Abstract
Bone marrow derived endothelial progenitor cells (EPCs) are known to play an important role in neovascularization and wound healing. We investigated the temporal effects of cutaneous wounding on EPC surface markers within the peripheral blood (PB) and bone marrow (BM), and better understand the role of the SDF-1α/CXCR4 axis on EPC mobilization after wounding. FVB/NJ mice were administered bilateral 8mm circular full thickness skin wounds. PB and BM were isolated at daily intervals post wounding through day 7 for EPC mobilization characteristics and levels of SDF-1α. Cutaneous wounding was found to cause a transient increase in EPC mobilization that peaked on day 3. In contrast, SDF-1α protein within blood plasma was observed to significantly decrease on days 3, 4, and 7 following cutaneous wounding. BM levels of SDF-1α protein decreased to a nadir on day 3, the same day as peak mobilization was observed to occur. The decrease in BM SDF-1α protein levels was also associated with a decrease in SDF-1α mRNA suggesting transcriptional down regulation as a contributing factor. This study for the first time characterizes EPC mobilization following cutaneous wounding in mice and supports a major role for the SDF-1α/CXCR4 axis in regulating mobilization within the BM, without evidence for systemic increases in SDF-1α.
Keywords: EPC, wound healing, SDF-1α, mobilization
Introduction
Optimal wound healing is dependent on neovascularization, or the formation of new blood vessels, at the site of injury. New blood vessels supply the ischemic tissue with adequate amounts of oxygen and nutrients to allow for cell proliferation and eventual wound closure. It is now well described that the bone marrow (BM) plays an important role in this process1–5. Following injury, locally ischemic tissue is known to produce a plethora of growth factors and cytokines that will recruit bone marrow derived stem cells to assist in neovascularization and tissue repair4,6. A subset of these bone marrow derived stem cells known as endothelial progenitor cells (EPCs) retain an increased degree of tissue plasticity, which allows for de novo contribution to the neovasculature by differentiating into endothelial cells, a process termed vasculogenesis7. EPCs have been shown to improve neovascularization in multiple injury models including woundhealing2,3,7–9 and may also facilitate neovascularization through secretion of various growth factors and cytokines2,10.
Before being recruited to sites of ischemia, EPCs within the bone marrow must first transition from a state of quiescence into an activated state where they migrate out of the stem cell niche and into peripheral blood (PB), a process called mobilization. Much effort has been focused on understanding this complex process, with multiple interactions and signaling pathways being identified11. One critical interaction in mobilization and homing of EPCs is between the G-protein-coupled receptor CXCR4 and its ligand, stromal cell-derived factor-1 alpha (SDF-1α, also known as CXCL12a)12. The CXCR4 receptor is highly expressed by endothelial cells and hematopoietic progenitor cells considered to include EPCs13,14, while SDF-1α is expressed within the BM, largely by stromal cells15. It is thought that SDF-1α secreted by the BM stromal cells has a retentive action on EPCs. This idea is supported by data in which administration of AMD3100, a bicyclam CXCR4 antagonist results in a rapid mobilization of stem cells from the BM16. Additionally, stem cell mobilizing agents such as granulocyte colony-stimulating factor (GCSF) cause an up-regulation of cell surface CXCR4 expression while decreasing BM SDF-1α levels17.
The contribution of EPCs to the neovasculature and wound healing has been well documented; however, the characteristics of EPC mobilization in these models have not been investigated. Additionally, studies focused on the SDF-1α/CXCR4 signaling in EPC mobilization have been largely performed using pharmacologic mobilizing agents with limited investigation in wounding models. The purpose of this study was to investigate the temporal effects of cutaneous wounding on EPC mobilization and better understand the role of the SDF-1α/CXCR4 interaction in this process.
Because no single cell-surface marker has been identified to accurately label EPCs, a combination of commonly used markers are used to enrich for EPC cell populations. Here we utilized two established marker combinations, CD133+/Flk-1+18,19 and Sca-1+/c-Kit+20–22, to identify populations enriched for EPCs. Additionally, we followed cells expressing the CXCR4 receptor, which is known to be expressed by EPCs, to help clarify the role of the CXCR4/SDF-1α axis during EPC mobilization.
Materials and Methods
Animal model
All experiments were approved by the Cincinnati Children’s Hospital Institutional Animal Care and Use Committee (IACUC). 8–10 week-old female FVB/NJ mice (Jackson Laboratory, Bar Harbor, ME; Stock Number, 001800) were anesthetized using isoflurane and then shaved with an electric shaver so as to avoid injury (Oster, McMinnville, TN). Shaved mice were cleaned with both betadine® surgical scrub (Purdue Products L.P., Stamford, CT) and isopropyl alcohol (Vedco, Inc., Saint Joseph, MO) prior to creating 8mm diameter, full thickness, circular wounds on bilateral flanks of each mouse. The skin wounds were then covered with a sterile transparent dressing (Tegaderm; 3M Healthcare, St. Paul, MN) before the mice were housed individually for recovery. Non-wounded but anesthetized, shaved, and bandaged mice were also utilized for comparison.
Tissue harvest
At the conclusion of the time course, mice were anesthetized using isofluorane and PB was collected from the retro-orbital space into EDTA coated tubes (BD, San Jose, CA). Following sacrifice by cervical dislocation, bilateral femurs were harvested into ice cold PBS. One femur was flushed with 3mL ice-cold PBS using a 21-gauge needle for flow cytometric analysis and RNA isolation, while the other was flushed with 40µL of ice-cold PBS containing 1% Igepal, 8µL protease inhibitor cocktail, 20mM NaF, and 1mM Na3VO4 (all from Sigma-Aldrich Co., St Louis, MO) for protein isolation. Femurs from one mouse per group were harvested into 10% neutral buffered formalin at room temperature (RT) for 24 hours for immunohistochemistry. Whole blood was centrifuged to collect plasma for SDF-1α quantitation. All samples were then stored at −80°C until use.
Flow cytometry
Immunostaining and flow cytometry analyses were performed according to standard procedures, and all cells were analyzed on a FACSCanto II flow cytometer (BD Biosciences). To label EPCs in PB and BM, cells were stained using anti-CXCR4 (biotinylated, clone 2B11, BD Bioscienes), anti-Sca-1 (PE-Cy7 conjugated, clone D7), anti-c-Kit (APC-AF750 conjugated, clone 2B8), anti-CD133 (PE conjugated, clone 13A4), and anti-Flk-1 (APC conjugated, clone Avas12A1) monoclonal antibodies followed by streptavidin FITC (all from eBioscience, San Diego, CA). For live/dead discrimination, cells were resuspended in medium containing 7-AAD (eBioscience) prior to analysis. Each data point included at least 100,000 events. Flow data were then analyzed using FlowJo software (Tree Star Inc., Ashland, OR)
Real time reverse transcription-PCR
Total RNA was isolated from bone marrow using the RNeasy mini kit (Qiagen Inc.,Valencia, CA) per manufacturer’s recommendations. Following cDNA conversion, SDF-1α expression was analyzed using an Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA) with forward primer 5’-GGT TCT TCG AGA GCC ACA TCG-3’ and reverse primer 5’-ACG GAT GTC AGC CTT CCT CG-3’. SDF-1α expression was normalized to beta-2-microglobulin using the forward primer 5’-GGCCTGTATGCTATCCAGAA-3’ and reverse primer 5’-GAAAGACCAGTCCTTGCTGA-3’. Real time PCR data were analyzed using the 2−ΔΔCT method23.
Enzyme-linked immunosorbent assay (ELISA)
SDF-1α protein levels were determined within BM and plasma samples using the mouse SDF-1 alpha Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) per manufacturer’s instructions. BM samples were then normalized to total protein, calculated using the Bio-Rad Protein Assay (Bio-Rad laboratories, Hercules, CA).
Immunohistochemistry (IHC)
Femurs were removed from 10% formalin after 24 hours, washed briefly in deionized (DI) water and decalcified in immunocal™ solution (Decal Chemical Corporation, Tallman, NY) for 3 days at RT. Femurs were rinsed in DI water, dehydrated through a series of alcohols and cleared with 3 changes of xylenes. Femurs were soaked in three changes of infiltrating paraffin wax, allowed to cool and embedded in paraffin blocks for sectioning. Five micron sections were placed onto SuperFrost Plus slides (Fisher, Pittsburgh, PA) and allowed to dry overnight. Sections were deparaffinized, and rehydrated in graded alcohols. Sections were blocked with 3% hydrogen peroxide in water for 10 minutes followed by a 10 minute Avidin and Biotin block (Dako Inc, Carpinteria, CA). A Protein Block solution (Dako Inc, Carpinteria, CA) was then placed on the sections for 15 minutes at RT. Sections were incubated with rabbit anti-mouse SDF-1α (1:500) (Abcam Inc., Cambridge, MA) for 30 minutes at RT, rinsed in 1X Wash Buffer (Dako Inc, Carpinteria, CA) and incubated with biotinylated goat anti-rabbit (1:200) (Vector Labs, Burlingame, CA) for 30 minutes at RT. After rinsing, tissues were incubated with Elite ABC reagent (Vector Labs, Burlingame, CA) for 30 min at RT. Slides were rinsed and incubated in DAB reagent (Dako Inc., Carpinteria, CA) for 5 min at RT. Sections were counterstained using hematoxylin, dehydrated, cleared, mounted using permount (Fisher, Pittsburgh, PA) and coverslipped.
Statistical analysis
The flow data were analyzed using the nonparametric Kruskal-Wallis test followed by Wilcoxon rank sum tests for pairwise comparisons. The remaining comparisons were performed using one-way ANOVA followed by Tukey post hoc means comparison test. All of the statistical analyses were carried out using SPSS for Windows (SPSS Inc., Chicago, IL). The data are expressed as the mean ± S.E.M. Differences at p < 0.05 were considered significant.
Results
Cutaneous wounding results in a transient increase in EPC mobilization, which peaks on day 3
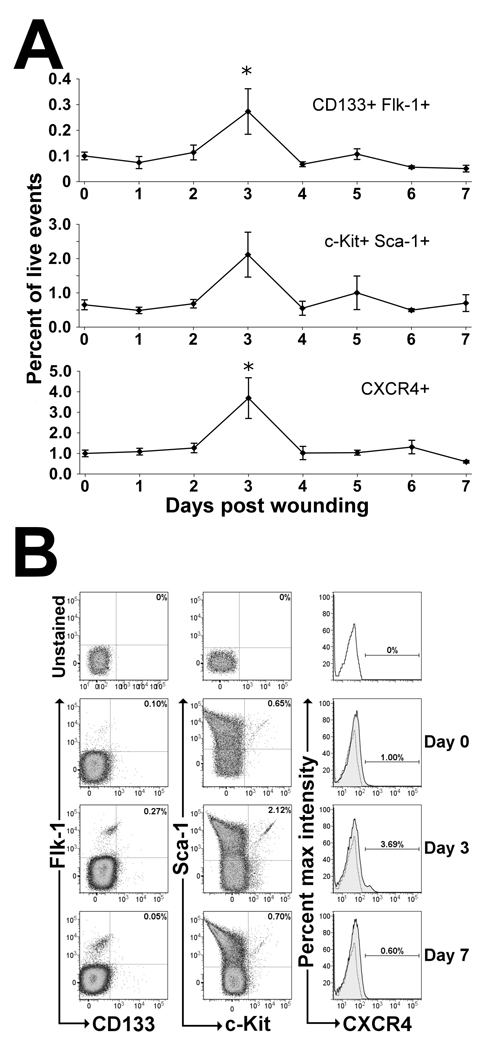
To determine the temporal effects of cutaneous wounding on EPC mobilization, flow cytometry was used to quantitate the number of cells in PB staining positive for known EPC markers. As shown in Figure 1a, cells expressing EPC surface markers within the blood were observed to transiently increase and peak on day 3 following wounding, with a return to baseline by day 4. Cells staining positive for both CD133 and Flk-1 were elevated by 2.7-fold on day 3 post wounding compared to non-wounded controls. Additionally, cells staining positive for both c-Kit and Sca-1 also increased by an average of 3.3-fold at day 3 compared to non-wounded mice. However, due to higher variability this difference was not statistically significant.
Figure 1.
EPC mobilization in response to cutaneous wounding in mice. (A) Flow cytometry was performed using peripheral blood (PB) from non-wounded mice (day 0) and mice wounded at 1-day intervals ending at day 7. EPCs were quantitated as the percentage of cells staining positive for EPC surface markers per total live events (7-AAD−). Cutaneous wounding was found to rapidly mobilize 3 populations of cells enriched for EPCs (CD133+Flk-1+, Sca-1+c-Kit+, and CXCR4+) on day 3 following wounding. Values are means ± SEM with 3–5 mice per group. *P < 0.05, vs. control by Wilcoxen rank sum test. (B) Representative dot plot distributions and histograms from flow cytometric analyses of wounded and non-wounded mice. PB was stained with antibodies against EPC cell surface markers CD133, Flk-1, Sca-1, c-Kit and CXCR4 and analyzed using flow cytometry. Representative dot plots for both CD133+/Flk-1+ and Sca-1+/c-Kit+ cells and histograms for CXCR4+ cells show the gating strategy used for cell quantitation at days 0, 3 and 7 after wounding with unstained controls (top) for comparison. Unstained controls are shown as stippled lines within histogram plots. All panels shown are gated on live cells as determined by 7-AAD dye exclusion.
To determine if the SDF-1α/CXCR4 axis was playing a role in EPC mobilization following cutaneous wounding, mobilization of CXCR4+ cells in PB was investigated using flow cytometry. As noted in the previous two EPC enriched populations, a rapid 3.7-fold increase in CXCR4+ cells within the PB was similarly observed on day 3 following wounding compared to non-wounded mice (Figure 1b).
A rapid increase and peak in EPC surface markers are found within the bone marrow on day 2 following cutaneous wounding
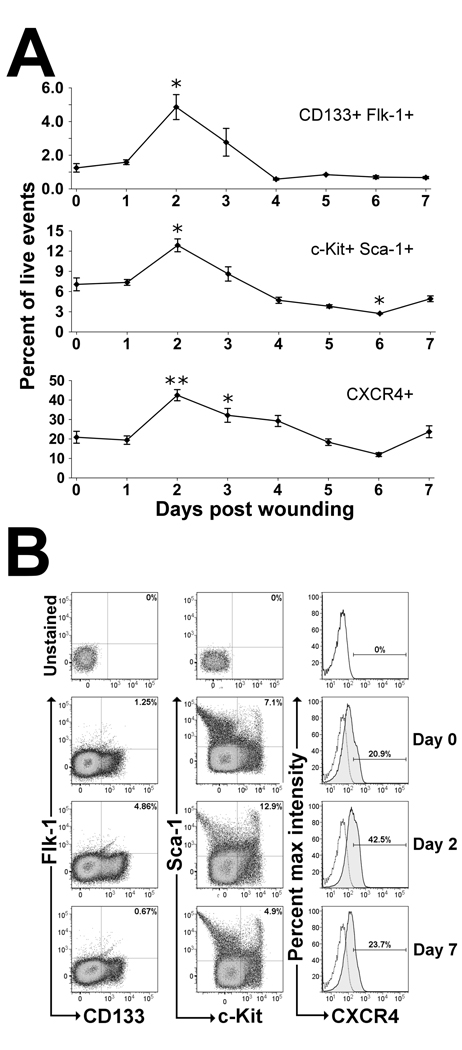
Because EPCs were found to rapidly mobilize into PB on day 3 following wounding, we hypothesized that cells expressing EPC surface markers within the BM must also increase prior to day 3 to allow for the observed increase in mobilization. To test this hypothesis, the number of cells expressing EPC surface markers within the BM was quantified using flow cytometry. As shown in Figure 2a, EPCs were observed to rapidly increase and peak on day 2 following wounding, with a subsequent decrease to levels below baseline on days 4–7. CD133+/Flk-1+ BM cells were found to be significantly increased by 3.9-fold on day 2 following wounding compared to non-wounded controls. Similarly, c-Kit+/Sca-1+ BM cells were significantly increased by 1.8-fold on day 2 following wounding compared to non-wounded controls. Additionally, BM cells expressing CXCR4 were significantly increased by 2.0-fold on day 2 and 1.5-fold on day 3 post wounding compared to non-wounded controls (Figure 2b).
Figure 2.
EPC surface markers found within the BM in response to cutaneous wounding in mice. (A) Flow cytometry was performed on BM cells flushed from femurs of non-wounded mice (day 0) and mice wounded at 1-day intervals ending at day 7. EPCs were quantitated as the percentage of cells staining positive for EPC surface markers per total live events (7-AAD−). Cutaneous wounding was found to cause a significant increase in EPC enriched populations (CD133+Flk-1+, Sca-1+c-Kit+, and CXCR4+) within the BM on day 2 following wounding. Values are means ± SEM with 3–5 mice per group. *P < 0.05, **P < 0.01 vs. control by Wilcoxen rank sum test. (B) Representative dot plot distributions and histograms from flow cytometric analyses of wounded and non-wounded mice. BM was flushed and stained with antibodies against EPC cell surface markers CD133, Flk-1, Sca-1, c-Kit and CXCR4 and analyzed using flow cytometry. Representative dot plots for both CD133+/Flk-1+ and Sca-1+/c-Kit+ cells and histograms for CXCR4+ cells show the gating strategy used for cell quantitation at days 0, 2 and 7 after wounding with unstained controls (top) for comparison. Unstained controls are shown as stippled lines within histogram plots. All panels shown are gated on live cells as determined by 7-AAD dye exclusion.
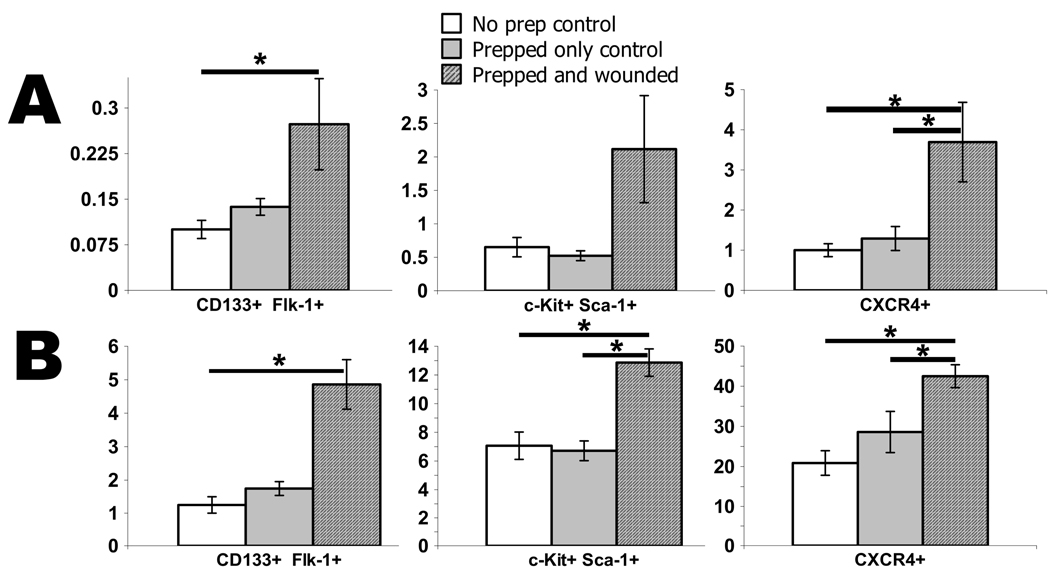
Animal and wound preparation does not significantly contribute to EPC mobilization
To be certain that the animal and wound preparation did not contribute significantly to EPC mobilization, we compared anesthetized, shaved and bandaged mice at 2 and 3 days post preparation to non-prepped mice and to the wounded and prepped test mice. Day 2 was used for comparing EPC markers found within the BM and day 3 for comparing EPC markers within the blood as those were the time points found to be statistically significant from non-wounded control mice. As shown in figure 3a and b, EPC markers found within the blood and BM were found to be statistically no different from mice within the prepped group and the non-prepped group. Additionally, with the exception of the CD133+/Flk-1+ blood and BM groups, the wounded test group had the same statistical outcome when compared to the prepped vs non-prepped control mice.
Figure 3.
Comparison of wounded mice to prepped and non-prepped control mice. To be certain that the animal and wound preparation did not significantly contribute to EPC mobilization, the number of EPCs within (A) the blood on day 3 and (B) the BM on day 2 following wounding or animal preparation were compared with the non-prepped control mice. Values are means ± SEM with 3–5 mice per group. *P < 0.05, vs. control by ANOVA.
Cutaneous wounding results in decreased bone marrow SDF-1α protein expression
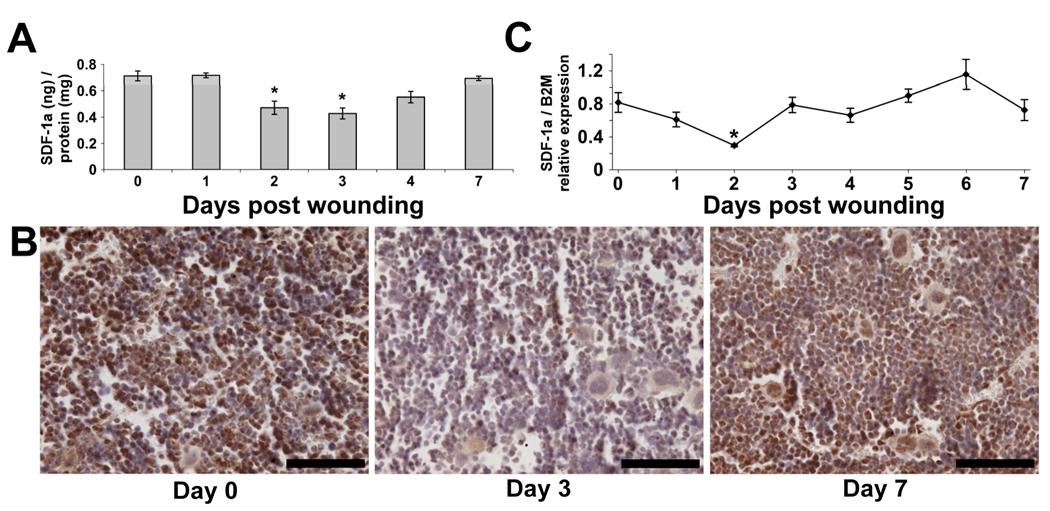
Accumulating evidence supports a major role for the SDF-1α/CXCR4 axis in EPC mobilization. To determine if this axis is important following cutaneous wounding, we analyzed the expression profile of SDF-1α protein within the bone marrow using an ELISA assay. As seen in Figure 4a, SDF-1α protein was observed to significantly decrease by 1.5-fold on day 2 and 1.7-fold on day 3 following wounding compared to non-wounded controls. SDF-1α protein was then found to increase approaching baseline levels by day 7 post wounding.
Figure 4.
The effect of cutaneous wounding on bone marrow (BM) levels of SDF-1α. (A) Following total protein isolation from femurs of non-wounded mice (day 0) and mice post-wounding days 1–4 and 7, SDF-1α was quantitated using ELISA and normalized to total protein. Concentration expressed as nanograms (ng) of SDF-1α protein per milligram (mg) of total protein. Values are means ± SEM with 3–5 mice per group. *P < 0.05, vs. control by ANOVA. (B) Representative images of femurs from non-wounded mice and mice post wounding days 3 and 7 following immunohistochemistry for SDF-1α. Notice less detection of SDF-1α (brown) at day 3 compared to day 0. By day 7, the amount of SDF-1α appears to recover to non-wounded levels. Scale bars = 70 microns. (C) Relative expression of SDF-1α mRNA from BM of non-wounded mice and mice post wounding days 1–7. Messenger RNA was isolated from total femoral BM and quantitated for the relative expression of SDF-1α using real-time RT-PCR and normalized to beta-2-microglobulin. Values are means ± SEM with 3–5 mice per group. *P < 0.05 vs. control by ANOVA.
This observation was also made qualitatively from the BM within sectioned femurs following IHC. As shown in Figure 4b, decreased staining intensity was observed in the BM of a sectioned femur from a mouse 3 days following wounding compared to a non-wounded mouse when probed with anti-SDF-1α antibodies. Consistent with the quantitative ELISA results, the degree of staining intensity appeared to recover by day 7 following wounding.
We next wanted to determine if the decrease in protein observed within the BM was due to a decrease in mRNA expression. As shown in Figure 4c, relative expression of SDF-1α mRNA was found to be significantly decreased by 2.7-fold on day 2 compared to non-wounded controls. This result suggests that the decrease in protein expression within the bone marrow is at least in part due to a decrease in SDF-1α mRNA expression.
Plasma levels of SDF-1α protein are decreased on days 3, 4 and 7 following cutaneous wounding
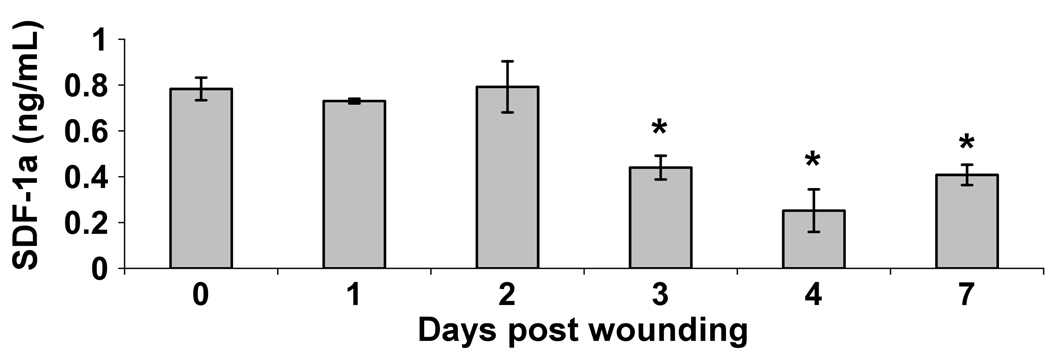
To determine if an increase in systemic SDF-1α protein initiates EPC mobilization as previous reports have suggested24, we measured the amount of SDF-1α protein within the plasma following wounding. Surprisingly, we observed a significant decrease in plasma levels of SDF-1α protein on days 3, 4 and 7 after wounding compared to non-wounded controls (Figure 5).
Figure 5.
Plasma levels of SDF-1α in response to cutaneous wounding. PB was collected from non-wounded mice and mice post wound days 1–4 and 7. Following plasma isoloation, levels of SDF-1α protein were assayed using ELISA. Data were expressed as nanogram (ng) of SDF-1α protein per milliliter (mL) of plasma. Values are means ± SEM with 3–5 mice per group. *P < 0.05 vs. control by ANOVA.
Discussion
Injury induced EPC mobilization has been documented in humans after coronary artery ischemia25,26 and burns27, as well as in mice following hind-limb ischemia28,29 and laparotomy30. However, no previously published reports exist that characterize mobilization of EPCs following cutaneous wounding. This study demonstrates that a significant increase in EPC mobilization occurs 3 days following cutaneous wounding in mice, which cannot be reproduced in the prepped only mouse group. This increase in EPC mobilization was observed to be transient as the number of cells expressing EPC surface markers within the PB rapidly returned to baseline the following day (day 4). Similar results were found by De Falco et al., who observed a peak in c-kit+ cells 3 days following hind-limb ischemia in mice, although they observed a longer mobilization period that occurred through day 7. In humans, Gill et al. reported a transient increase in Flk-1+AC133+ cells following coronary bypass grafting and burns that began at 6 to 12 hours and returned to baseline by 48 to 72 hours. These variations in peak mobilization and duration are likely attributable to the differences in the type, species and severity of injury models being described. This timeline of EPC mobilization also coincides with findings by Asahara et al., who reported finding EPCs incorporated into foci of neovascularization at high frequency at days 4 and 7 following cutaneous wounding in mice3.
In our study, we observed a rapid increase in the number of cells expressing EPC surface markers within the BM on day 2 following cutaneous wounding, one day prior to mobilization, which was also not reproducible in the prepped only mouse group. This phenomenon was also described by Condon et al., using a laparotomy induced injury model in mice. The authors found a significant increase in Sca-1+c-Kit+ cells within the BM at 24 hours with peak EPC mobilization noted at 48 hours following laparotomy. These observations reveal a preparation by the BM to expand the population of EPCs in order to allow for mobilization.
The experiments presented here also support a major role for the SDF-1α/CXCR4 axis in EPC mobilization following cutaneous wounding. The CXCR4 receptor, highly expressed by hematopoietic progenitor cells13, was found to be present on rapidly mobilized cells on day 3 following wounding. The mobilization of these cells from the BM correlated with a significant decrease in BM SDF-1α protein that also reached a nadir on day 3. Although this difference was only a modest 1.7-fold change in BM SDF-1α protein levels on day 3, it is unknown to what magnitude an effect this may create on any downstream cellular processes.
This pattern of SDF-1α protein expression is consistent with current theories, which postulate that SDF-1α produced by BM stromal cells has a retentive action on stem cells within the BM niche preventing their egress into circulation. However, it is well known that mobilization of stem cells is a complex event requiring the interaction of multiple cell surface receptors, ligands and signaling molecules and the SDF-1α/CXCR4 axis is only one of many relationships important for mobilization to occur.
This phenomenon has also been described by Petit et al. using a pharmacologic model for stem cell mobilization. Following administration of G-CSF, a known stem cell mobilizing agent, Petit et al. observed a similar decrease in BM SDF-1α protein during stem cell mobilization17. However, with respect to mRNA expression, the same authors observed an increase in BM SDF-1α mRNA at 24 hours after administration of G-CSF compared to no difference at 24 hours in our model of stem cell mobilization. The same authors provide additional data showing that the observed decrease in BM SDF-1α protein may be due to proteolytic degradation by neutrophil elastase. This difference at 24 hours may likely be multifactorial in that the two models are significantly different in the stimulus provoking stem cell mobilization. However, since Petit et al. only published a 24 hour in vivo time point, they may in fact have also observed a similar down regulation of BM SDF-1α mRNA at 48 hours following G-CSF administration. Similarly, in our injury model, we cannot rule out any additional down regulation of BM SDF-1α protein due to proteolytic degradation, as this was not a mechanism under study.
It has also been suggested that secretion of SDF-1α by ischemic tissue following injury may induce EPC mobilization through reversal of the BM SDF-1α gradient. In the current model, we were unable to demonstrate this occurrence as the concentration of SDF-1α was actually observed to decrease on days 3, 4 and 7. EPC mobilization following cutaneous wounding may in fact be initiated through alternate cytokines or growth factors produced from the injury, as proteins such as angiopoietin-1 and VEGF24 have also been implicated in mobilization.
This study for the first time characterizes EPC mobilization following cutaneous wounding in mice. These data also support a major role for the SDF-1α/CXCR4 axis in regulating mobilization within the BM, without evidence of systemic increases in SDF-1α. Gaining further knowledge of normal EPC mobilization during cutaneous wounding will help to understand states of impaired wound healing that may in part be due to impaired EPC mobilization such as diabetes31. With this increased knowledge and mechanistic insight we may eventually discover optimal methods at restoring or enhancing EPC mobilization to help in healing wounds or other injuries.
ACKNOWLEDGMENT
The authors would like to thank Dr. Jose Cancelas and Deidre Daria for assistance with flow cytometry.
This work was supported by NIH grants RO1 DK074055 TMC and RO1 DK72446 TMC.
References
- 1.Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P. Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol. 2003;196:245–250. doi: 10.1002/jcp.10260. [DOI] [PubMed] [Google Scholar]
- 2.Suh W, Kim KL, Kim JM, Shin IS, Lee YS, Lee JY, Jang HS, Lee JS, Byun J, Choi JH, Jeon ES, Kim DK. Transplantation of endothelial progenitor cells accelerates dermal wound healing with increased recruitment of monocytes/macrophages and neovascularization. Stem Cells. 2005;23:1571–1578. doi: 10.1634/stemcells.2004-0340. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 5.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–822. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isner JM. Tissue responses to ischemia: local and remote responses for preserving perfusion of ischemic muscle. J Clin Invest. 2000;106:615–619. doi: 10.1172/JCI10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 8.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;7:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 9.Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–1536. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 11.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 12.Sainz J, Sata M. CXCR4, a key modulator of vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:263–265. doi: 10.1161/01.ATV.0000256727.34148.e2. [DOI] [PubMed] [Google Scholar]
- 13.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Isner JM. Endothelial progenitor cells for vascular regeneration. J Hematother Stem Cell Res. 2002;11:171–178. doi: 10.1089/152581602753658385. [DOI] [PubMed] [Google Scholar]
- 15.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 18.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 19.Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- 20.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rütten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eguchi M, Masuda H, Asahara T. Endothelial progenitor cells for postnatal vasculogenesis. Clin Exp Nephrol. 2007;11:18–25. doi: 10.1007/s10157-006-0448-1. [DOI] [PubMed] [Google Scholar]
- 22.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion 45-37. [DOI] [PubMed] [Google Scholar]
- 25.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 26.Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol. 2004;24:684–690. doi: 10.1161/01.ATV.0000124104.23702.a0. [DOI] [PubMed] [Google Scholar]
- 27.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel H, Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 29.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 30.Condon ET, Wang JH, Redmond HP. Surgical injury induces the mobilization of endothelial progenitor cells. Surgery. 2004;135:657–661. doi: 10.1016/j.surg.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]