Abstract
Background
Although the role of the osteoclast in bone resorption is becoming better understood, much remains to be learned about osteoclastogenesis and the exact mechanism of action of anti-resorbing agents such as 17β-estradiol. This study investigated bone and morphologic osteoclast alterations following long-term estrogen administration to the B6D2F1 mouse. B6D2F1 mice aged 4-5 weeks were exposed to high levels of estrogen via implanted silastic tubing for at least 12 weeks; controls received empty tubing. Femurs of control and treated mice were assessed with radiology, quantitative histomorphometry and transmission electron microscopy.
Results
After 8 weeks of treatment, there was radiologic evidence of severe osteosclerosis and 86% of femoral marrow space was replaced with bone. After 12 weeks histologic studies of treated animals revealed that osteoclasts were positive for tartrate-resistant acid phosphatase but showed markedly abnormal ultrastructure which prevented successful bone resorption.
Conclusions
Findings extend our understanding of osteoclast structure and function in the mouse exposed in vivo to high doses of estrogen. Ultrastructural examination showed that osteoclasts from estrogen-treated mice were unable to seal against the bone surface and were unable to form ruffled borders.
Background
Although the role of the osteoclast in bone resorption is becoming better understood [1], much remains to be learned about osteoclastogenesis and the exact mechanism of action of anti-resorbing agents on the functional osteoclast. The anti-resorbing agent 17β-estradiol is especially noteworthy because of the association of its decline at menopause with the development of postmenopausal osteoporosis. As previously noted by Liu and Howard, the underlying cellular changes responsible the increased bone formation which follows estrogen administration are still not well characterized [2].
In the present report we report findings on alterations in osteoclast morphology following long term administration of high doses of 17β-estradiol to B6D2F1 mice. Development of osteosclerosis and the disappearance of the marrow space in these estrogen-treated mice is an interesting and useful model since marrow stromal cells not only contain the precursors for osteogenic cell lineages, but they also exert important effects on osteoclastogenesis and lymphopoiesis, and modulate the effects of some systemic factors of bone turnover. Osteoclasts, as well as osteoblasts, possess estrogen receptors [3, 4, 5, 6]. Hematopoietic cells also influence osteogenic cell differentiation, and some evidence suggests that mature lymphocytes influence osteoclast and osteoblast function. In the estrogen-treated mouse model used in the studies reported here, natural killer (NK) cells, which rely upon an intact bone marrow for full marrow maturation, are arrested in a nonlytic state [7]; since marrow space is lessened due to the osteosclerosis, the spleen provides the source for T and B cells and macrophages.
The objective of the present study was to assess the functional state of osteoclasts in estrogen-treated mice by determining if osteoclasts retained tartrate-resistant acid phosphatase activity (TRAP) and normal ultrastructural features.
Results
Radiographic examination of excised specimens from control and estradiol-treated mice confirmed the presence of osteosclerosis in the femurs of estradiol-treated mice. Diaphyseal cortices were prominent in the midshaft of control specimens (Figure 1A and 1C), but could not be identified in specimens from estradiol-treated animals (Figure 1B, 1D and 1E). These changes were not due to differences in radiographic technique since control and estradiol-treated femurs shown in Figure 1 were exposed side-by-side on the same film.
Figure 1.
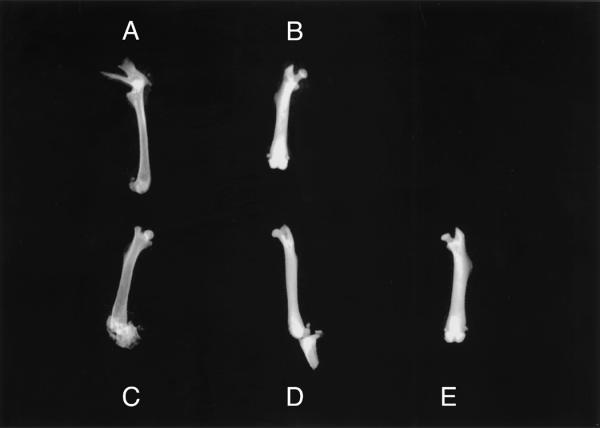
Radiographs of femurs from control (A and C) and estradiol-treated (B, D and E) mice. In control femurs (A and C), note that the marrow space and cortical margins are apparent but cannot be seen in specimens from estradiol-treated mice (B, D and E). Femurs marked A and B, and those marked C, D and E were radiographed as a group on the same X-ray film using one exposure to eliminate variability in exposure and development of the films.
Histologic examination also confirmed osteosclerotic features in femurs from estradiol-treated mice. Specimens from estradiol-treated mice contained only small regions of bone marrow (Fig. 2B) in contrast to control bones (Figure 2A). Since the normal, pre-estradiol treatment endosteal margin could still be histologically identified on these specimens, specimens were evaluated with quantitative histomorphometry by first measuring the old, normal marrow area and then measuring the small marrow cavity areas present at time of harvest. On average 86% of mid-shaft marrow space was replaced by bone in estradiol-treated mice at 8 weeks (p < 0.0001 control vs estradiol-treated).
Figure 2.
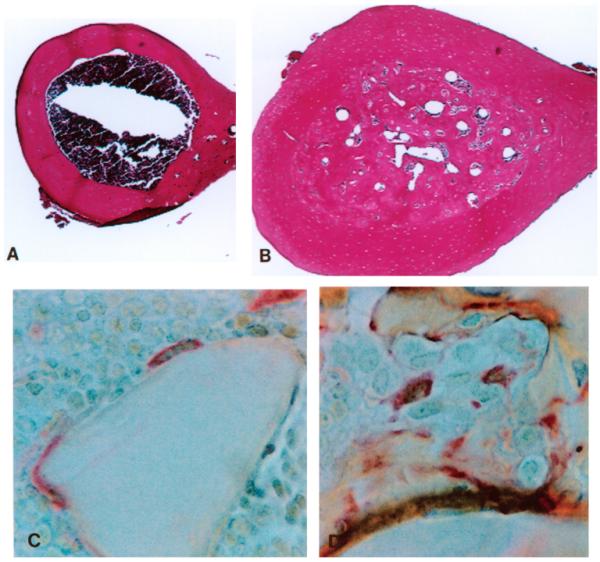
Photomicrographs of decalcified (A and B) and methacrylate-embedded (C and D) femoral specimens. Cross-sections of normal mice show a normal marrow cavity (Fig. 2A) which is in sharp contrast to the appearance of the cross-section from an estradiol-treated mouse (Fig. 2B) in which only small islands of marrow are present. Localization of Ocl using tartrate-resistant acid phosphatase (TRAP) localization in metaphyseal bone from a normal mouse (Fig. 2C) and from an estradiol-treated mouse (Fig. 2D). (Fig. 2A and 2B, H&E, X 43; Fig. 2C and 2D, TRAP, X 640).
Osteoclasts (Ocl) present in both control and estradiol-treated mice showed positive localization of TRAP (Figure 2C). In contrast to the close apposition of Ocl to the bone surface seen in control specimens, in estradiol-treated animals Ocl extended away from the bone surface with little bone contact (Figure 2D). In order to further investigate this observation, transmission electron microscopy was performed to examine the fine structure of the Ocl in estradiol-treated and control specimens.
Figure 3A illustrates the normal ultrastructural appearance of an Ocl from a control femur; note the normal ruffled border and snug apposition of the Ocl to the bone surface. Ocl from estradiol-treated animals, however, frequently showed only one margin of the cell perimeter involved with small areas of ruffed border formation, with the remainder of the cell surface displaying a smooth profile (Fig. 3B). Some Ocl from estradiol-treated animals had no ruffled borders in spite of the appearance of some attempt to form sealing podosome regions (Fig. 3C).
Figure 3.
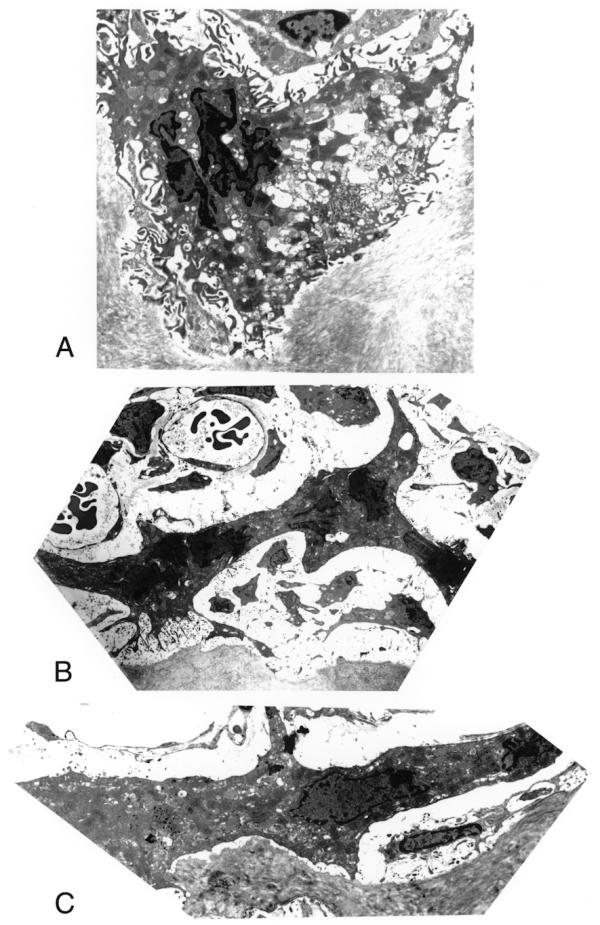
Transmission electron micrographs of Ocl from control (Fig. 3A) and from estradiol-treated (Fig. 3B and 3C) mice. Note the normal, extensive ruffled border present on the margin of an Ocl from a control femur (Fig. 3A). In estradiol treated animals, Ocl show either very small portions of the cell perimeter involved with ruffled border formation and the remainder of the cell surface reveals a smooth profile (Fig. 3B) or no ruffled border formation (Fig. 3C). (Fig. 3A, X 3,290; 3B, X 1,310; 3C, X 4,230)
Discussion
The present study indicated that severe changes in Ocl morphology are associated with the cells' inability to carry out resorption in the osteosclerotic estradiol-treated mouse. Although osteoclasts retained TRAP activity, ultrastructural alterations were identified in estradiol-treated animals in both the sealing patterns of osteoclasts against the bone surface and in altered brush border morphology. Proper sealing of the osteoclast against the bone surface and a functional brush border are two ultrastructural components believed to be of major importance in Ocl function. The ultrastructural alterations identified in estrogen-treated mice were very likely the cellular functional basis for the decreased resorption and development of osteosclerosis in estrogen-treated mice. Osteoclast function and ultrastructural morphology have been shown to be highly responsive to anti-resorbing agents such as ethane-1-hydroxy-1,1-diphosphonate (EHDP) [8, 9], dichloromethylene diphosphanate (Cl2MDP) [10], calcitonin [11,12,13], other anti-resorptive agents, such as mithramycin [14], and to gallium nitrate [15].
Although histochemical TRAP localization provides an accurate and sensitive histologic method for identification of Ocl, understanding osteoclastogenesis and Ocl function remain a challenging area of bone research. The current understanding of Ocl function is that the Ocl seals its perimeter along the bone surface by means of the podosome [16, 17, 18] . Within this sealed margin, projections from the cytoplasm form the ruffled border, a specialized Ocl-extracellular space in which bone resorption occurs. In the present study in the mouse, differentiation of osteoclast progenitors appears to be dependent on the direct interaction of bone marrow stromal cells with Ocl precursor cells [19, 20]. Recent data of Baylink [21] and Linkhart [22] provide evidence that two inbred strains of mice with different bone densities may have fundamental differences in bone resorption (not bone formation), and that genes affecting the bone marrow Ocl precursor population may contribute to relative differences in the C57BL/6J and C3H/HeJ strains. In vivo and in vitro studies of avian osteoclasts led Pederson et al to suggest that modulation of the Ocl response to estrogen may be controlled by alterations in the Ocl estrogen receptor level [3].
Maturation of osteoclasts from their macrophage precursors requires marrow stromal cells or their osteoblast progeny as shown by Udagawa et al [23]. These cells produce macrophage colony-stimulating factor (M-CSF) and the receptor for activation of nuclear factor kappa b (NF-kB) (RANK) ligand (RANKL). M-CSF is essential for macrophage maturation, but formation of osteoclasts also requires contact between osteoclast precursors and stromal cells or osteoblasts [23]. As described by Hofbauer et al [24], the quantity of bone resorbed depends on the balance between expression of RANKL and of its inhibitor, osteoprotegerin (OPG). Hofbauer et al suggest that the stimulation of the pool of M-CSF precursors to committed osteoclastogenesis by RANKL may be one of the central pathophysiologic pathways involved in increasing the number of osteoclasts in osteoporosis.
Futures studies of the estrogen-treated mouse model are needed which are designed to address osteoclastogenesis and osteoprotegerin (OPG) secretion by osteoblasts. OPG is currently hypothesized to be an important paracrine mediator of the antiresorptive action of estrogen on bone cells [25]. In osteoblast cell lines exposed to estrogen, OPG mRNA and protein levels were increased compared to controls. Hofbauer et al have suggested that estrogen may exert its anti-resorptive effects by enhancement of OPG secretion by osteoblasts [11].
Future studies should also assess the temporal development of functional changes in the osteoclast populations during estrogen treatment, and the patterns of osteoclast death with special attention to apoptosis. Estradiol has been shown to modulate IL-1 action on human osteoclasts in vitro; estrogen administration inhibited IL-1-mediated cytokine (IL-8) mRNA induction and caused increased Ocl apoptosis [26]. When avian Ocl were exposed to estradiol in vitro, the Ocl plasma membrane became depolarized and there was a marked decrease in potential, suggesting that estrogen may regulate osteoclasts through ion channel activities [27]. Estradiol has also been shown to inhibit acid production in avian Ocl [28]. The human pre-Ocl cell line FLG 29.1 has specific binding sites to 17β-estradiol on the cell surface, and estradiol exposure increases the cellular pH, cAMP, cGMP and intracellular calcium [29].
In vivo work by Jilka et al has shown that Il-6 can prevent the bone loss associated with ovariectomy in the mouse model [30]. Girasole et al have recently shown that production of IL-6 is inhibited in vitro by exposure of marrow cells to 17β-estradiol; cultures of mouse bone cells showed suppressed osteoclast development when exposed to either 17β-estradiol or a neutralizing antibody to Il-6 [31]. Future in vivo studies combining estradiol and selected interleukins may further elucidate how the network of signals from the marrow microenvironment influences both Ocl development and Ocl function.
Conclusions
Findings extend our understanding of osteoclast structure and function in the mouse exposed in vivo to high doses of estrogen. Ultrastructural examination showed that osteoclasts from estrogen-treated mice were unable to seal against the bone surface and were unable to form ruffled borders.
Materials and Methods
B6D2F1 mice were selected for this study based upon the following criteria. The C57BL/6 mouse is positive for the NK 1.1 epitope but from previous unpublished data we found that the C57BL/6 responded poorly to estradiol. From previous hybrid resistance studies we had historical data that the B6D2F1 mouse would be positive for the NK 1.1 epitope and respond well to the estradiol. B6D2F1 male mice aged 4-5 weeks were implanted for at least 12 weeks with silastic tubing filled with 15 mg 17β-estradiol [32, 33, 34, 35]; age-matched controls received empty tubing. NK cytolytic functions disappeared in estradiol-treated mice by 8 weeks. Previous work with the model by Roubinian et al showed that administration of 5-6.5 mg estradiol by this method returned estrogen levels to normal in ovariectomized mice for more than 3 months [36]. Seaman et al has also reported that administration 15 mg estradiol in this model was capable of raising estradiol to 9 times the normal level [37, 38].
At 8-18 weeks post-implantation, euthanasia was performed (CO2 inhalation) and femurs were harvested from 13 control and 33 estradiol-treated mice. Cross-sections of the femur and sections of femoral metaphyses and epiphyses were studied. For light microscopy, specimens were either fixed in 10% neutral buffered formalin, decalcified and embedded in paraffin or processed non-decalcified and embedded in methyl methacrylate. For ultrastructural studies, specimens were fixed in Karnovsky's fixative, post-fixed in osmium tetroxide, decalcified, and embedded in Spurr resin. Thin sections were prepared and grids were stained with uranyl acetate and lead citrate. Sections were viewed on a Phillips CM10 transmission electron microscope. Light microscopic sections were evaluated with quantitative histomorphometry using OsteoMeasure software (OsteoMetrics, Inc., Decatur, GA). Tartrate-resistant acid phosphatase (TRAP) localization was performed to identify osteoclasts with light microscopy. Protocols were approved by the appropriate Institutional Animal Care and Use Committee.
Statistical analysis of quantitative histomorphometric data used standard statistical methods; means and standard deviations were calculated. Unpaired t-tests were used to test for differences between means; a p-value of <0.05 was considered statistically significant.
Acknowledgments
Acknowledgements
The authors thank Audrey Stasky, Jane Ingram and Tracie McClain for assistance with light microscopy, and Winston Wiggins, Pat Hill and Daisy Ridings for assistance with electron microscopy.
Contributor Information
Helen E Gruber, Email: hgruber@carolinas.org.
Igor J Puzanov, Email: puzanov.igor@pathology.swmed.edu.
Michael Bennett, Email: Bennett.michael@pathology.swmed.edu.
Vinay Kumar, Email: Vkumar@delphi.bsd.uchicago.edu.
Brian Gordon, Email: Brian-Gordon@mail.omrf.ouhsc.edu.
References
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Liu C-C, Howard GA. Bone-cell changes in estrogen-induced bone-mass increase in mice: Dissociation of osteoclasts from bone surfaces. Anat Rec. 1991;229:240–250. doi: 10.1002/ar.1092290211. [DOI] [PubMed] [Google Scholar]
- Pederson L, Kremer M, Foged NT, Winding B, Ritchie C, Fitzpatrick LA, Oursler MJ. Evidence of a correlation of estrogen receptor level and avian osteoclast estrogen responsiveness. J Bone Mineral Res. 1997;12:742–752. doi: 10.1359/jbmr.1997.12.5.742. [DOI] [PubMed] [Google Scholar]
- Eriksen E, Colvard D, Berg N, Graham M, Mann K, Spelsberg TC, Riggs BL. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–87. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Komm BS, Terpening C, Benz D, Graeme K, Gallegos A, Kore M. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988;241:81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- Oursler MJ, Osdoby P, Pyfferoen J, Riggs BL. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci USA. 1991;88:6613–6617. doi: 10.1073/pnas.88.15.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzanov IJ, Bennett M, Kumar V. Il-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. The Journal of Immunology. 1996;157:4282–4285. [PubMed] [Google Scholar]
- Miller SC, Jee WSS. Ethane-1-hydroxy-1,1-diphosphonate (EHDP) effects on growth and modeling of the rat tibia. Calcified Tissue Res. 1975;18:215–231. doi: 10.1007/BF02546241. [DOI] [PubMed] [Google Scholar]
- Plasmans CMT, Jap PHK, Kuijpers W, Slooff TJHH. Influence of a diphosphonate on the cellular aspect of young bone tissue. Calcified Tissue Int. 1980;32:247–256. doi: 10.1007/BF02408548. [DOI] [PubMed] [Google Scholar]
- Miller SC, Jee WSS. The effect of dichloromethylene diphosphonate, a pyrophosphate analog, on bone and bone cell structure in the growing rat. Anat Res. 1979;193:439–462. doi: 10.1002/ar.1091930309. [DOI] [PubMed] [Google Scholar]
- Lucht U. Effects of calcitonin on osteoclasts in vivo. Z Zellforsch. 1973;145:75–87. doi: 10.1007/BF00307190. [DOI] [PubMed] [Google Scholar]
- Kallio DM, Garant PR, Minkin C. Ultrastructural effects of calcitonin on osteoclasts in tissue culture. J Ultrastructural Res. 1972;39:205–216. doi: 10.1016/s0022-5320(72)90017-2. [DOI] [PubMed] [Google Scholar]
- Accardo G, Aleotti A, Ricci D, Pazzi A. Support for the clinical use of calcitonin: Electron microscope study of the functional state of bone cells of rats after chronic treatment with calcitonin. Curr Therapeutic Res. 1982;31:422–433. [Google Scholar]
- Minkin C. Inhibition of parathyroid hormone stimulated bone resorption in vitro by the antibiotic mithramycin. Calcified Tissue Res. 1973;13:249–257. doi: 10.1007/BF02015415. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Norton HJ, Singer FR. In vivo morphologic changes in the rat osteoclast induced by gallium nitrate: The result of toxicity or other effects? Mineral and Electrolyte Metabolism. 1999;25:127–134. doi: 10.1159/000057436. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Advances in bone biology: The osteoclast. Endocr Rev. 1996;17:308–332. doi: 10.1210/edrv-17-4-308. [DOI] [PubMed] [Google Scholar]
- Väänäen HK, Horton M. The osteoclast clear zone is a specialized cell-extracellular matrix adhesion structure. J Cell Science. 1995;108:2729–2732. doi: 10.1242/jcs.108.8.2729. [DOI] [PubMed] [Google Scholar]
- Teti A, Marchisio PC, Zallone AZ. Clear zone in osteoclast function: role of podosomes in regulation of bone-resorbing activity. Amer J Physiol. 1991;261:C1–C7. doi: 10.1152/ajpcell.1991.261.1.C1. [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Baylink DJ, Farley J, Donahue L, Rosen CJ, Barr B, Lee J, Beamer W. Evidence that high peak bone density is due to decreased resorption not increased formation in a murine model. J Bone Mineral Res. 1995;10 (Suppl.):338–338. [Google Scholar]
- Linkhart TA, Linkhart SG, Kodama Y, Farley JR, Dimai HP, Wright KR, Wergedal JE, Sheng M, Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Osteoclast formation in bone marrow cultures from two inbred strains of mice with different bone densities. J Bone Miner Res. 1999;14:39–46. doi: 10.1359/jbmr.1999.14.1.39. [DOI] [PubMed] [Google Scholar]
- Udagawa N, Takashashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer L.C., Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382–4389. doi: 10.1210/endo.140.10.7034. [DOI] [PubMed] [Google Scholar]
- Hofbauer L.C., Khosla S., Dunstan D.R., Lacey D.L., Spelsbert T.C., Riggs B.L. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology. 1999;140:4367–4370. doi: 10.1210/endo.140.9.7131. [DOI] [PubMed] [Google Scholar]
- Sunyer T, Lewis J, Collin-Osdoby P, Osdoby P. Estrogen's bone-protective effects may involve differential IL-1 receptor regulation in human osteoclast-like cells. J Clin Invest. 1999;103:1409–1418. doi: 10.1172/JCI4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker KD, Gay CV. Depolarization of osteoclast plasma membrane potential by 17b-estradiol. J Bone Miner Res. 1999;14:1861–1866. doi: 10.1359/jbmr.1999.14.11.1861. [DOI] [PubMed] [Google Scholar]
- Gay CV, Kief NL, Bekker PJ. Effect of estrogen on acidification in osteoclasts. Biochem Biophys Res Commun. 1993;192:1251–1259. doi: 10.1006/bbrc.1993.1551. [DOI] [PubMed] [Google Scholar]
- Fiorelli G, Gori F, Frediani U, Franceschelli FTA, Tosti-Guerra C, Benvenuti S, Gennari L, Becherini L, Brandi ML. Membrane binding sites and non-genomic effects of estrogen in cultured human pre-osteoclastic cells. J Steroid Biochem Mol Biol. 1996;59:233–240. doi: 10.1016/S0960-0760(96)00092-1. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Hangoc G, Firasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Girasole G, Jilka RL, Passeri G, Boswell S, Boder G, Williams DC, Manolagas SC. 17b-estradiol inhibits interleukin-6 production by bone marrow-derived stromal cells and osteoblasts in vitro: a potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992;89:883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett T, Jr., Tutt M, Lipscomb M, Bennett M, Koo G, Kumar V. Origin and differentiation of natural killer cells. II. Functional and morphologic studies of purified NK-1.1+ cells. J Immunology. 1986;136:3124–3131. [PubMed] [Google Scholar]
- Bennett M, Baker EE, Eastcott JW, Kumar V, Yonkosky D. Selective elimination of marrow precursors with the bone-seeking isotope 89Sr: implications for hemopoiesis, lymphopoiesis, viral leukemogenesis and infection. J of the Reticuloendothelial Society. 1976;20:71–87. [PubMed] [Google Scholar]
- Seaman WE, Gindhart TD, Greenspan JS, Blackman MA, Talal N. Natural killer cell, bone, and the bone marrow: studies in estrogen-treated mice and in congenitally osteopetrotic (mi/mi) mice. J Immunology. 1979;122:2541–2547. [PubMed] [Google Scholar]
- Kumar V., Ben-Ezra J, Bennett M, Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunology. 1979;123:1832–1838. [PubMed] [Google Scholar]
- Roubinian JR, Talal N, Greenspan JS, Goodman JR, Siiteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW mice. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman WE, Gindhart TD, Greenspan JS, Blackman MA, Talal N. Natural killer cells, bone, and the bone marrow: Studies in estrogen-treated mice and in congenitally osteopetrotic (mi/mi) mice. J Immunol. 122:2541–2547. [PubMed] [Google Scholar]
- Seaman WE, Gindhart TD. Effect of estrogen on natural killer cells. Arthritis and Rheumatism. 1979;22:1234–1240. doi: 10.1002/art.1780221110. [DOI] [PubMed] [Google Scholar]


