Wnt-1 strongly induced OPN expression in DPSCs. (C) Expression of ON was similar in both DPSC/V and DPSC/Wnt-1 cells. The total RNA from DPSC/Wnt-1 and DPSC/V cells was probed with 32P-labeled cDNA probes for type I alpha collagen, OPN, and ON. (D) Wnt-1 partially inhibited BSP expression in DPSCs, as determined by qPCR analysis. The results represent average values from triplicate assays, and the experiments were repeated twice. OPN, osteopontin; ON, osteonectin; BSP, bone sialoprotein.
Wnt-1 Inhibits Odontoblastic Differentiation of DPSCs
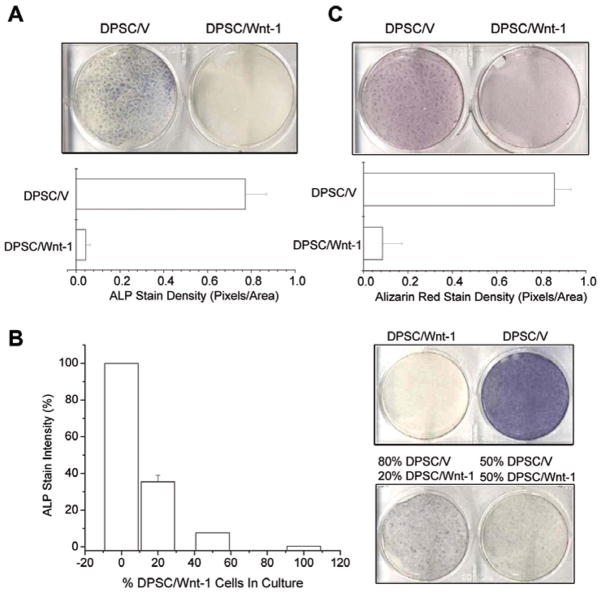
ALP activity is an early marker of both odontoblastic and osteoblastic differentiation and plays an important role in mineralization. We examined whether Wnt-1 affected ALP activity in DPSCs. ALP activity was strongly detected in DPSC/V cells after 5 days of culture in differentiation medium, but not in DPSC/Wnt-1 cells (Fig. 3A). ALP activity in DPSC/Wnt-1 cells was suppressed by more than 90% when compared with that in DPSC/V cells. Wnts are secreted glycoproteins that act by binding to cell-surface receptors (Reya and Clevers, 2005). Thus, we generated co-cultures of DPSC/V and DPSC/Wnt-1 cells to determine whether secreted Wnt-1 could inhibit ALP activity in DPSC/V cells. DPSC/V cells were co-cultured with DPSC/Wnt-1 cells, with initial DPSC/V cells plated ranging from 0% to 100% of the total culture (Fig. 3B). For comparison, the intensity of the staining in the pure DPSC/V cells was set to 100%, and the intensity of the pure DPSCs/Wnt-1 cells was set to 0%. The mixture of 20% DPSC/Wnt-1 cells with 80% control DPSC/V cells significantly inhibited over 65% of the ALP activity. Similarly, in a culture with 50% DPSC/V cells and 50% DPSC/Wnt-1 cells, over 90% of the ALP activity was inhibited (Fig. 3B). These results suggest that secreted Wnt-1 can inhibit ALP activity in DPSCs.
Figure 3.
Wnt-1 inhibits mineral formation by DPSCs. (A) Wnt-1 inhibits ALP activity in DPSCs after 5 days of culture in differentiating medium. The experiments were performed twice, and the results represent average values from triplicate assays. (B) Secreted Wnt-1 inhibited ALP activity. Mixed cultures were established with various ratios of DPSC/V to DPSC/Wnt-1 cells as indicated and grown in differentiating medium for 12 days. Results were quantified by densitometry to compare staining intensities. Sample staining was repeated in duplicate. DPSC/V results were set to 100% staining and DPSC/Wnt-1 results to 0% staining for standardization of the mixed cultures. (C) Wnt-1 inhibited mineral formation of DPSCs after 14 days of culture in differentiation medium. Both DPSC/V and DPSC/Wnt-1 cells were induced to differentiate with dexamethasone and β-GP. Two wks after induction, cells were stained with Alizarin red. The experiments were performed twice, and the results represent average values from triplicate assays. ALP, alkaline phosphatase; β-GP, β-glycerophosphate.
Since ALP activity plays a critical role in mineralization, we examined whether Wnt-1 impaired mineralization of DPSCs. After the prolonged induction of differentiation, we stained cells with Alizarin red to detect the level of calcium mineral deposition. While DPSC/V cells exhibited a staining density of 0.86 ± 0.073 pixels per area, DPSCs/Wnt-1 cells demonstrated a staining density of only 0.086 ± 0.088 pixels per area (Fig. 3C). Moreover, we found that secreted Wnt-1 could also inhibit the mineral formation of DPSCs in a manner similar to that demonstrated above with ALP (data not shown).
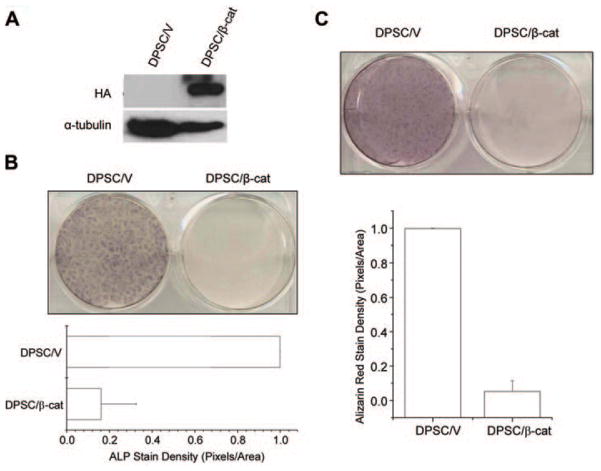
β-catenin Inhibits Odontoblastic Differentiation of DPSCs
Wnt-1 is a founder member of the Wnt family of proteins. Previously, studies from our group and others have demonstrated that Wnt-1 signals through β-catenin. To determine whether β-catenin was sufficient to suppress the differentiation of DPSCs, we stably transduced DPSCs with a mutant S37Aβ-catenin (Fig. 4A). The S37Aβ-catenin contains a serine-to-alanine mutation that protects the β-catenin from being phosphorylated and targeted for degradation by GSK3-β. Therefore, it is constitutively active within the cell. As was observed with DPSC/Wnt-1 cells, DPSC/β-cat cells failed to express ALP when compared with control cells after induction (Fig. 4B). While DPSC/V cells were highly mineralized, with a staining density of 0.999 pixels per area, S37Aβ-catenin-expressing DPSCs (DPSC/β-cat) showed poor mineralization and had a staining density of only 0.053 pixels per area (Fig. 4C).
Figure 4.
β-catenin inhibits mineral formation by DPSCs. (A) DPSCs stably expressing HA-tagged β-catenin were established. The expression of β-catenin was determined by Western blot analysis. (B) β-catenin inhibited ALP activity. The experiments were repeated twice, and the results represent average values from triplicate assays. (C) β-catenin inhibited mineral formation of DPSCs, as determined by Alizarin red staining. Cells from panels B and C were cultured in differentiation medium for 10 days. The experiments were repeated twice, and the results represent average values from triplicate assays. ALP, alkaline phosphatase; HA, hemagglutinin.
DISCUSSION
The results reported here are the first demonstration that canonical Wnt signaling can inhibit the odontoblast-like differentiation of DPSCs. Recent work from several groups has elucidated the importance of the canonical Wnt/β-catenin signaling pathway in skeletal development and the post-natal maintenance of bone mass (Krishnan et al., 2006). Loss-of-function mutations in human LRP5, the canonical Wnt co-receptor, result in osteoporosis-pseudoglioma syndrome, in which persons have a low bone mineral density and skeletal fragility (Gong et al., 2001). In contrast, activating mutations in human LRP5 have resulted in increased bone density in affected persons (Boyden et al., 2002). Several elegant studies with knock-out mice have also confirmed that the canonical Wnt/β-catenin signaling pathway is essential for bone development as well as the post-natal maintenance of bone mass (Day et al., 2005; Holmen et al., 2005; Krishnan et al., 2006). These important discoveries suggest that the activation of the Wnt/β-catenin signaling pathway may help to improve bone or dental tissue engineering. Because human adult bone marrow MSCs are one of the most promising adult stem cell populations for bone regeneration and repair, several studies have examined whether the canonical Wnt proteins, such as Wnt-1 or Wnt-3a, could enhance the osteogenic differentiation of MSCs. Paradoxically, several studies have found that Wnt-1 or Wnt-3a strongly inhibited the osteogenic differentiation of MSCs (Boland et al., 2004; de Boer et al., 2004; Cho et al., 2006). Consistent with these studies, we found that canonical Wnt signaling inhibits the odontoblast-like differentiation of DPSCs. Thus, targeting the canonical Wnt/β-catenin signaling pathway to enhance mineralized tissue regeneration may require further investigation.
Odontoblast-like differentiation of DPSCs has been assumed to be similar to that observed with bone marrow MSCs. Osteogenic differentiation is characterized by an increase in ALP expression within the first 4 days (Cheng et al., 1994). This is followed by a deposition of collagenous matrix and the subsequent maturation and mineralization of the matrix (Jaiswal et al., 1997). Since Wnt-1 did not inhibit the production of extracellular matrix, it is likely that the inhibition of mineralization by Wnt-1 is due to the lack of ALP activity. Studies of crystal nucleation have suggested that OPN is a potent inhibitor of mineral formation and mineral crystal growth and proliferation at high concentrations (Boskey et al., 2002). Interestingly, Wnt-1 strongly induces OPN expression in DPSCs. Therefore, it is possible that the induction of OPN by Wnt-1 may play a role in the suppression of mineral formation in DPSCs. Interestingly, we could not find that β-catenin induced OPN expression (data not shown). Thus, it is unlikely that OPN is an essential molecule in the inhibition of DPSC differentiation mediated by the canonical Wnt signaling pathway. While Wnt-1 did not affect ON expression, we found that Wnt-1 partially inhibited BSP expression. Thus, Wnt signaling may impair mineralization by inhibiting BSP. Finally, since the Wnt/β-catenin signaling pathway regulates adult stem cell properties, it is possible that canonical Wnt signaling may help to maintain MSCs in an undifferentiated state, thereby inhibiting the odontoblast-like differentiation of DPSCs. In the future, it will be interesting to develop a model system to test whether canonical Wnt signaling regulates the stem cell properties of DPSCs.
Acknowledgments
This work was supported by grants R01DE 016513 (C.-Y. W) and T32DE 007057 (E. S.) from the National Institute of Dental and Craniofacial Research.
Abbreviations
- DPSC
dental pulp stem cell
- ALP
alkaline phosphatase
- BSP
bone sialoprotein
- MSC
mesenchymal stem cell
- β-GP
β-glycerophosphate
- APC
adenomatous polyposis coli
- GSK-3β
glycogen synthase kinase-3β
- LRP
LDL receptor-related protein
- Tcf
T-cell factor
- LEF
lymphoid enhancer factor
- FCS
fetal calf serum
- AA
L-ascorbic acid 2-phosphate
- α-MEM
α-modified Eagle’s medium
- PBS
phosphate-buffered saline
- HA
hemagglutinin
- ON
osteonectin
- OPN
osteopontin
References
- Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. doi: 10.1002/jcb.20284. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, et al. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SL, Yang JW, Rifas L, Zhang SF, Avioli LV. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277–286. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- Cho HH, Kim YJ, Kim SJ, Kim JH, Bae YC, Ba B, et al. Endogenous Wnt signaling promotes proliferation and suppresses osteogenic differentiation in human adipose derived stromal cells. Tissue Eng. 2006;12:111–121. doi: 10.1089/ten.2006.12.111. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- de Boer J, Siddappa R, Gaspar C, van Apeldoorn A, Fodde R, van Blitterswijk C. Wnt signaling inhibits osteogenic differentiation of human mesenchymal stem cells. Bone. 2004;34:818–826. doi: 10.1016/j.bone.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Gaur T, Lengner CJ, Hovhannisyan H, Bhat RA, Bodine PV, Komm BS, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132–33140. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, et al. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci USA. 2006;103:18627–18632. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, Macdougald OA. WNT signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein alpha and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:14515–14524. doi: 10.1074/jbc.M700030200. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(−/−) mice. Genes Dev. 2002;16:3173–3185. doi: 10.1101/gad.1035602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–1209. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Jin T, Ritchie HH, Smith AJ, Clarkson BH. In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol Anim. 2005;41:232–238. doi: 10.1290/0502014.1. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Ito Y, Xu X, Han J, Bringas P, Jr, Maeda T, et al. LEF1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol. 2005;278:130–143. doi: 10.1016/j.ydbio.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Yang F, Yamashita J, Tang E, Wang HL, Guan K, Wang CY. The zinc finger mutation C417R of I-kappa B kinase gamma impairs lipopolysaccharide- and TNF-mediated NF-kappa B activation through inhibiting phosphorylation of the I-kappa B kinase beta activation loop. J Immunol. 2004;172:2446–2452. doi: 10.4049/jimmunol.172.4.2446. [DOI] [PubMed] [Google Scholar]
- You Z, Ouyang H, Lopatin D, Polver PJ, Wang CY. Nuclear factor-kappa B-inducible death effector domain-containing protein suppresses tumor necrosis factor-mediated apoptosis by inhibiting caspase-8 activity. J Biol Chem. 2001;276:26398–26404. doi: 10.1074/jbc.M102464200. [DOI] [PubMed] [Google Scholar]
- You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, et al. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157:429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, Shi S, Fan M, Jansen JA. Multilineage differentiation potential of stem cells derived from human dental pulp after cryopreservation. Tissue Eng. 2006;12:2813–2823. doi: 10.1089/ten.2006.12.2813. [DOI] [PubMed] [Google Scholar]