Abstract
The overall purpose of this study was to establish human head and neck squamous cell carcinoma (HNSCC) xenografts in mice by transplantation of surgical tumor tissue and to characterize the growth, histologic and vascular properties of these xenografts. Primary surgical specimens of HNSCC were xenografted into eight-to-twelve week old severe combined immunodeficiency (SCID) mice. Histologic features of primary HNSCC specimens, initial and established xenografts were compared for tumors established from three different head and neck subsites, namely, oral cavity, larynx and base of tongue (one tumor per site). Growth rates of xenografts were compared along with magnetic resonance imaging (MRI) measures of tumor vascularity and correlative CD31-immunostaining. Initial and established xenografts from all three sites demonstrated a squamous phenotype similar to the original patient tumor histology. Established xenografts of oral cavity and larynx exhibited increased keratinization (H&E) compared to initial xenografts and the primary tumor. No differences in tumor growth rates were observed between established xenografts from the different subsites. Xenografts established from SCC of the larynx exhibited increased microvessel density and lumen area (CD31 staining) along with enhanced permeability to the MR contrast agent compared to oral cavity and base of tongue tumors. Our results show that the combination of non-invasive imaging along with histologic evaluation of patient tumor xenografts offers a valuable platform for preclinical investigations in head and neck cancer. However, it is important to recognize the influence of tumor-host interactions on the histologic phenotype of transplanted tumors.
Keywords: Head and neck cancers, squamous cell carcinoma, patient tumor xenografts, magnetic resonance imaging, histopathology
INTRODUCTION
Squamous cell carcinomas constitute a majority of head and neck cancers and are etiologically linked to tobacco and alcohol exposure.1 Despite highly aggressive therapeutic intervention, loco-regional recurrence is a major challenge and the predominant cause of mortality.2 It is therefore crucial to evaluate and develop novel targeted therapeutic strategies for head and neck cancers.
Clinical trials provide the definitive evidence of safety and efficacy of any investigational therapeutic agent. However, preclinical investigations provide an important platform for (i) understanding pathophysiology of tumors, (ii) dissecting critical molecular pathways involved in tumor progression and metastasis, (iii) identifying potential therapeutic targets and (iv) examining the biological activity and toxicity profiles of experimental drugs prior to initiation of clinical trials in patients.3-5 A majority of preclinical models currently being used in cancer research are based on establishing tumors from cell lines passaged in culture. These models are widely employed due to their ease of use and economic feasibility for performing large-scale therapeutic studies; however, human tumor cell lines often do not recapitulate tumor biologic characteristics typically observed in the clinical setting.3,4 An ideal preclinical model system should adequately reflect the biological heterogeneity observed in the patient population, an important variable that influences the potential for therapeutic success. In this regard, the engrafting of surgical tumor tissue specimens into animals is considered to be a more appropriate tumor model system for preclinical assays compared to inoculation of tumor cell lines.6-8
The overall goal of this study was to establish and characterize head and neck squamous cell carcinoma (HNSCC) xenografts by transplantation of patient tumor specimens into severe combined immunodeficiency (SCID) mice. Surgical specimens of HNSCC were initially xenografted into SCID mice to determine the ‘take-rate’ in vivo. To investigate the biologic and angiogenic heterogeneity in head and neck cancer, SCC xenografts established from a primary tumor of the oral cavity, larynx and base of tongue were examined. Histological features of the primary tumor specimens were compared to initial and established xenografts. Microvessel density and lumen size were calculated from CD31-stained sections of established SCC xenografts from all 3 sites. Contrast-enhanced magnetic resonance imaging (CE-MRI) was used to estimate differences in blood volume and permeability between the xenografts.
RESULTS
Establishment of HNSCC xenografts from patient tumor tissues
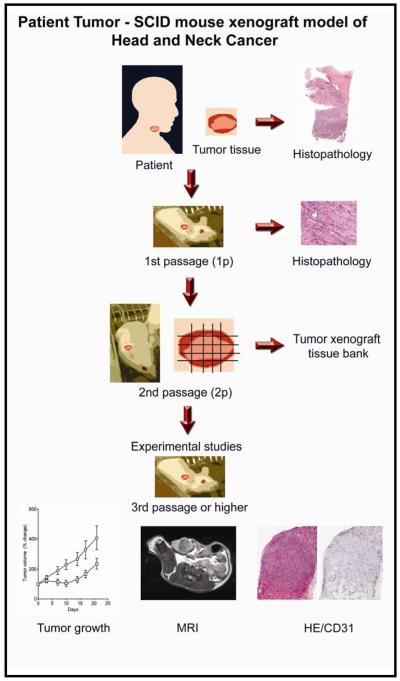
The basic work flow involved in establishing HNSCC xenografts in SCID mice at Roswell Park is illustrated in Figure 1. Surgically resected tumor tissue specimens are initially transplanted into animals to examine tumor ‘take-rate’ in vivo. Successfully established xenografts are then harvested from donor mice and subsequently transplanted into recipient mice for further in vivo passaging. Tissue sections of the original patient tumor specimen, the initial passage (1p) and a later passage (3p-6p) are evaluated and compared for histologic characteristics. Tumor xenografts from the initial passage are also frozen to maintain a tumor bank from the patient specimens and potentially provide a source for isolation and future expansion of tumor cell population. A total of 29 primary HNSCC specimens were grafted subcutaneously into SCID mice with a successful tumor ‘take-rate’ of ~60%. From this group, 3 xenografts representing different SCC sites, namely, oral cavity, larynx and base of tongue were selected for further evaluation of in vivo growth, histology and vascular properties.
Figure 1. Patient tumor-SCID mouse model of head and neck cancer.
The figure depicts the basic workflow algorithm involved in establishing and characterizing patient tumor xenografts in SCID mice. Surgical samples of head and neck squamous cell carcinomas (HNSCC) confirmed by histopathologic assessment were serially passaged in vivo. Engraftments were considered successful when tumors could be established over 2 passages (2p). For experimental studies, 3-5p tumors were utilized. Histologic assessment was performed at all stages of tumor transplantation for comparison between original patient tumors and xenografts. Non-invasive magnetic resonance imaging (MRI) based assessment of tumor vascularity was correlated with immunohistochemistry and tumor growth.
Histologic comparison of initial passage and established HNSCC xenografts with patient specimens
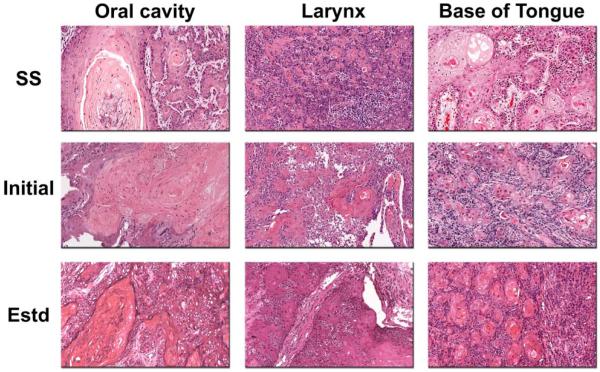
We first examined the histologic characteristics of the three HNSCC xenografts and compared them to the original patient tumor histology. Figure 2 shows digitized images of H&E sections of the primary tumor (surgical specimen; SS), initial passage and established xenografts of the three different SCC subtypes. Histologic assessment of both initial and established passage xenografts revealed a squamous phenotype with the formation of keratin pearls similar to the original patient tumor histology. Both initial and established xenografts maintained the cellular differentiation status observed in the primary patient tissue specimen with minimal areas of tumor necrosis visible in established xenografts (Table 1). However, the degree of keratin production by the neoplastic cells was higher in the xenografts compared to the primary tumor. As shown in Table 1, the keratinization scores of SCC xenografts of the oral cavity [initial – 5; established – 4.3 (mean from 3 tumors)] and larynx [initial – 3; established – 4.75 (mean from 4 tumors)] were greater than the original primary tumor (oral cavity – 2; larynx −2).
Figure 2. Comparative histology of the original patient tumors with initial passage and established xenografts of HNSCC.
Histologic sections of the original surgical specimen (SS), initial passage and established (estd) xenografts established in SCID mice are shown for three HNSCC samples. The samples represent SCC of the oral cavity, larynx and base of tongue. The degree of differentiation and keratinization was assessed using a grading scale as described in the Materials and Methods section. Initial passage and established xenografts from all three samples retained the histologic characteristics of the original surgical specimen.
Table 1. Comparative histologic analysis of keratinization, differentiation and necrosis in primary tumor tissues and xenografts established in SCID mice.
| Xenograft ID | Specimen | Keratinization† | Differentiation‡ | Necrosis§ |
|---|---|---|---|---|
| Oral cavity | Surgical specimen (n=1) | 2 | 1 | 1 |
| Initial passage (n=1) | 5 | 1 | 1 | |
| Established xenografts (n=3) | 4.33 | 1.33 | 1 | |
| Larynx | Surgical specimen (n=1) | 2 | 2 | 2 |
| Initial passage (n=1) | 3 | 2 | 2 | |
| Established xenografts (n=4) | 4.75 | 1.25 | 1.25 | |
| Base of Tongue | Surgical specimen (n=1) | 4 | 1 | 1 |
| Initial passage (n=1) | 3 | 2 | 1 | |
| Established xenografts (n=5) | 4.4 | 1 | 1 | |
Grading scale:
- 0 = non-keratinizing
- 1 = minimal keratinization (intracellular keratin production)
- 2 = focal extracellular keratin production (<25% tumor volume )
- 3 = moderate extracellular keratin production (25-50% tumor volume )
- 4 = extensive extracellular keratin production (51-75% tumor volume )
- 5 = marked extracellular keratin production (>75% tumor volume )
- 1 = Well differentiated; 2 = Moderately differentiated; 3 = Poorly differentiated
- 1=0-5%; 2=5-25%; 3=26-50% 4= >50%
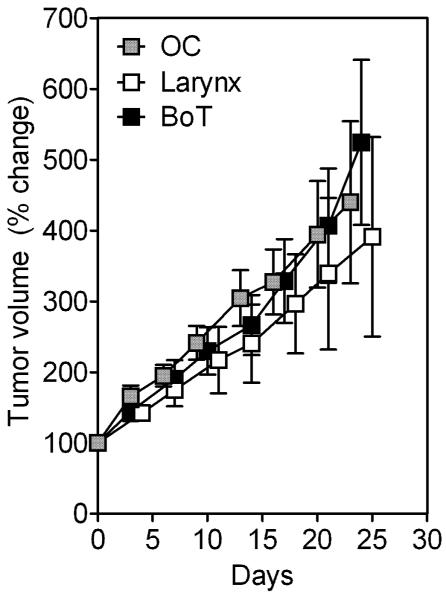
We then examined differences in growth rates of established (3rd passage) SCC xenografts of the oral cavity (OC, n=8), larynx (n=7) and base of tongue (BoT, n=8) by comparing the change in tumor volume over a 30-day period following implantation (Figure 3). Although larynx SCC xenografts exhibited slower growth rates compared to BoT or OC xenografts, this difference was not statistically significant (p>0.5).
Figure 3. Growth of HNSCC xenografts in SCID mice.
Growth curves of three HNSCC xenografts (3rd passage) plotted as a percent change in tumor volume over time. Xenografts were established from patient specimens of SCC of the oral cavity (OC, n=8), larynx (n=7) and base of tongue (BoT, n=8). No significant difference was observed between the growth curves of the three tumor types.
Immunohistochemical assessment of vascular differences between SCC xenografts
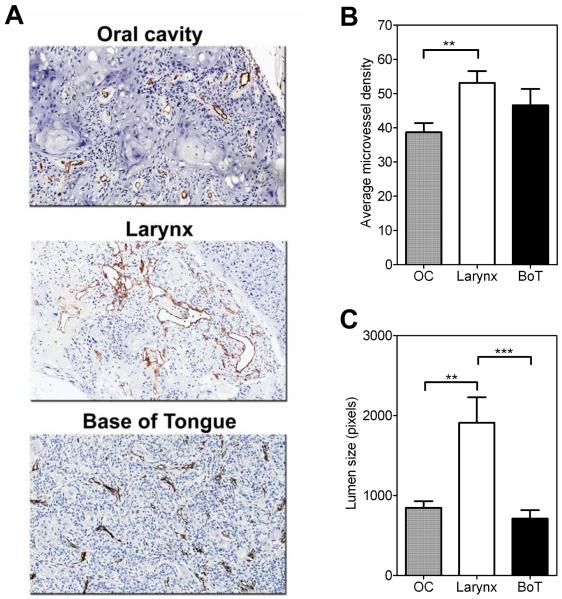
To compare differences in vascular characteristics between the three SCC xenografts immunostaining of tissue sections for the endothelial cell marker CD31 (PECAM-1) was performed. Tissue sections from all 3 xenograft types showed strong CD31-immunoreaction with distinctly visible sharp vessel outlines (Figure 4A). Microvessel density (MVD) counts and vessel lumen size were estimated in CD31-immunostained sections of established xenografts of all three tumor types (total of 15 fields/tumor type). The MVD counts for the three SCC xenograft types were as follows: OC (38.67 ± 2.7), larynx (53.13 ± 3.4), BoT (46.60 ± 4.7). Statistical analysis revealed a significant difference in MVD counts (Figure 4B) between larynx and OC xenografts (two-tailed t test p<0.01). No statistically significant difference was observed between the MVD counts of established SCC xenografts of the larynx and BoT or between BoT and OC xenografts (p>0.05). Vessel lumen size measurements of SCC xenografts of the larynx were also significantly greater than BOT (p<0.001) or OC (p<0.01) SCC xenografts (Figure 4C).
Figure 4. Immunohistochemical assessment of vascular differences between OC, larynx and BoT xenografts.
(A) Digitized images of CD31-immunostained sections of the three HNSCC xenografts. Average microvessel density counts (B) and vessel lumen size (C) obtained by analysis of CD31 stained sections of the 3 HNSCC xenograft subtypes (OC, larynx and BOT) are also shown. CD31+ endothelial clusters (brown) with clearly visible lumina were counted in 5 randomly selected fields at 20x magnification in 3 samples per xenograft type (a total of 15 fields/xenograft type) and analyzed for statistical significance using unpaired t tests. **p<0.01; ***p<0.001
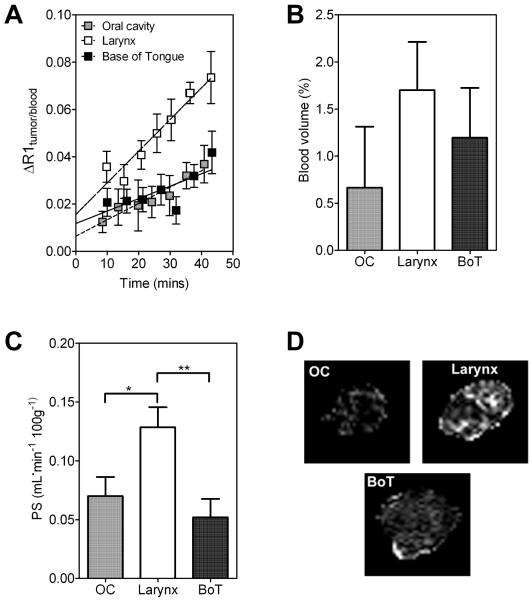
MRI-based detection of differences in vascular function between HNSCC xenografts
Contrast-enhanced MRI was utilized to examine differences in vascular function between the three SCC xenografts in vivo (Figure 5). Quantitative estimates of the contrast agent concentration within tumors were indirectly obtained by measuring the change in longitudinal relaxation rate (ΔR1) in all three SCC xenograft types. The methodology for measuring vascular parameters based on a macromolecular contrast-enhanced MRI has been previously described in several reports.9-11 Following intravenous administration of a large molecular weight Gd-based contrast agent, the initial enhancement of relaxation in tumor voxel is related to its fractional blood volume (BV) and the subsequent increase in relaxation is a result of leakage of the contrast agent from the blood vessels into the extravascular compartment.9,10 Therefore, plotting the ratio of ΔR1 in the tumor to the enhancement in blood over time provides an estimate of the BV (y-intercept) and the permeability of the tumor vessels to the contrast agent (slope). Mean normalized signal responses (ΔR1tumor/blood) over time for all 3 SCC xenografts are shown in Figure 5A. Linear regression analysis of the normalized change in ΔR1 (tumor/blood) revealed a greater rate of contrast enhancement (slope) in SCC xenografts of the larynx compared to OC (p<0.01 between slopes) and BoT (p<0.05 between slopes) tumors. Values of BV of individual tumors were calculated for all 3 SCC sub types from the ratio of tumor-to-blood signal at t=0 by extrapolating the dynamic signal response curves (ΔR1). Although a trend towards lower BV values was seen in OC xenografts compared to larynx or BoT xenografts, this was not statistically significant (p>0.1, Figure 5B). Similarly, permeability surface area product (PS) values of individual tumors were calculated and analyzed for statistical significance between the three different xenograft types. As shown in Figure 5C, significantly higher PS values were observed for larynx SCC xenografts (0.124 ± 0.01) compared to OC xenografts (0.067 ± 0.01, p<0.05) and BoT xenografts (0.050 ± 0.01, p<0.01). Corresponding PS maps calculated on a pixel-by-pixel basis revealed increased extravasation of the contrast agent in larynx SCC xenografts compared to OC and BoT tumors (Figure 5D).
Figure 5. Contrast-enhanced MRI of HNSCC xenografts in vivo.
(A) Change in longitudinal relaxation rate (ΔR1tumor:blood) following administration of the intravascular contrast agent, albumin-GdDTPA in established HNSCC xenografts from all 3 sites (OC, larynx and BoT). Estimates of blood volume (BV) and permeability surface area product (PS) were obtained by kinetic analysis of the normalized normalized ΔR1 (tumor/blood) as shown in bar graphs (B) and (C) respectively. (D) Panel of images represent calculated PS maps (scale 0-0.5 ml/min/100g) of three tumors from each of the HNSCC subsites. Increased extravasation of the contrast agent was observed over the post contrast imaging period in SCC xenografts of the larynx compared to OC and BoT.
DISCUSSION
The biological diversity of SCCs of the head and neck region is well-recognized.2 It is therefore important to develop research models that adequately reflect this diversity. In this study, subcutaneous HNSCC xenografts were established by transplantation of primary surgical tumor specimens into immunocompromised mice. Histologic and growth curve analyses of established xenografts were performed along with MRI based assessment of tumor vascularity. Our results demonstrate the feasibility of establishing SCC patient tumor xenografts from minimal surgical tissue. The combination of non-invasive imaging and histologic assessment allowed for assessment of the influence of tumor-host interactions on the histologic and vascular characteristics of transplanted tumors.
Patient tumor xenograft models of cancer have been previously described by us and others.6-8,12,13 Consistent with these published reports for other cancer sites, our results demonstrate that HNSCC xenografts obtained by transplantation of patient tissues retain the histologic phenotype and morphology of the original tumor (Figure 2). Both initial and established xenografts retained the histologic phenotype and the differentiation status of the original patient tumor specimen. In our study, established SCC xenografts of the OC and larynx showed increased degree of keratinization (Table 2) compared to the original tumor specimen. Similar observations on the influence of the host on the growth and biology of transplanted xenografts have been previously reported.14-17 Chen et al., showed that tumors established in nude mice were more differentiated and exhibited less cellular pleomorphism than their original patient tumors suggesting that the gnotobiotic model system either selects or induces a more differentiated phenotype of tumor growth.14 The exact mechanism(s) that contribute to differences in keratinization between the primary tumor specimen and the associated xenografts is not clear. However, it has been suggested that the change in differentiation status of the xenografts is likely related to both the inherent proliferative potential of the tumor cell population and the supporting host.14,15 Keratin expression could also be influenced by the growth factors and extracellular matrix proteins in the tumor tissue milieu.15A study by Wennerberg et al., showed that the size and histologic grade of the primary tumor influenced the successful ‘take’ of xenografts in vivo with higher frequency of tumor cell lines generated from T4 and poorly differentiated tumors.16 At the time of preparation of this manuscript, a total of 29 primary HNSCC specimens were grafted subcutaneously into SCID mice with a successful tumor ‘take-rate’ of ~60%. This is comparable to findings from the published literature in which take rates ranging from 25-85% have been reported for head and neck SCC xenografts.17,18 Braakhuis and colleagues evaluated a total of 130 human head and neck cancers transplanted subcutaneously into nude mice and observed that poorly differentiated SCCs grew more readily than moderately or well-differentiated tumors.17 In our study, we did not observe any significant growth differences between the three xenografts (Figure 3). However, none of the original tumors evaluated in this study were poorly differentiated, i.e., comprised of highly proliferative, immature cells. In our experience, while the ‘take rate’ does not appear to correlate with tumor site, careful evaluation of the viability of the primary surgical specimen appears to be an important factor.
Studies comparing the vascular morphology of primary tumors and xenografts have also been reported in the literature.19 Lauk and coworkers observed an increase in the distance between tumor cells and blood vessels in histologic sections of xenografts compared to the original patient biopsy material.19 It is well known that HNSCCs exhibit considerable variability in clinical behavior and aggressiveness depending on the specific anatomic location of the primary tumor. Heterogeneities in vascularization exist among head and neck cancer sites and have been previously been shown to influence tumor response to therapy.20-22 Given the biological heterogeneity of HNSCC, we examined the angiogenic characteristics of xenografts established from primary tumors of the OC, larynx and BoT. Vascular differences between the three HNSCC xenografts were assessed by estimating MVD counts from CD31-immunostained tumor sections (Figure 4). Our results showed increased MVD in larynx SCC xenografts compared to OC xenografts. No difference was seen between SCC xenografts of the larynx and base of tongue tumors. Vessel lumen measurements also showed increased lumen size in SCC xenografts of the larynx compared to OC or BOT tumors. Although we did not investigate the possible mechanisms behind the observed vascular differences, similar findings on angiogenic heterogeneity within HNSCC have been reported.23-26 Kyzas et al., demonstrated higher VEGF immunostaining in SCCs of larynx and the oral cavity compared to tumors in the lower lip with a strong correlation to overall survival.23 The switch to the angiogenic phenotype has also been reported as an early event in laryngeal cancers with alterations in vessel morphology observed in relation to the growth pattern and differentiation status.24 Beatrice et al., have shown microvessel counts to be an important biological parameter that can prognosticate disease relapse and survival in patients with SCCs of the larynx.25 Recently, using gene expression arrays, Hasina et al., have demonstrated inter-tumoral variation in the angiogenic mediators among various anatomic subsites of HNSCC.26
Several preclinical and clinical studies investigating tumor angiogenesis have utilized MVD as a surrogate marker of angiogenesis. While some studies have shown strong correlation between MVD and disease progression, others have shown no correlation with prognosis.23,27,28 This discrepancy can at least in part be attributed to technical factors such as staining procedure utilized, endothelial epitope chosen and variable analytical criteria (hot spots versus entire tumor sections) applied to obtain the MVD counts.23,25,27,28 Furthermore, the invasive nature of the technique precludes serial assessment and does not provide information on vascular function. In this regard, imaging methods such as MRI offer the ability to provide anatomic and functional information of living tissues in a non-invasive manner with high spatial and temporal resolution. Therefore, in addition to immunohistochemistry, we utilized non-invasive contrast-enhanced MRI to examine differences in vascular function between the three SCC xenografts in vivo (Figure 5).
Contrast-enhanced MRI (CE-MRI) is a widely used advanced imaging technique that enables visualization and quantitation of tumor perfusion/oxygenation without the use of radioactive tracers.29 In CE-MRI, the change in signal enhancement following intravenous administration of a contrast agent can be calculated to provide an indirect estimate of the functional vasculature within the tumor.9,11,29 In HNSCC patients, changes in MRI parameters of blood flow have been shown to predict tumor response to radiotherapy.30 We have previously demonstrated the usefulness of contrast-enhanced MRI in characterizing the response of HNSCC xenografts to antivascular therapy.11 Using the same technique, we assessed the BV and PS of the three SCC xenografts. No differences in BV were observed between the three xenografts evaluated; however, xenografts of the primary SCC of the larynx exhibited increased PS values compared to OC and BoT tumors (Figure 5). While vascular differences between head and neck tumor sites have been demonstrated in patients, a majority of these studies have utilized immunohistochemical markers of tissue perfusion and hypoxia.20-23 Only a few recent studies have utilized functional imaging techniques to study tumor vascular parameters in patients.31,32 Van Cann et al., have reported the utility of dynamic contrast-enhanced MRI (DCE-MRI) in the assessment of mandibular invasion in squamous cell carcinoma patients.31 Bisdas and colleagues have recently shown that pretreatment estimates of tumor microcirculatory parameters such BV and PS based on perfusion CT may allow for prediction of outcome in patients undergoing chemoirradiation for SCCs of the oral cavity, oropharynx and hypopharynx.32 Further investigation into the implications of vascular differences between the head and neck subsites in clinical studies would be useful, particularly in response to therapies targeted towards the vasculature.
In summary, the patient tumor xenograft system combined with the use of an imaging based approach provides a clinically-relevant in vivo platform that recapitulates the biological heterogeneity of HNSCC and could serve as a valuable preclinical model for evaluation of novel experimental therapeutics. However, several limitations of the present study have to be recognized. Firstly, while we have demonstrated the utility of establishing a head and neck tumor model from primary patient tumor specimens, tumors were studied in an ectopic (subcutaneous) environment rather than their true orthotopic tissue microenvironment. Previous studies have clearly demonstrated the influence of the microenvironment on histologic, angiogenic and metastatic ability of tumors.3,4,33 The value of orthotopic tumor models has also been demonstrated with the use of patient specimens.3,4,33. Therefore, the influence of the non native environment on the phenotype of the xenografts cannot be ruled out. Secondly, although human tissues were grafted into mice, the blood vessels supporting the tumor are still believed to be derived from the host, i.e. murine in nature (34). We are currently developing immunohistochemical methods that will allow detection of mouse CD31 and human CD34 in the same tissue sample. Once the methods are optimized, we plan on carrying out a systematic analysis of the vascular characteristics of primary patient specimens and their associated xenografts. These studies could provide useful information on the sensitivity of human vessels to antiangiogenic or vascular disrupting agents. And finally, experiments are currently underway to perform a comparative evaluation of the activity of novel targeted therapies in the patient tumor xenograft system and tumors established from HNSCC cell lines. The results of these studies could provide relevant information on the efficacy of targeted agents in vivo prior to clinical evaluation.
MATERIALS AND METHODS
Patient tissue procurement
All surgical procedures in patients were performed in the operating rooms at Roswell Park Cancer Institute (RPCI) and tumor tissues processed through the Pathology Resource Network. Tissue specimens were obtained from patients following written consent and under a research protocol approved by the Institutional Review Board and the research ethics committee. 3 primary patient tumor specimens representing 3 head and neck subsites were studied. The tumor site/stage of the specimens were as follows: (i) Oral cavity (OC) - SCC Right retromolar trigone, T3N0M0, (ii) Larynx - SCC Larynx, T1 and (iii) Base of Tongue (BoT) - SCC base of tongue, T1N2b. Gross histologic examination of samples was performed for identification of abnormal/normal areas prior to transplantation into animals.
Animals
Experimental studies were carried out using six-to-eight week old CB.17 SCID/SCID with an average body weight ~20 g. Mice were kept in sterile cages (4-5 mice per cage) in a pathogen-free environment and provided with food and water ad libitum. Animals were housed in the vivarium within the laboratory animal resource at RPCI in air conditioned and light controlled rooms (12 hour cycles).
Patient tumor xenografts
We have previously described the use of patient tumor xenografts in SCID mice.9,10 Briefly, surgical HNSCC specimens were obtained shortly after resection and cut into multiple smaller fragments (~2 mm in size) and placed in tissue culture medium (RPMI 1640) under sterile conditions. Individual pieces of tumor tissue were then implanted subcutaneously into SCID mice under isoflurane anesthesia. Three-to-four animals per tumor specimen were used for this initial passage of tumors. Following implantation, animals were monitored periodically for tumor growth. When tumors reached ~1 cm in diameter, animals were euthanized and tumors were subsequently passaged into naïve mice (2nd passage). Tumors were considered to have successfully engrafted when these tumors grew in vivo. For experimental studies, established tumors (3rd passage or higher) were utilized. All surgical implantation procedures were carried out under aseptic conditions in a laminar flow hood.
Tumor growth measurements
Tumor growth was monitored by periodic visual inspection at the site of implantation and the dimensions of the xenografts were measured with a Vernier caliper once every 2-3 days. Tumor volume was calculated using the formula
where V is the tumor volume, LD is the longest tumor diameter and SD is the shortest tumor diameter. Tumor growth curves were plotted as percent change in tumor volume relative to initial tumor volume over a 30-day period.
Histologic Assessment and Immunohistochemical Evaluation
All slides were evaluated by a board-certified pathologist (M.M.) with a practice focus in head and neck pathology. Tissue sections were stained with Harris hematoxylin (Poly Scientific, Bay Shore, NY). Immunostaining of tissue sections for the endothelial cell adhesion molecule, CD31, was performed as previously described.11 Briefly, excised tissues were placed in zinc-formalin fixative for 18 hours and subsequently transferred to 70% ethanol, dehydrated, and embedded in paraffin. 5-μm thick sections were stained with rat anti-mouse CD31 MAb (PECAM-1; MEC 13.3; BD Biosciences, San Jose, CA) at 10μg/ml concentration for 60 min at 37°C. Duplicate slides stained with an isotype match (rat IgG2a also at 10μg/ml) instead of the primary antibody were used as negative controls. All slides were scanned and digitized using the ScanScope XT system and ImageScope software (Aperio Technologies, Version 9.1; Vista, CA) at a magnification of 20X.
The degree of keratinization was evaluated based on a scoring system ranging from 0-5 on the following scale: 0 - non-keratinizing, 1 - minimal keratinization (intracellular keratin production), 2 - focal extracellular keratin production (<25% tumor volume), 3 - moderate extracellular keratin production (25-50% tumor volume), 4 - extensive extracellular keratin production (51-75% tumor volume), 5 - marked extracellular keratin production (>75% tumor volume). Tumor differentiation was scored as follows: 1 - well differentiated tumors, 2 - moderately differentiated tumors and 3 - poorly differentiated tumors. Tissue necrosis was assessed on a scale of 1-4 based on the percentage of necrosis visible on the tissue sections: 1 = 0-5%; 2 = 5-25%; 3 = 26-50%, 4 = >50%.
To obtain an estimate of the average microvessel density in the three different xenograft types (OC, larynx, BoT), CD31+ endothelial clusters (brown) with clearly visible lumina were counted in 5 randomly selected fields at 20x magnification using ImageScope software (Version 9.1; Aperio Technologies, Vista, CA). Tissue sections were analyzed from 3 samples per xenograft type (a total of 15 fields/xenograft type) and analyzed for statistical significance using an unpaired t test. Vessel lumen size measurements were performed on CD31-stained tissue sections (15 fields/xenograft type at 20x magnification) using the medical imaging software Analyze™ (AnalyzeDirect, Overland Park, KS) and reported as average number of pixels.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed in a 4.7T scanner (General Electric, Fremont, CA) with AVANCE digital electronics (Bruker Medical, Billerica, MA), a removable gradient coil insert (G060) and a custom designed 35 mm radiofrequency transmit/receive coil. MRI examinations were performed in mice bearing established xenografts (3rd passage or higher) with matched tumor volumes for all 3 tumor types approximately 30-50 days post implantation. Anesthetized mice were placed on an MR-compatible sled equipped with temperature and respiratory sensors and positioned in the scanner. Preliminary scout images were acquired on sagittal and axial planes for positioning of slices for contrast-enhanced MRI. T1-weighted (T1W) contrast-enhanced MRI was performed using the macromolecular contrast agent, albumin-gadopentetate dimeglumine (albumin-GdDTPA) as described previously (11). T1-relaxation rates (R1=1/T1) were calculated using a saturation recovery, fast spin echo scan with variable repetition times (TR) 360-6000ms with the following parameters: TEeff = 25 ms, matrix size 128 × 96, field-of-view (FOV) 3.2 × 3.2 mm, 1mm thick slices. A series of ten T1-weighted images (3 precontrast and 7 post contrast) were acquired over a 45 minute period. Albumin-Gd-DTPA was administrated at a dose of 0.1 mmol/kg as a bolus via tail-vein injection after completion of 3 baseline precontrast images. To accurately determine the vascular relaxation enhancement after administration of albumin-GdDTPA, a balanced steady state precession, inversion recovery technique (TrueFISP) was used. R1 measurements were obtained in the vena cava of 5 SCID mice before and after albumin-GdDTPA over ~45 minutes using the TrueFISP sequence using the following parameters: matrix size 128 × 128, FOV 3.2 × 3.2 cm, number of slices 1, slice thickness 1.5mm, TR 3ms, TE 1.5ms, number of averages 1, number of frames 100, number of segments 16, acq time 2m40s.
Raw image datasets were transferred to a workstation for post processing using Analyze™ (AnalyzeDirect, Overland Park, KS) and MATLAB (Version 7.0, Mathworks Inc., Natick, MA). Regions of interest (ROI) were manually defined for the entire tumor, blood vessel, kidneys and murine muscle tissues at each time point. At least 2-3 slices were evaluated for each tumor type. The change in R1 (ΔR1) following contrast agent injection was assumed to be proportional to the tissue concentration of the contrast agent. Blood R1 curves were fitted to monoexponential decay. Kinetic analysis of ΔR1tumor (normalized to the fit values of ΔR1 in blood) was performed to estimate the fractional blood volume (BV; y-intercept) and the permeability surface area product per unit volume of tissue (PS; slope) as described previously.9-11 Fit parameters of BV and PS were calculated for each individual tumor and values and expressed as percentage (BV) and mL/min/100g of tissue (PS) assuming a tissue density of 1g/cc. PS maps were calculated on a pixel-by-pixel basis using MATLAB.
Statistical considerations
All statistical analyses were performed using GraphPad Prism Version 5.00 for Windows (GraphPad Software, San Diego, CA). Measured values are reported as the mean ± standard error of the mean and p-values <0.05 were considered statistically significant. Histologic evaluation of established xenografts was performed using tissue sections obtained from 3-5 animals per group. Tumor growth measurements were performed in 23 mice bearing tumors (oral cavity, n=8; larynx, n=7; base of tongue, n=8). Unpaired t tests were used to compare changes in tumor volume between the three xenograft types at different times post implantation. Microvessel density counts and lumen size measurements were estimated in 3 samples per tumor type (15 fields total/xenograft type) and analyzed for statistical significance using unpaired t tests. A total of 22 mice bearing tumors were examined using MRI (SCC oral cavity, n=6; SCC larynx, n=6; SCC base of tongue, n=10). Differences in BV and PS values were analyzed using unpaired t test.
Acknowledgments
Funding/Support: This work was supported by a grant from the Roswell Park Alliance Foundation (Dr. Seshadri) and Roswell Park Cancer Institute’s NCI-funded Cancer Center Support Grant P30CA16056.
Abbreviations
- HNSCC
head and neck squamous cell carcinoma
- MRI
magnetic resonance imaging
- SCID
severe combined immunodeficiency
REFERENCES
- 1.Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135:451–7. doi: 10.1016/j.otohns.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Conley BA. Treatment of advanced head and neck cancer: what lessons have we learned? J Clin Oncol. 2006;24:1023–25. doi: 10.1200/JCO.2005.05.0682. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev. 1991;10:229–243. doi: 10.1007/BF00050794. Review. [DOI] [PubMed] [Google Scholar]
- 4.Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci U S A. 1991;88:9345–9. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollingshead MG. Antitumor efficacy testing in rodents. J Natl Cancer Inst. 2008;100:1500–10. doi: 10.1093/jnci/djn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinders RJ, Hollingshead M, Khin S, Rubinstein L, Tomaszewski JE, Doroshow JH, et al. Preclinical modeling of a phase 0 clinical trial: qualification of a pharmacodynamic assay of poly (ADP-ribose) polymerase in tumor biopsies of mouse xenografts. Clin Cancer Res. 2008;14:6877–85. doi: 10.1158/1078-0432.CCR-08-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutz JC, Guan J, Bayani J, Yoshimoto M, Xue H, Sutcliffe M, et al. Establishment in severe combined immunodeficiency mice of subrenal capsule xenografts and transplantable tumor lines from a variety of primary human lung cancers: potential models for studying tumor progression-related changes. Clin Cancer Res. 2006;12:4043–54. doi: 10.1158/1078-0432.CCR-06-0252. [DOI] [PubMed] [Google Scholar]
- 8.Marangoni E, Vincent-Salomon A, Auger N, Degeorges A, Assayag F, de Cremoux P, et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin Cancer Res. 2007;13:3989–98. doi: 10.1158/1078-0432.CCR-07-0078. [DOI] [PubMed] [Google Scholar]
- 9.Demsar F, Roberts TP, Schwickert HC, Shames DM, van Dijke CF, Mann JS, et al. A MRI spatial mapping technique for microvascular permeability and tissue blood volume based on macromolecular contrast agent distribution. Magn Reson Med. 1997;37:236–42. doi: 10.1002/mrm.1910370216. [DOI] [PubMed] [Google Scholar]
- 10.Turetschek K, Preda A, Novikov V, Brasch RC, Weinmann HJ, Wunderbaldinger P, et al. Tumor microvascular changes in antiangiogenic treatment: assessment by magnetic resonance contrast media of different molecular weights. J Magn Reson Imaging. 2004;20:138–44. doi: 10.1002/jmri.20049. [DOI] [PubMed] [Google Scholar]
- 11.Seshadri M, Mazurchuk R, Spernyak JA, Bhattacharya A, Rustum YM, Bellnier DA. Activity of the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid against human head and neck carcinoma xenografts. Neoplasia. 2006;8:534–42. doi: 10.1593/neo.06295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naka T, Sugamura K, Hylander BL, Widmer MB, Rustum YM, Repasky EA. Effects of tumor necrosis factor-related apoptosis-inducing ligand alone and in combination with chemotherapeutic agents on patients’ colon tumors grown in SCID mice. Cancer Res. 2002;62:5800–6. [PubMed] [Google Scholar]
- 13.Hylander BL, Pitoniak R, Penetrante RB, Gibbs JF, Oktay D, Cheng J, et al. The anti-tumor effect of Apo2L/TRAIL on patient pancreatic adenocarcinomas grown as xenografts in SCID mice. J Transl Med. 2005;3:22. doi: 10.1186/1479-5876-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JC, Shuler CF, Zhang CX, Schuller DE, Milo GE. Histopathologic comparison between human oral squamous cell carcinomas and their xenografts in nude mice. Oral Surg Oral Med Oral Pathol. 1991;71:457–63. doi: 10.1016/0030-4220(91)90430-k. [DOI] [PubMed] [Google Scholar]
- 15.Tilgen W, Boukamp P, Breitkreutz D, Dzarlieva RT, Engstner M, Haag D, et al. Preservation of morphological, functional, and karyotypic traits during long-term culture and in vivo passage of two human skin squamous cell carcinomas. Cancer Res. 1983;43:5995–6011. [PubMed] [Google Scholar]
- 16.Wennerberg J, Willén R, Biörklund A, Tropé C. Histopathological characteristics predictive for growth of squamous cell carcinomas of the head and neck heterotransplanted to nude mice. Anticancer Res. 1986;6:1165–70. [PubMed] [Google Scholar]
- 17.Braakhuis BJ, Sneeuwloper G, Snow GB. The potential of the nude mouse xenograft model for the study of head and neck cancer. Arch Otorhinolaryngol. 1984;239:69–79. doi: 10.1007/BF00454264. [DOI] [PubMed] [Google Scholar]
- 18.Robbins KT, Rosenberg W, Weiss B, Varki NM. Growth characteristics of human laryngeal carcinoma in athymic mice. J Otolaryngol. 1991;20:117–22. [PubMed] [Google Scholar]
- 19.Lauk S, Zietman A, Skates S, Fabian R, Suit HD. Comparative morphometric study of tumor vasculature in human squamous cell carcinomas and their xenotransplants in athymic nude mice. Cancer Res. 1989;49:4557–61. [PubMed] [Google Scholar]
- 20.Le QT, Kong C, Lavori PW, O’byrne K, Erler JT, Huang X, et al. Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2007;69:167–75. doi: 10.1016/j.ijrobp.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 21.Kaanders JH, Wijffels KI, Marres HA, Ljungkvist AS, Pop LA, van den Hoogen FJ, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–74. [PubMed] [Google Scholar]
- 22.Galmarini FC, Galmarini CM, Sarchi MI, Abulafia J, Galmirini D. Heterogeneous distribution of tumor blood supply affects the response to chemotherapy in patients with head and neck cancer. Microcirculation. 2000;7:405–10. [PubMed] [Google Scholar]
- 23.Kyzas PA, Stefanou D, Batistatou A, Agnantis NJ. Prognostic significance of VEGF immunohistochemical expression and tumor angiogenesis in head and neck squamous cell carcinoma. J Cancer Res Clin Oncol. 2005;131:624–30. doi: 10.1007/s00432-005-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laitakari J, Nöyhö V, Stenböck F. Size, shape, structure, and direction of angiogenesis in laryngeal tumour development. J Clin Pathol. 2004;57:394–401. doi: 10.1136/jcp.2002.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beatrice F, Cammarota R, Giordano C, Corrado S, Ragona R, Sartoris A, et al. Angiogenesis: prognostic significance in laryngeal cancer. Anticancer Res. 1998;18:4737–40. [PubMed] [Google Scholar]
- 26.Hasina R, Whipple ME, Martin LE, Kuo WP, Ohno-Machado L, Lingen MW. Angiogenic heterogeneity in head and neck squamous cell carcinoma: biological and therapeutic implications. Lab Invest. 2008;88:342–53. doi: 10.1038/labinvest.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martone T, Rosso P, Albera R, Migliaretti G, Fraire F, Pignataro L, et al. Prognostic relevance of CD105+ microvessel density in HNSCC. Oral Oncol. 2005;41:147–55. doi: 10.1016/j.oraloncology.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Lentsch EJ, Goudy S, Sosnowski J, Major S, Bumpous JM. Microvessel density in head and neck squamous cell carcinoma primary tumors and its correlation with clinical staging parameters. Laryngoscope. 2006;116:397–400. doi: 10.1097/01.MLG.0000195286.29613.E1. [DOI] [PubMed] [Google Scholar]
- 29.Padhani AR. Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging. 2002;16:407–22. doi: 10.1002/jmri.10176. Review. [DOI] [PubMed] [Google Scholar]
- 30.Hoskin PJ, Saunders MI, Goodchild K, Powell ME, Taylor NJ, Baddeley H. Dynamic contrast enhanced magnetic resonance scanning as a predictor of response to accelerated radiotherapy for advanced head and neck cancer. Br J Radiol. 1999;72:1093–98. doi: 10.1259/bjr.72.863.10700827. [DOI] [PubMed] [Google Scholar]
- 31.Van Cann EM, Rijpkema M, Heerschap A, van der Bilt A, Koole R, Stoelinga PJ. Quantitative dynamic contrast-enhanced MRI for the assessment of mandibular invasion by squamous cell carcinoma. Oral Oncol. 2008;44:1147–54. doi: 10.1016/j.oraloncology.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Bisdas S, Nguyen SA, Anand SK, Glavina G, Day T, Rumboldt Z. Outcome prediction after surgery and chemoradiation of squamous cell carcinoma in the oral cavity, oropharynx, and hypopharynx: use of baseline perfusion CT microcirculatory parameters vs. tumor volume. Int J Radiat Oncol Biol Phys. 2009;73:1313–8. doi: 10.1016/j.ijrobp.2008.06.1956. [DOI] [PubMed] [Google Scholar]
- 33.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89:5645–49. doi: 10.1073/pnas.89.12.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehr HA, Skelly M, Buhler K, Anderson B, Delisser HM, Gown AM. Microvascular endothelium of human tumor xenografts expresses mouse (=host) CD31. Int J Microcirc Clin Exp. 1997;17:138–42. doi: 10.1159/000179221. [DOI] [PubMed] [Google Scholar]