Abstract
Recently, disruption of the endogenous cannabinoid (endocannabinoid, eCB) system was found to impair extinction in delay and contextual fear conditioning models. However, conditioning procedures used in that work precluded investigation of possible eCB effects on acquisition of learned fear. We therefore examined the role of eCBs in modulating fear responses using multiple-trial versions of trace (hippocampal-dependent) and delay (amygdala–dependent) Pavlovian fear conditioning. By administering the CB1 receptor antagonist AM251 (5 mg/kg, i.p) to C57/Bl/6 mice at various times, we systematically identified the stages of learning and memory (i.e. acquisition, consolidation, recall and extinction) that are modulated by eCB signaling. During tone (CS) – footshock (US) conditioning, AM251 enhanced acquisition of freezing behavior for both trace- and delay-conditioning protocols. CB1 antagonism also enhanced generalized fear (baseline freezing) and cued (CS) freezing during memory recall tests in a state-dependent manner for both trace and delay conditioned animals. Furthermore, in trace-conditioned animals, AM251 impaired extinction performance of both cued and generalized fear. CB1 antagonism did not affect short-term memory (STM) or long-term memory (LTM) consolidation processes. Together, these results suggest that during acquisition and recall of aversive learning, eCBs prevent the expression and retention of inappropriate generalized and learned responses. These findings have important implications for the therapeutic use of CB1 antagonists.
Keywords: CB1 receptor, learning, anxiety, hippocampus, amygdala
Introduction
The plant-derived cannabinoid, Δ9-THC (the psychoactive constituent of marijuana) and the endocannabinoids (eCBs) anandamide and 2-AG, modulate a variety of behavioral phenomena such as learning and memory, cognition, mood and stress (Viveros et al., 2005; Martin et al., 2002; Ameri, 1999). In the brain, cannabinoids mainly exert their actions through the cannabinoid receptor, CB1, which is the most abundant G-protein-coupled receptor in the central nervous system. It is densely localized in many brain areas, including the hippocampus, cortex, amygdala and the basal ganglia, that are responsible for learning and memory and other cognitive functions.
Several behavioral studies provide evidence that eCBs are involved in certain aspects of learning and memory. For example, in animal models of cognition, either genetic deletion or pharmacologic antagonism of CB1 reportedly enhances memory. Terranova et al. (1996) found that the CB1 antagonist, SR 141716A, improved social recognition memory of both normal and aged rats and a similar effect was found for CB1 KO mice in an object recognition task (Reibaud et al., 1999). SR 141716A also enhanced performance of rats in spatial memory tasks such as the delay eight-arm radial maze (Wolff and Leander, 2003; Lichtman, 2000) and the elevated T-maze (Takahashi et al., 2005). On the other hand, studies using operant conditioning paradigms that rely on working (short-term) memory do not find any effect of CB1 antagonism (Hampson and Deadwyler, 2000; Mallet and Beninger, 1998; Mansbach et al., 1996).
One prominent function of eCBs appears to be the modulation of behavioral extinction. Marsicano et al. (2002) reported that genetic or pharmacologic disruption of CB1 in mice impaired extinction of fear responding to a CS-tone previously paired with a footshock in an amygdala-dependent fear model. No effect on learning acquisition or consolidation was reported, that is, initially all mice responded equally to the CS. These results were confirmed in other models of conditioned fear including, fear-potentiated startle (Chhatwal et al., 2005) and contextual fear conditioning (Suzuki et al., 2004). CB1 antagonism also impairs reversal learning in a Morris water maze task where the animals have to learn the location of the hidden platform that is different from the originally learned location (Varvel et al., 2005). These findings confirm and extend the evidence that eCBs facilitate the process of extinction learning, because the animals must suppress the knowledge of the old platform location in order to learn the new one. Reversal learning impairment in the Morris water maze task also occurs in rats that had been subjected to a 21-day chronic stress procedure (Hill et al., 2005), which, interestingly, also down regulates CB1 receptors by ∼50%.
The published work suggested that, depending on the behavioral task being tested, CB1 antagonism might have either facilitatory or inhibitory effects. However, studies on eCB modulation of learned fear have used either conditioning procedures (Mariscano et al., 2002) or contextual conditioning (Suzuki et al., 2004) that preclude extensive investigation of possible eCB effects on acquisition. Previous evidence that eCBs act generally as endogenous anxiolytics (Bortolato et al., 2006; Patel and Hillard, 2006; Haller et al., 2004; Haller et al., 2002; Martin et al., 2002; Navarro, 1997) suggests that deficiency in the eCB system might enhance acquisition in addition to impairing extinction. Supporting this hypothesis is evidence that pre-exposure to either acute or chronic stress facilitates acquisition of fear conditioning (Rau, et al., 2005). Furthermore, if eCBs are anxiolytics, then the effects of CB1 agonists and antagonists on learning and memory may be attributable to a general modulation of anxiety. We therefore examined the role of eCBs in modulating fear responding using multiple-trial versions of both trace (hippocampal-dependent) and delay (amygdala-dependent) fear conditioning. Administering the CB1 receptor antagonist AM-251 to C57/Bl/6 mice at various time points (as in Rodrigues et al., 2004) allowed for identification of the stages of aversive learning and memory (i.e., acquisition, consolidation, recall and extinction) that are modulated by eCB signaling.
Materials and methods
Subjects
All experimental procedures were carried out in accordance with protocols established by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. Upon arrival in the University animal facility, male C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) were single-caged in hanging plastic cages for 5–10 days. All animals were between 6–8 weeks old at the beginning of experimental procedures. Animals were maintained on a 12-h/12-h light–dark cycle with lights on at 8:00 a.m. Food and water were available ad libitum in the home cages.
Pharmacological treatment
The CB1 antagonist AM-251(1-2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxanide, Tocris-Cookson, St. Louis, MO, USA) was dissolved in a vehicle solution (0.9% saline, dimethylsulphoxide and Tween-80 in a 7:3:1 ratio). AM-251 (1, 5 or 10 mg/kg body weight) or an equivalent volume of vehicle was injected i.p., 20 min prior to any experimental procedures.
Apparatus
Experiments were performed using an automated, computerized, fear-conditioning system (San Diego Instruments, San Diego, CA, USA). The system consisted of four conditioning chambers (25.4 × 25.4 × 19.05 cm) constructed from white acrylic, and having removable stainless steel grid floors. Footshock was delivered through the floor grid via a shocker-scrambler unit controlled by custom-designed software (San Diego Instruments). Locomotor and freezing activities were monitored through a 16 × 16 array (∼1.25 cm spacing) of infrared photobeams and detectors located along the sides of the chambers. Each chamber was equipped with a house light and speaker (broadband, white noise, 80 dB) in the chamber lid (distance from light and speaker to grid floor is ∼15 cm). In addition, each chamber was contained in a sound-attenuating cubicle equipped with a ventilation fan (60 dB).
Fear conditioning
All fear conditioning procedures were performed in an isolated testing room. Animals were transported in their home cages to the testing room. The cages were covered to blind the animals during transport to and from the colony room. On conditioning day, mice were injected either with AM-251 or vehicle ∼20 min before being placed in a chamber. For trace fear conditioning each animal had a 60 s baseline acclimation period before receiving eight presentations of a tone CS (15 s) followed by a foot-shock US (1 s, 0.8 mA) that occurred 30 s after the CS offset (i.e, trace period). As injection stress may facilitate aversive learning, footshock duration and intensity were minimized to avoid ceiling effects. CS–US presentations were separated by 180 s inter-trial-intervals (ITI). For delay fear conditioning, all procedures were the same except that the CS co-terminated with the US (0.8 mA). Immediately after the end of the session, each animal was returned to its home cage in the colony room. Each chamber was carefully cleaned with Formula 409 cleaner at the end of each session.
To test for unspecific effects of AM-251 on behavior, another set of animals was injected with AM-251 or vehicle and conditioned with either the CS only, the US only or with no stimuli, to assess locomotor differences.
Fear retention and extinction testing
The strength of the CS–US association was assessed 24, 48 or 72 h after the conditioning sessions. The conditioned animals were initially subdivided into four groups that received either: 1) AM-251 on both conditioning and testing days (designated A_A); 2) AM-251 on conditioning day and vehicle on testing days (A_V), and 3) vehicle on conditioning and AM-251 on testing days (V_A) or 4) vehicle on both conditioning and testing days (V_V). For the 72-h test the A_A and V_A groups were further subdivided into two groups each depending on the treatment prior to the 72-h test session: hence, on that day, two groups received AM-251 (A_A_A and V_A_A) and two received vehicle (A_A_V and V_A_V). See Table 1. Mice were injected either with AM-251 or vehicle ∼20 min prior to the start of all testing sessions. Retention/extinction trials consisted of a 60-s baseline acclimation period followed by either six or three presentations of the CS only at 180 s ITI. These spaced extinction trials were designed to minimize habituation to the altered (smaller, see below) chamber.
Table 1.
Experimental protocol and treatment
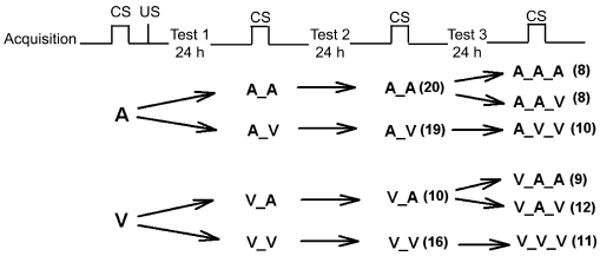 |
A = AM-251; V = vehicle; n's for each group are listed in parentheses beside group letter.
To minimize inadvertent contextual conditioning, the chambers were altered during the testing sessions. The grid floor was replaced with smooth Plexiglas and another piece of Plexiglas was used to bisect the chambers (Weitmier and Ryabinin, 2003). The outer walls were covered in multicolored construction paper to further eliminate spatial cues. In addition the chambers were cleaned with orange-scented 409 cleaner and the chamber assignments were shuffled from the previous day.
Behavioral measure and data analysis
Automated measures of horizontal activity, similar with the ones we use, are reliable indices of freezing behavior (Weitmier and Ryabinin, 2003). In the present study, freezing was defined as a lack of change in beam state (beams broken remained broken, beams unbroken remained unbroken) for ≥ 3 s. This method represents an indirect measure of freezing behavior, defined as the absence of all movement except for respiration (Fanselow, 1980). However, preliminary visual observations indicated that this measure closely matched the traditional definition.
For all experimental sessions time was binned into 15-s intervals. The percentage of the freezing time in each consecutive 15-s bin was calculated. To determine the learning acquisition curves during conditioning sessions, freezing scores in the 15-s bins were averaged over a given segment of the ITI (150 s) across animals after the USA. Animals that had baseline freezing scores ≥ 2.5 standard deviations from the group mean were removed from further analysis. For the retention/extinction trials, freezing scores in 15-s bins were averaged over the first minute following the CS offset. Peak freezing to a CS tone occurs during this period (Weitmier and Ryabinin, 2003).
Data analysis was performed by repeated measures, one-way and two-way ANOVAs of group means with Student–Neuman–Keuls post-hoc tests (P < 0.05) as indicated.
Results
CB1 antagonism affects acquisition of trace fear conditioning
As 5 mg/kg of AM-251 represents the lowest dosage of CB1 antagonists used in human clinical studies (Pi-Sunyer et al., 2006) and is comparable to doses used in many animal studies, we initially tested the effects of this dose on trace fear conditioning. In addition, we found that 5 mg/kg of AM-251 completely prevented the hypothermia caused by injection of 5 mg/kg of the CB1 agonist WIN 551212-2 (data not shown).
Average freezing behavior during each ITI (150 s) of the eight-trial conditioning session is shown in Figure 1a. Animals injected with AM-251 exhibited higher levels of freezing than did vehicle-injected animals (F7,140 = 2.00, P < 0.05). The AM-251 group froze significantly more during trials 1,2,3,5 and 8 than did vehicle-injected animals (P < 0.02). Freezing during trial 1 was already significantly different from baseline freezing (19.1 ± 1.57 versus 4.7 ± 1.40%, P < 0.01) in AM-251-treated animals, and reached a maximum level by trial 3 (51.7 ± 4.24%). Freezing responses in the vehicle group were not significantly different from baseline until trial 2 (25.8 ± 2.93 versus 1.1 ± 0.50%, P < 0.01) with the maximum response being reached by trial 4 (47.2 ± 3.90%).
Figure 1.
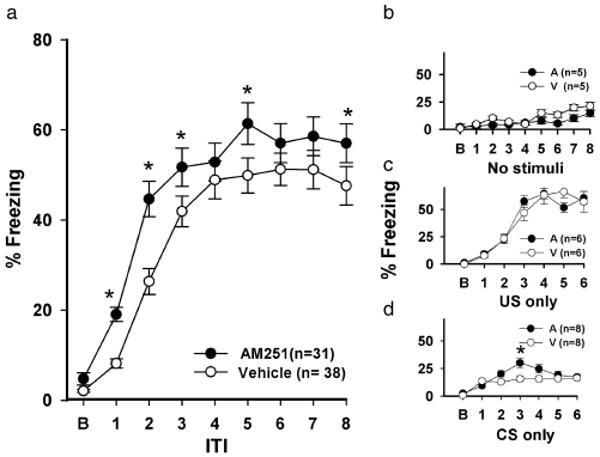
AM-251 enhances trace fear-conditioning. a) Average freezing responses during the ITIs (150 s). b) AM-251- and vehicle- treated animals showed similar performance during a locomotor test, and c) showed no differences in freezing responses to presentations of the footshock US alone. d) AM-251-treated animals tended to freeze more in response to repeated presentations of the tone CS alone compared with vehicle-injected animals. In this and all subsequent figures, bars represent mean ± SEM. Asterisks indicate significant differences between the AM-251 and vehicle groups (P < 0.05).
The CB1 antagonist affected the behavioral learning performance specifically. When animals injected with either AM-251 or vehicle were placed in the chambers for the same duration as the conditioning sessions (33.33 min) and no stimuli were delivered, there were no differences between the two groups (Figure 1b, P > 0.30), indicating that AM-251 (at 5 mg/kg) does not significantly affect locomotion. When animals treated with either AM-251 or vehicle were exposed to six presentations of the US-alone, both groups exhibited a rise in freezing, suggesting an association between the US and background context, that is, a form of contextual conditioning. No differences were detected between the two groups (Figure 1c, P > 0.25). However, during repeated CS-alone presentations, a significant interaction was found between the two groups (Figure 1d, (F5,100) = 3.40, P < 0.007). The AM-251-treated animals froze more during trial 3 than vehicle-treated animals (P < 0.003), suggesting that CB1 antagonism may affect freezing behavior in part by sensitizing responses to startling or other potentially anxiogenic stimuli.
Given the above findings, we tested AM-251 for dose-response effects on fear responding. During eight trials of trace fear conditioning, no differences were found between a 1 mg/kg dose of AM-251 (n = 10) and vehicle (n = 10) (F1, 20 = 0.100, P > 0.5, data not shown). However, a 10 mg/kg dose severely affected freezing behavior during the eight trials (average freezing = 63.4 ± 23.1%, 10 mg/kg (n = 10) versus 38.9 ± 15.1%, vehicle (n = 10), (F1, 20 = 16.51, P < 0.001). Baseline freezing rates were also affected by the 10 mg/kg dose (26.8 ± 5.9% versus 0 ± 0.0% (vehicle), F1, 6 = 20.50, P < 0.004). This suggests that the 10 mg/kg dose significantly affects locomotion and thus accounts for the observed high freezing levels. In fact, during a locomotion test the 10 mg/kg dose produced significantly higher levels of freezing compared with vehicle-treated animals (average freezing = 66.40 ± 1.41% (n = 3) versus 6.50 ± 1.30% (vehicle, n = 5), F1,6 = 178.70, P < 0.001). These results do demonstrate a dose-dependence of the AM-251 effect, but also reveal a strong generalized depressant effect of the highest dose of AM-251. Therefore, we elected to use only the 5 mg/kg dose of AM-251 for the remainder of the experiments.
eCBs modulate expression of generalized- and cued-fear memory
To assess the effects of CB1 antagonism on fear acquisition and recall, a memory retention test was performed 24 h after the conditioning session. The two groups treated with either AM-251 or vehicle on conditioning day were each subdivided into groups that received either AM-251 or vehicle 20 min prior to testing on the retention test (see Table 1).
We first compared the cued freezing scores (60 s after the first CS offset) among the different groups, and observed a main effect of group (F3, 12 = 9.10, P < 0.002). Post-hoc analysis revealed that cued freezing for the V_V group was significantly lower than all other groups (37.5 ± 8.26%, P < 0.001, Figure 2a). In addition, the A_A group (75.2 ± 2.85%) had cued freezing scores significantly higher than the A_V group (54.2 ± 3.37%, P < 0.03). Scores for the V_A group were not significantly lower than A_A (63.4 ± 5.4%, P > 0.1), although V_A and A_V groups did not differ (P > 0.1). Freezing scores did not significantly differ across the six trials for any group (P > 0.3, data not shown), thus indicating that no short-term extinction occurred.
Figure 2.
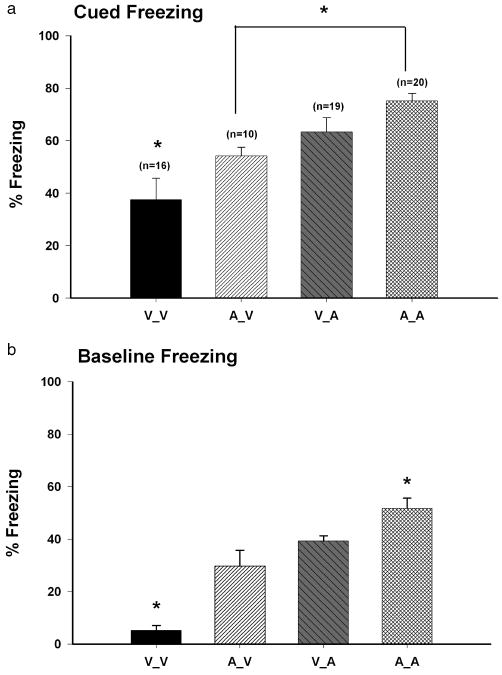
CB1 antagonism modulates fear expression during recall test. a) Percent freezing during the first post-CS (60 s) interval. The A_A group froze significantly more than the A_V and V_V groups. The V_V group froze significantly less than any group. Asterisks indicate significant differences among groups, (P < 0.05). b) Baseline freezing (60 s) for each pharmacological treatment group. The A_A group froze the most and the V_V froze the least. Asterisks indicate significant differences among the different treatment groups (P < 0.05).
These findings would appear to suggest that CB1 antagonism either before acquisition or before memory recall enhances the expression of a cued fear response, consistent with a recent report (Arenos et al., 2006) that cued fear responses in rats were enhanced by AM-251. However, the actual situation may be more complex. When we compared the average freezing scores during the baseline period (60 s), prior to the cuing, among the four groups by one-way ANOVA, we also found a main effect of group (F3, 12 = 26.20, P < 0.0002). As shown in Figure 2b, the animals that received AM-251 on both conditioning and the test days (A_A) froze significantly more than the other three groups (51.6 ± 3.94%, P < 0.001), and the V_V group froze the least (5.2 ± 1.96%, P < 0.01 Figure 2a). The V_A (39.3 ± 1.96%) and A_V (29.8 ± 6.00%) groups had intermediate freezing scores and did not differ from each other (P > 0.1). High levels of baseline freezing are usually attributed to the generalization of the fear response. That is, training procedures that produce strong freezing result in high freezing levels upon introduction into a novel environment (Baldi et al., 2004; Radulovic et al., 1998). The observation that groups administered AM-251 (either prior to conditioning or testing) had higher freezing levels than the V_V group suggests that eCBs modulate fear generalization. Hence, the retention test differences among the groups (Figure 2a), may primarily reflect an increase in generalized fear caused by CB1 antagonism during the conditioning trials (Figure 1) rather than an effect of CB1 on cued trace conditioning per se. Indeed, a naive approach of simply subtracting the baseline freezing scores (Figure 2b) from the cued freezing scores (Figure 2a) would lead to the conclusion that actually AM-251 had no effect on trace fear acquisition. The undoubted complexity of the underlying neural-behavioral mechanisms precludes lending much credence to this simplification, although the data do highlight the need for cautious interpretation.
AM-251 impairs expression of extinction not extinction learning
After the initial 24-h retention test, animals were tested for two more days (24-h apart) for long-term extinction. For the third test, the A_A and V_A groups were each subdivided into two groups: one that received AM-251 (A_A_A and V_A_A) and the other that received vehicle (A_A_V and V_A_V). Groups (A_V and the V_V) were also tested on the third day, but were not subdivided. For clarity, they are designated A_V_V and V_V_V, respectively for the third test (see Table 1).
Cued freezing
A two-way ANOVA (group × Test day) of cued freezing responding on repeated test days resulted in a significant interaction (F6, 24 = 5.71, P < 0.001). Only in the absence of AM-251 (A_V and V_V groups) was there evidence of extinction (P < 0.05) during Test 2, whereas animals that continued to receive AM-251 (A_A_A and V_A_A) maintained high freezing scores on test days 2 and 3, respectively (Figure 3a).
Figure 3.
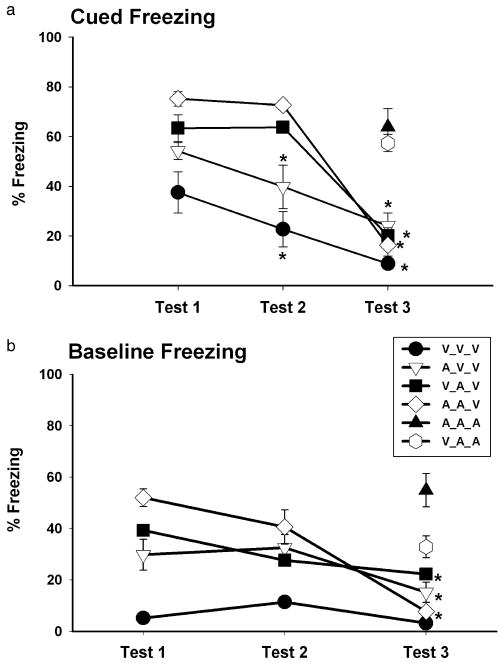
AM-251 modulates fear extinction performance. a) CS-elicited freezing during the three sessions. The A_V_V and V_V_V groups displayed significant extinction during Test 2. Letters in parentheses indicate pharmacological treatment during Test 3 (see Table 1). Again, in the absence of AM-251 the A_A_V and V_A_V groups (see Table 1) showed extinction during Test 3, but freezing responses remained high for the A_A_A and V_A_A groups. Asterisks indicate significant differences within groups, (P < 0.05). b) Baseline freezing responses during three daily test sessions. In the absence of AM-251, the A_A_V, V_A_V and A_V_V groups exhibited significant extinction during Test 3. However, the continued presence of AM-251, the A_A_A and V_A_A groups, maintained high baseline freezing levels. Asterisks indicate significant differences within groups, (P < 0.05).
To determine if CB1 blockade specifically impaired extinction (i.e., new learning) to cued fear or had some other effect, subgroups of the A_A and V_A animals were tested a third day without receiving AM-251(groups A_A_V and V_A_V). Both of these subgroups displayed significantly less freezing than during Tests 1 and 2 (P < 0.001). In comparison, animals that were administered AM-251 for the third test day (A_A_A and V_A_A subgroups) maintained freezing scores similar to Tests 1 and 2 (P > 0.3, planned comparison). These results suggest that CB1 antagonism during the CS testing per se maintains fearful responsiveness, and suggests that CB1 antagonism may not interfere with the neural processes underlying extinction learning.
Baseline freezing
During Test 2, baseline freezing for all groups did not significantly differ from their respective Test 1 levels (P > 0.1, ANOVA, Figure 3b). However, the two-way ANOVA (group × Test day) on baseline freezing yielded a significant interaction (F6, 24 = 7.55, P < 0.001). In Test 3, freezing responses were significantly reduced with respect to Test 1 (P < 0.03), for groups not receiving AM-251 on that day. The scores were: A_A_V (7.6 ± 1.61%), A_V_V (12.6 ± 2.63%) and V_A_V (22.3 ± 1.28%). Continued administration of AM-251 (subgroups A_A_A and V_A_A) maintained the same high level of freezing in these animals (55.9 ± 6.50% and 32.8 ± 6.50%, respectively) that was observed during Tests 1 and 2 (P > 0.1). These observations imply that blocking CB1 induces a state of enhanced fearful responding to the testing situation that might represent generalized fear or anxiety, rather than disruption of the active process of learned extinction.
Post-training effects of AM-251 administration
Recently, de Oliveira Alvares et al., (2005) reported that post-training intrahippocampal infusions of AM-251 caused amnesia of an inhibitory avoidance task. We therefore asked whether post-training AM-251 injections would modulate cued fear responses in short-term (STM) and long-term memory (LTM) tests. We trained a group of animals (n = 18) using the trace procedure discussed earlier. Following the training session, the animals were removed from the chambers and injected with either the AM-251 (n = 12) or vehicle (n = 6) solution. We then tested for cued fear by presenting three CS-tone presentations either three (STM) or 24 h later (LTM). There were no differences in cued freezing between the AM-251 and vehicle-treated animals on either the STM or LTM tests (data not shown). In the STM condition, freezing during the 60 s following CS offset were 36.7 ± 9.00 and 37.0 ± 5.50%, for AM-251 and vehicle-treated groups, respectively (data not shown). In the LTM test, AM-251 and vehicle animals also froze similarly (42.2 ± 4.13% versus 37.5 ± 8.26%, P > 0.5). Hence, post-training CB1 disruption did not affect either short-term or consolidation of long-term fear memory.
Delay fear conditioning
Marsicano et al., (2002) found that neither genetic nor pharmacologic disruption of CB1 affected freezing in animals trained with a delay conditioning paradigm. However, that study employed a single-trial conditioning session, which did not allow for observation of learning acquisition. Our findings from the trace conditioning sessions suggested that CB1 antagonism might also enhance fear responding in a multiple-trial version of delay conditioning. Indeed, we found that AM-251-treated animals displayed greater freezing responses over the course of the eight delay-conditioning acquisition trials (Figure 4a, F7,140 = 7.14, P < 0.0001). The AM-251 group froze significantly more (P < 0.0001) than vehicle-treated animals during all trials. Significant freezing to the CS_US pair for the AM-251 animals was evident by trial 1 (32.2 ± 2.60%, P < 0.001) and was maximal at trial 2 (54.9 ± 8.30%). In contrast, significant freezing for the vehicle group did not begin until trial 2 (21.3 ± 2.50%, P < 0.001) with maximal freezing not seen until trial 8 (46.1 ± 2.80%).
Figure 4.
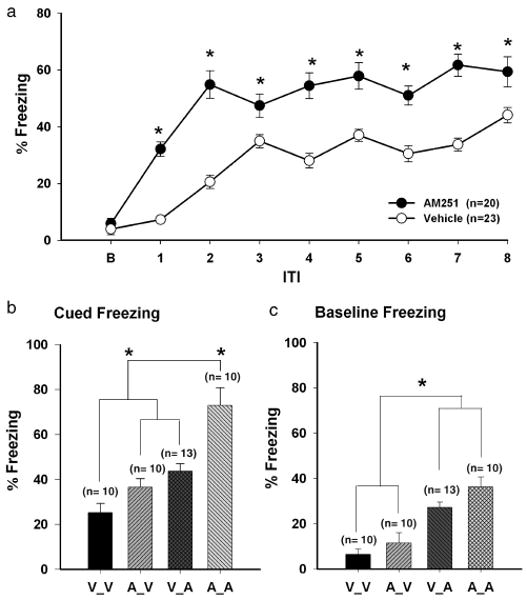
AM-251 enhances delay fear-conditioning. a) Percent freezing during the ITI periods during a delay fear protocol (see text). Asterisks indicate significant differences between the AM-251 and vehicle group (P < 0.05). b) 60-s period following the first CS presentation. During baseline, the A_A and V_A group froze more than the A_V and V_V group. The A_A group froze the most during CS-elicited freezing and the V_V group froze the least, whereas the V_A and A_V groups did not differ. Asterisks indicate significant differences among the pharmacological treatment groups (P < 0.05) and c) Baseline freezing.
For the 24-h memory retention test, the two groups were divided into four subgroups: A_A, V_A, A_V and V_V. The retention test consisted of one session with six CS-only presentations. To capture the period of maximal responsiveness, freezing was measured during a one-minute period beginning with CS onset. Cued responses during the first trials were analysed to determine the associative strength of the CS–US pairings; freezing scores were significant for all four groups (F3,12 = 27.84, P < 0.000), thus indicating that memory for the CS–US pairing was intact. Interestingly (Figure 4b), freezing responses for A_A were significantly higher than the other three groups (73.89 ± 10.50%, P < 0.003). The V_A (47.7 ± 3.10%) and A_V (37.0 ± 3.42%) groups did not differ significantly, however, both of these groups had higher freezing scores than the V_V group (25.2 ± 4.16%, P < 0.05). We did not find any evidence of short-term extinction in any group after delay conditioning during the six trials (P > 0.1).
During the baseline period, a one-way ANOVA revealed a main effect of group (F3,12 = 8.94, P < 0.002). The two groups that received AM-251 prior to the test (A_A, V_A) displayed elevated levels of freezing compared with the vehicle-treated animals (Figure 4c, P < 0.01). This is in general agreement with the results from the trace experiments that eCBs modulates the ‘generalized’-fear response. In summary, our data suggest that eCBs regulate the expression of fear responses during both the acquisition and memory recall of generalized and cued fear. As fear responding was affected in both the trace- and delay-fear conditioning protocols, it appears that eCBs modulate (in a general fashion) aversive behavioral responses.
Discussion
This study suggests that eCBs acting via the CB1 receptor modulate expression of both generalized- and cued-fear responses during hippocampal (trace) and amygdala (delay) dependent forms of fear conditioning. Our findings also argue that eCBs play a vital role in the extinction process following trace conditioning, consistent with results from delay (Marsicano et al., 2002), contextual fear conditioning (Suzuki et al., 2004) and fear-potentiated startle (Chhatwal et al., 2005) studies. Yet, despite these similarities with previous studies, our data have a number of novel facets. This is the first study to suggest an anxiolytic-like function of eCBs in both the acquisition and recall of aversive memories. This hypothesis is supported by recent evidence demonstrating that eCBs function as anxiolytics in animal models of anxiety (Bortolato et al., 2006; Patel and Hillard, 2006). It is also the first study to suggest that the effects of CB1 antagonism on learning and memory may vary in a state-dependent manner. Finally, this is the first systematic comparison of the effects of eCBs on trace and delay-conditioning models of fear conditioning.
Antagonizing CB1 activation significantly accelerated learning curves, and enhanced fear responding during retention trials in both the trace- and delay-conditioning protocols. AM-251 (5 mg/kg) did not affect baseline locomotion or sensitivity to the US footshock, although there was a tendency in AM-251-treated animals towards enhanced freezing behavior in response to repeated presentations to the CS-tone alone. In general the data suggest that normally, during repeated exposures to aversive or stressful stimuli, eCBs dampen aversive responding. Buttressing this conclusion is evidence that animals treated with the CB1 antagonist, SR 141716A, or CB1 KO mice, are more anxious than control animals, as assessed by models of anxiety or stress (Bortolato et al., 2006; Patel and Hillard, 2006; Haller et al., 2002; Martin et al., 2002; Haller et al., 2004; Navarro, 1997). The anxiolytic-like effects (Griebel et al., 2005; Rodgers et al., 2003) or anxiogenic-like responses induced by SR 141716A (Haller et al., 2004, 2002) are seen only in certain behavioral contexts, and may therefore not represent a truly generalized anxiety state. Paradoxically, CB1 agonists like Δ9-THC also produce anxiolytic effects at low doses and anxiogenic effects at midrange to high doses (Viveros et al., 2005; Ameri, 1999). Agonist-induced receptor downregulation or G-protein uncoupling of the CB1 receptor may account for the anxiogenic effects of higher doses (Sim-Selley, 2003).
During the first memory retention test of trace-conditioned animals, the A_V group displayed higher freezing levels than the V_V group, suggesting that AM-251 administration prior to conditioning could have increased memory acquisition. However, the A_A group froze more than the A_V group, suggesting that retention may be modulated in a state-dependent fashion or that CB1 antagonism has behavioral effects not solely related with acquisition and retention. Indeed, the V_A and A_V groups, although showing more freezing than V_V, did not differ from each other suggesting that disruption of CB1 signaling may simply enhance freezing in fearful animals. Results for delay-conditioned animals were similar, although the greater degree of freezing in the A_A group relative the other groups may mean that state-dependency was stronger for delay-conditioning. The data do argue that CB1 antagonism did not negatively interfere with memory acquisition.
High levels of baseline freezing are usually attributed to the ‘generalization’ of the fear response, and are evident when animals that have been strongly fear-conditioned are simply exposed to novel environments (i.e, those not used for conditioning). Factors such as handling, injections and placement into an unfamiliar environment, all contribute to fear generalization (Radulovic et al., 1998; Fanselow, 1990), suggesting this is a subtle form of ‘contextual’ conditioning. This could have occurred with AM-251 treatments in our experiments, implying that eCBs may also modulate generalized fear. However, it should be noted that Arenos et al. (2006) reported that AM-251 administered to Long-Evans rats prior to conditioning or to testing reduced contextual fear responses relative to vehicle controls. Interestingly, the same group observed that AM-251 did increase CS freezing when tested in a novel (non-training) context. Nevertheless, our investigation leads to a novel interpretation of the role of eCBs in fear conditioning in mice: in addition to interacting to some degree with the cellular basis of learning, they impact learning by affecting a basal state of responsiveness. A recent study by Kamprath et al. (2006) demonstrating that eCBs are released in the hippocampus and amygdala during fear-memory recall and that eCB-sensitive extinction depends on nonassociative memory processes, is consistent with this broader interpretation eCB function.
CB1 antagonism impairs extinction in several fear conditioning paradigms (Chhatawal et al., 2005; Suzuki et al., 2004; Marsicano et al., 2002) and even in a model of rodent spatial memory (Varvel et al., 2005). We now report that AM-251-treatment also retarded or prevented extinction in trace-fear conditioning. However, in our hands AM-251 needed to be present to block the expression of extinction; substantial behavioral extinction was apparent as soon as AM-251 was removed. This finding contrasts with Marsicano et al. (2002) and Chhatawal et al. (2005), who both report deficits in extinction expression in the absence of the CB1 antagonist SR 141716A. This discrepancy may be due to differences in experimental protocols (e.g., trace versus delay conditioning or fear-potentiated startle, mass versus spaced extinction trials, time of drug injection or time between extinction learning and testing). Nevertheless, in our study, had the underlying neural mechanism of extinction learning been affected by AM-251, a more gradual recovery of normal behavior, reflecting the decay of extinction itself, would have been expected when the AM-251 was removed. The effects of AM-251 on the nervous system may constitute a definite behavioral ‘state’, essentially a behavioral context (Bouton, 2004). This ‘anxious’ state may initially enhance aversive responding in fear-conditioned animals, and then maintain responding by masking the extinction process. In other words, the learned process of extinction of the tone-shock pairing could have proceeded normally, but, would only become evident during testing in the nondrug context. This interpretation represents a novel alternative to the hypothesis that eCBs specifically modulate the neural processes underlying extinction learning.
Our findings are consistent with studies of spatial memory using the radial arm maze (Wollf and Leander, 2003; Lichtman, 2000) and social recognition memory (Terranova, 1996) that showed that the CB1 antagonist SR 141716A enhanced memory. In addition, Martin et al. (2002) reported that, during an active avoidance test, CB1−/− mice displayed an enhancement in performance, which translates into a facilitation of learning. CB1−/− mice exhibit enhanced long-term-potentiation (LTP) of excitatory transmission in both the hippocampus (Bohme, 2000) and the amygdala (Marsicano, 2002), although to date, pharamacological blockade of CB1 has not been reported to enhance LTP. In contrast, activation of CB1 by eCBs enhances LTP in hippocampal slices (Carlson et al., 2002; Chevaleyre and Castillo, 2003). Inasmuch as LTP is the most widely accepted neural mechanism underlying learning and memory, it will be important to investigate both the differences between genetic and pharmacological antagonism of CB1 on synaptic plasticity as well as learning and memory.
A major implication of our work is that, normally, eCBs may counteract tendencies towards over-reaction to aversive events. The ability of peripheral administration of CB1 antagonists to enhance behavioral manifestations of fear conditioning may have important implications for the therapeutic use of CB1 antagonists (Mackie, 2005). The CB1 antagonist that we used, AM-251, is chemically very similar to SR141716A (Gatley et al., 1996), which is produced by Sanofi Aventis and is under review by the US Federal Drug Administration for use in weight management. Chronic administration of the drug should be carefully monitored.
Acknowledgments
This work was supported by NIH grants RO1 NS30219, and RO1 DE14625 to BEA. CGR was supported in part by the Cellular and Integrative Neuroscience Training Grant (NS07375) to the University of Maryland, and MHM was supported by a graduate stipend from the Department of Physiology, University of Maryland School of Medicine. We would like to thank Dr. Miranda Karson, Dr. David Edwards and Carlos LaFourcade for their critical reading of a draft of this report.
References
- Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Arenos JD, Musty RE, Bucci DJ. Blockade of cannabinoid receptors alters contextual learning and memory. Eur J Pharmacol. 2006;539:177–183. doi: 10.1016/j.ejphar.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Baldi E, Lorenzini CA, Bucherelli C. Footshock intensity and generalization in contextual and auditory-cued fear conditioning in the rat. Neurobiol Learn Mem. 2004;81:162–166. doi: 10.1016/j.nlm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Bohme GA, Laville M, Ledent C, Parmentier M, Imperato A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience. 2000;95:5–7. doi: 10.1016/s0306-4522(99)00483-2. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, La Rana G, Russo R, Calignano A, Gessa GL, Cuomo V, Piomelli D. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31(12):2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear 2. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- de Oliveira Alvares L, de Oliveira LF, Camboim C, Diehl F, Genro BP, Lanziotti VB, Quillfeldt JA. Amnestic effect of intrahippocam-pal AM-251, a CB1-selective blocker, in the inhibitory avoidance, but not in the open field habituation task, in rats. Neurobiol Learn Mem. 2005;83:119–124. doi: 10.1016/j.nlm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learn Behav. 1990;18:264–270. [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan R, Markriyannis A. 125I-labeled AM-251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Scatton B. Effects of the cannabinoid CB1 receptor antagonist rimonabant in models of emotional reactivity in rodents. Biol Psychiatry. 2005;57:261–267. doi: 10.1016/j.biopsych.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Haller J, Bakos N, Szirmay M, Ledent C, Freund TF. The effects of genetic and pharmacological blockade of the CB1 cannabinoid receptor on anxiety. Eur J Neurosci. 2002;16:1395–1398. doi: 10.1046/j.1460-9568.2002.02192.x. [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci. 2004;19:1906–1912. doi: 10.1111/j.1460-9568.2004.03293.x. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids reveal the necessity of hippocampal neural encoding for short-term memory in rats. J Neurosci. 2000;20:8932–8942. doi: 10.1523/JNEUROSCI.20-23-08932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Mariscano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol. 2000;404:175–179. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Ann Rev Pharmacol Toxicol. 2005 doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology (Berl) 1998;140:11–19. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Rovetti CC, Winston EN, Lowe JA., III . Psychopharmacology (Berl) Vol. 124. 1996. Effects of the cannabinoid CB1 receptor antagonist SR141716A on the behavior of pigeons and rats; pp. 315–322. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di MV, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Navarro M, Hernandez E, Munoz RM, Del AI, Villanua MA, Carrera MR, Rodriguez DF. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318(1):304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Kammermeier J, Spiess J. Generalization of fear responses in C57BL/6N mice subjected to one-trial foreground contextual fear conditioning. Behav Brain Res. 1998;95:179–189. doi: 10.1016/s0166-4328(98)00039-4. [DOI] [PubMed] [Google Scholar]
- Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29:1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Reibaud M, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A. Enhancement of memory in cannabinoid CB1 receptor knockout mice. Eur J Pharmacol. 1999;379:R1–R2. doi: 10.1016/s0014-2999(99)00496-3. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Haller J, Halasz J, Mikics E. ‘One-trial sensitization’ to the anxiolytic-like effects of cannabinoid receptor antagonist SR141716A in the mouse elevated plus-maze. Eur J Neurosci. 2003;17:1279–1286. doi: 10.1046/j.1460-9568.2003.02548.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sim-Selly L. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Fernandes MS. The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett. 2005;380:270–275. doi: 10.1016/j.neulet.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology (Berl) 1996;126:165–172. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- Wolff MC, Leander JD. SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharmacol. 2003;477:213–217. doi: 10.1016/j.ejphar.2003.08.025. [DOI] [PubMed] [Google Scholar]


