Abstract
Medical imaging technologies have become increasingly important in the clinical management of cancer, and now play key roles in cancer screening, diagnosis, staging, and monitoring response to treatment. Standard imaging modalities such as MRI, PET, and CT require significant financial resources and infrastructure, which limits access to these modalities to those patients in high-resource settings. In contrast, optical imaging strategies, with the potential for reduced cost and enhanced portability, are emerging as additional tools to facilitate the early detection and diagnosis of cancer. This article presents a vision for an expanding role for optical imaging in global cancer management, including screening, early detection at the point-of-care, biopsy guidance, and real-time histology. Multi-modal optical imaging – the combination of widefield and high resolution imaging - has the potential to aid in the detection and management of precancer and early cancer for traditionally underserved populations. Several recent widefield and high-resolution optical imaging technologies are described, along with requirements for implementing such devices into lower-resource settings.
Keywords: clinical diagnostics, high-resolution imaging, widefield imaging, microscopy
Medical Imaging and Cancer
Medical imaging plays a central role in the detection and diagnosis of cancer. Screening, diagnosis, and staging all rely on the ability of imaging technologies to identify molecular and morphologic alterations associated with the onset and progression of neoplastic disease. Current treatment paradigms also rely heavily on imaging, for image-guided therapy, minimally-invasive surgery, and to monitor treatment response. Computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI) are well-established imaging modalities that can provide in situ anatomical data on the size, shape, and location of tumors. Relatively newer technologies such as positron emission tomography (PET) and single-photon-emission CT (SPECT) add the ability to detect and monitor functional activity of tumors, revealing both the location and metabolic activity of disease. Each of these imaging platforms can also take advantage of the growing array of molecularly-targeted contrast agents to detect and monitor distinct biochemical processes involved in neoplastic transformation (1, 2).
While the enhanced capabilities of these imaging systems will continue to play a key role in improving patient care, the financial cost and infrastructure required to establish and maintain them restricts their use largely to regional healthcare centers in industrialized countries. This is clearly underscored by the disparity in the availability of these technologies. As an example, a 2006 estimate placed the number of MRI machines in the US at around 11,000. Two years later in 2008, the first MRI machine was installed in Malawi, and the system is currently shared with neighboring Mozambique and Zambia, two countries without a single MRI scanner. Even in developing countries with rapidly emerging economies such as India, there are only 0.5 MRI machines per million inhabitants, compared to 37 machines per million persons in the US (3).
Today, more than 70% of the world's cancer deaths occur in these low- to middle-income countries, where 80% of patients present with advanced disease at the time of diagnosis (4, 5). These figures are unlikely to be affected by a widespread infusion of high-level imaging technology in the foreseeable future. Even in developed countries, the presence of diagnostic imaging technology alone does not equate to widespread access. Patients in the US who are uninsured or who have Medicaid insurance are significantly more likely to be diagnosed with late stage cancer, and have significantly lower survival rates than patients with private insurance (6). At a time when the global incidence of cancer is rapidly increasing and 47 million Americans lack health insurance (7), there is an urgent need for effective and affordable tools and technologies to facilitate early detection, prevention, and treatment of cancer in low resource settings.
Optical imaging is a new technology which may provide a potential solution to the global need for affordable imaging tools to aid in the early detection and management of cancer. While healthcare providers have traditionally used optical tools such as endoscopes, colposcopes, and surgical microscopes in cancer management, a new generation of instruments is being developed which can detect not just reflected white light, but additional signals arising from cancer biomarkers, carried in the fluorescence, polarization, and narrowband reflectance of light. These systems are capable of examining tissue over a wide range of spatial scales, with widefield macroscopic imaging typically spanning several square-centimeters, and high-resolution in vivo microscopy techniques enabling cellular and subcellular features to be visualized (1, 8, 9). Optical instrumentation is relatively inexpensive, using mass-fabricated components developed by the telecommunications and consumer electronics industries. A second key factor, which we focus on here, is the recent emergence of multimodal optical imaging systems, simultaneously providing wide-field and high-resolution optical imaging, within cost-effective, portable, and even battery-powered formats.
This article presents our vision of an expanding role for optical imaging in global cancer management, through the example of a dual-resolution portable device that has potential to aid in early cancer detection and management for traditionally underserved populations.
Current Optical Imaging Techniques for Cancer Management
Widefield Optical Imaging: An Emerging Technology for Cancer Screening
Over 80% of malignancies occur in epithelial surfaces, most of which can be directly visualized (10). Thus, many current procedures for cancer screening begin with visual inspection of the entire tissue surface at risk under white light illumination, often with additional low-power magnification. These techniques can be improved with narrow-band reflectance imaging (NBI) and autofluorescence imaging (AFI) to enhance visualization of the microvasculature and monitor alterations in epithelial and stromal fluorescence associated with the early development of neoplasia (11). Figure 1 illustrates the application of this technology to improve screening for lung cancer; white light and autofluorescence images acquired with light-induced fluorescence endoscopy (Xillix LIFE, Pentax 3000) show representative image features associated with atypical cells. The white light images (Fig. 1a,c) represent the clinician's conventional viewing mode, allowing anatomical inspection at low magnification. Autofluorescence images (Fig 1b,d) are sensitive to the natural fluorescence of tissue, which originates predominantly from stromal collagen. When illuminated with blue light, non-neoplastic tissue emits green fluorescent light.
Figure 1.
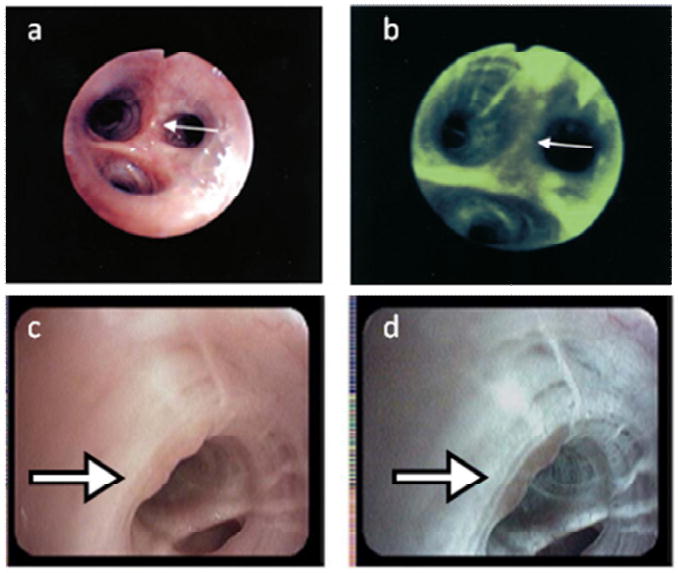
These images illustrate the application of light induced fluorescence endoscopy to improve screening for lung cancer with a widefield optical imaging system; white light and autofluorescence images acquired with light-induced fluorescence endoscopy (top, Xillix LIFE; bottom, Pentax 3000) show typical image features associated with atypical cells, with suspicious areas indicated by white arrows (12, 13). The white light images (A, C) represent the clinician's conventional viewing mode, allowing anatomical inspection at low magnification. Autofluorescence images (B, D) are sensitive to the natural fluorescence of tissue, which originates predominantly from stromal collagen. The development of neoplasia is associated with loss of stromal autofluorescence and this loss of fluorescence intensity can be used to assist clinicians in identifying pre-cancerous and suspicious cancerous lesions (as shown by decreased fluorescence intensity within areas in B, D).
The development of neoplasia is associated with loss of stromal autofluorescence and this loss of fluorescence intensity can be used to assist clinicians in identifying pre-cancerous and suspicious cancerous lesions (as evident in Fig. 1b,d). This technique has been used in over 1600 patients worldwide; studies support an improved sensitivity of autofluorescence bronchoscopy over white light bronchoscopy alone (80% versus 40%) (12, 13).
Imaging systems based on similar principles have been developed and tested for cancer detection in several other organ sites, including the esophagus, cervix, and oral cavity. In 2008, Curvers et al. reported the results of a multicenter study that used white-light endoscopy with the addition of NBI and AFI to detect neoplastic lesions in 84 patients with Barrett's esophagus, a precursor of esophageal cancer (11). Use of AFI identified 11 patients with early neoplasia in addition to the 16 identified by white-light alone, raising the targeted detection rate from 53% to 90%. These and other encouraging data from pre-clinical studies have led to the development of several commercially-available widefield multi-spectral imaging systems with FDA approval for early cancer detection, including the VELscope (oral), the Trimira-3000 (oral), the Olympus Lucera (GI), and the Xillix LIFE scope (lung). Newer spectral imaging techniques are being developed to measure other cancer-related biomarkers such as total hemoglobin content and hemoglobin oxygen saturation, as a tool to detect tumor margins in vivo during surgery (14).
High-Resolution Optical Imaging: Applications in Early Detection, Margin Detection, and Real-time Histology
In current practice, tissue sites appearing suspicious for disease on visual inspection with white light are typically biopsied, enabling a pathologist to evaluate cellular morphology at the microscopic level. Recently, high-resolution optical techniques have been developed that can image tissue with sub-cellular resolution in situ, revealing morphologic indicators of neoplasia such as increased pleomorphism, changes in nuclear size and nuclear density and the presence of abnormal mitotic figures, in addition to functional indicators when used in combination with targeted optical contrast agents (9). A high-resolution confocal endoscope for imaging the mucosal lining of the esophagus and colon was evaluated during in vivo clinical studies by Polglase et al., demonstrating the system's ability to visualize microscopic features including goblet cells and columnar epithelial cells, traditionally used by the pathologist in the diagnosis and staging of disease (15). In Figure 2, a confocal microendoscope was used to image excised ovarian tissue after the application of acridine orange (16). Fluorescence imaging enabled visualization of individual nuclei and the underlying stroma (Fig 2a-c). Images from a healthy ovary were characterized by a homogeneous distribution of nuclei, whereas images from carcinoma revealed a disordered tissue structure and variable nuclear size (Fig 2a,d). Other more subtle pathologic changes such as sclerosis and endometriosis were also detectable (Fig 2e,f). These optical methods have been adapted for in vivo use at several organ sites, with a focus on guiding biopsy site selection to those locations with the highest diagnostic potential. As with the wide-field imaging systems described earlier, several high-resolution optical imaging systems have been commercialized and received FDA approval for cancer detection, including the Lucid confocal microscope, the Mauna Kea fiber-optic system, and the Pentax/Optiscan confocal endoscope.
Figure 2.
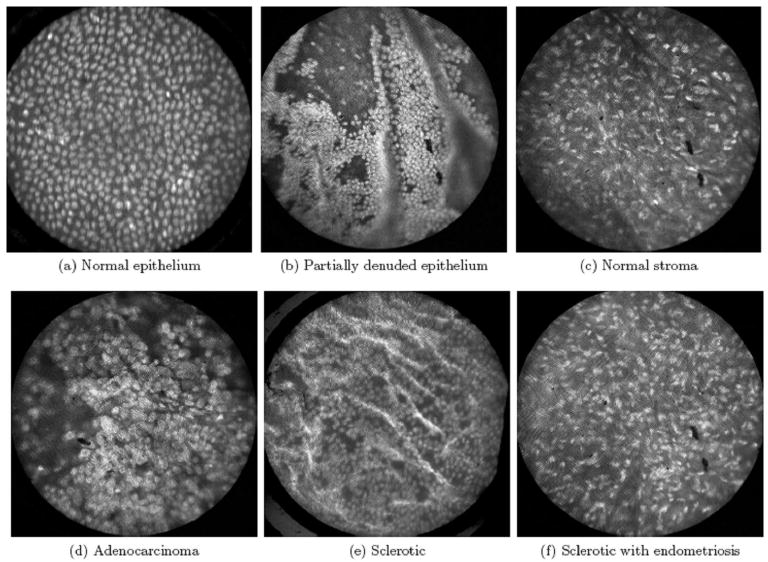
High-resolution confocal microendoscope images of excised ovarian tissue after the application of acridine orange (16). Fluorescence imaging enabled visualization of individual nuclei and the underlying stroma (A-C). Images from a healthy ovary were characterized by a homogeneous distribution of nuclei, whereas images from carcinoma revealed a disordered tissue structure and variable nuclear size (A, D). Other more subtle pathologic changes such as sclerosis and endometriosis were also detectable (E, F).
The same high resolution imaging techniques can potentially address the wider need for histopathology in settings where a pathology laboratory is either not available, less developed, or lacking expertise. Gareau et al. demonstrated that high-resolution optical imaging in the form of confocal microscopy can be used for margin evaluation during Mohs surgery for patients with basal cell carcinomas, providing an accurate diagnostic result in a much reduced time of frozen section histopathology (17).
Multimodal Optical Imaging
Each of these widefield and high-resolution systems has recognized strengths and weaknesses. In the same way that radiological imaging modalities have been combined to provide complementary diagnostic information (such as PET-CT, PET-MRI), widefield and high-resolution optical imaging systems have begun to be integrated into single platforms. In a 148-patient clinical study, Lam et al. performed high-resolution optical coherence tomography (OCT) imaging at sites initially inspected by widefield autofluorescence bronchoscopy (18). Following guidance to suspicious sites by widefield imaging, OCT was then used to measure the local epithelial thickness and identify enlarged nuclei, allowing invasive cancer to be distinguished from carcinoma in situ, and dysplasia to be distinguished from metaplasia, hyperplasia, and normal tissue. The autofluorescence endoscope described by Curvers et al. incorporated the additional use of narrowband reflectance imaging to improve diagnostic specificity in Barrett's esophagus, as supported by a drop in the false positive rate from 81% to 26% (11). It is likely that comprehensive screening and diagnosis of cancer will require the development of such multi-modal imaging platforms, with the ability to initially survey large tissue areas at risk to identify abnormal regions with high diagnostic sensitivity. Targeted follow-up of those suspicious sites with high-resolution imaging can then be used to eliminate confounding factors such as inflammation that lead to false positive results, improving the overall specificity of cancer detection.
Portable Optical Systems: Translating Optical Imaging to Low-Resource Settings
While these optical imaging systems continue to demonstrate encouraging performance in clinical studies, they remain primarily a subject of research and validation in large referral hospital settings in industrialized countries. However, by leveraging advances in optoelectronic components, low-power processing hardware, and digital image analysis, these imaging systems have the potential to be successfully adapted and integrated into low-resource settings, addressing the current gap in cancer screening and surveillance strategies in this environment. A few examples of compact, battery-powered optical systems have been demonstrated, including portable colposcopes for cervical cancer screening (19, 20), and a hand-held visual inspection device for the oral cavity (21), each of which require the user to observe the tissue by eye.
We recently developed a battery-powered, portable system, capable of both widefield and high-resolution digital imaging, and have begun clinical studies to compare its performance against large-scale counterparts. The widefield imaging component was previously described by Rahman et al., consisting of a commercially available surgical headlight system modified to include LED illumination for both white light and fluorescence excitation, and a high-sensitivity CCD camera for digital image acquisition (Fig. 3a) (22). This portable screening system weighs only 3 lb and can alternatively be mounted on a camera tripod. The high-resolution imaging capability is provided by epi-illumination of a flexible 1 mm diameter fiber-optic bundle, with the distal end of the fiber placed in contact with the tissue site to be imaged, following topical application of a fluorescent contrast agent. Fluorescent light emitted from the tissue returns through the same fiber and is imaged onto a high-sensitivity CCD camera (23). This system was engineered into a lightweight (6 lb) and portable package (Fig. 3b). Both the widefield imager and the high-resolution microendoscope systems connect to a single laptop computer via IEEE-1394 (Firewire) ports, enabling simultaneous imaging within a LabVIEW-based user interface (Fig. 3c). The total cost of components for the combined imaging platform was under $10,000.
Figure 3.
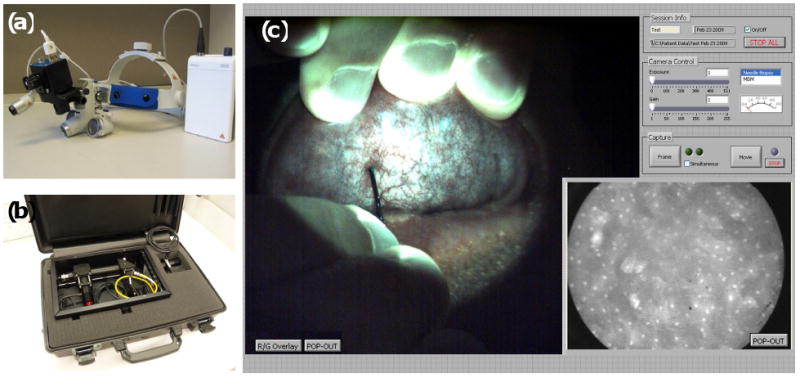
Combined portable widefield and high-resolution imaging systems. (A) The portable screening system weighs only 3 lb. (B) High-resolution microendoscope contained in a briefcase. (C) Widefield and high-resolution images of normal human oral mucosa acquired with the multimodal imaging system. In the widefield image (left frame), the green autofluorescence of normal tissue is apparent, as well as the microvascular network. The high-resolution fiber-optic probe can be seen in contact with the mucosal surface, approaching from the base of the widefield frame. The high-resolution image, acquired simultaneously, is displayed in the lower right frame, with the 800 μm diameter field-of-view corresponding to the tissue located beneath the tip of the fiber-optic probe. Following topical application of 0.05% proflavine solution to the probe tip, nuclei appear as discrete bright regions within each epithelial cell.
Figure 3c presents widefield and high-resolution images of normal human oral mucosa acquired with the multimodal imaging system. In the widefield image (left frame), the autofluorescence of normal tissue is apparent, as well as the microvascular network. The high-resolution fiber-optic probe can be seen in contact with the mucosal surface, approaching from the base of the widefield frame. The high-resolution image, acquired simultaneously, is displayed in the lower right frame, with the 800 μm diameter field-of-view corresponding to the tissue located beneath the tip of the fiber-optic probe. Following topical application of 0.05% proflavine solution to the probe tip, nuclei appear as discrete bright regions within each epithelial cell. The diagnostic performance of this integrated imaging system is currently being evaluated in pre-clinical studies, in comparison with research-grade optical imaging systems, and against the gold-standard of histopathology.
The Future of Optical Imaging for Global Cancer Management
Emerging clinical data suggest that optical imaging may play a key role in cancer management from two perspectives. First, optical methods can be used in vivo to acquire anatomical and functional images of tissue over a wide range of spatial scales, rapidly surveying large mucosal surfaces at risk to identify suspicious areas, and then interrogating those regions with sub-cellular spatial resolution. This capability offers practical flexibility in diagnostic use, enabling optical methods to be used in areas ranging from early-stage screening, to supplementing histopathology. Second, technological advances made by the optoelectronics industry have produced high-performance components which can be used to build portable, cost-effective imaging devices. These features are necessary to support the sustainable dissemination of diagnostic imaging tools in remote areas and for traditionally underserved populations.
We contend that these advantages of optical imaging technologies can be used to impact cancer management in several practical ways. The availability of optical imaging devices has the potential to streamline medical procedures, provide guidance to biopsy sites, and expedite margin determination to help reduce procedure costs, and improve surgical outcomes. Cost-effective, portable devices such as the optical systems described here can potentially help fill crucial technological gaps in settings where the infrastructure to support traditional radiological imaging and pathology services available in industrialized countries, are neither available, nor affordable.
Potential Roles and Requirements for Optical Imaging
Expanding Access to Screening and Early Detection
Screening high risk populations and early detection of cancer are critical to prevention, effective treatment and decreased morbidity of several types of malignancies. In low- and medium-resource countries, cancers of the stomach, liver, oral cavity, and cervix are common. Changes in diet and smoking habits are leading to increases in cancers of the lung, breast, esophagus, prostate, and colon (24). Optical imaging is most applicable to cancers that develop in epithelial mucosal surfaces, due to the limited penetration depth of light. Optical screening methods have been applied to and are currently the subject of significant research efforts for cancers of the oral cavity, cervix, lung, ovary, breast, esophagus, urinary bladder, skin, and colon (11, 12, 14, 15, 16, 21, 28), and these technologies can potentially be translated to low-resource settings. Without improved screening programs in these settings, it is likely that most of these cancer patients will continue to be diagnosed at late stages when limited treatment options exist. For instance, in Africa there is an overwhelming shortage of expertise for the operation and maintenance of medical equipment to deliver radiation therapy, and as a result, radiation treatment is only available to approximately 20% of the patients who need it (3). Early diagnosis can reduce the burden on these limited resources by enabling simpler treatments to be provided for patients with early-stage disease. In this respect, cervical cancer screening can serve as a positive model for prioritizing early detection of other cancers in developing countries. It has been shown that screening women just once for cervical cancer and its precursors at age 35 can reduce the lifetime risk of developing cervical cancer by 25-36% (25). When effective and economically viable treatment (cryosurgery in the case of cervical pre-cancer) can be administered, access to even minimal screening can clearly have a considerable benefit to an at-risk population.
Clinical studies using widefield optical imaging have shown that inexpensive optical systems can be used for early detection of cancer in the oral cavity, with the VELscope specifically targeting the dentist's office as a site for routine screening (21). In low-resource settings where healthcare providers are unlikely to have specific expertise in cancer detection, it is particularly important to provide practitioners with objective tools to guide diagnosis. Most imaging devices require subjective evaluation, but current research on image processing techniques enable optical devices with digital image capture to allow for objective assessment for pathologic abnormalities. For example, Roblyer et al. recently demonstrated a simple technique to detect and delineate oral neoplasia based on quantitative analysis of autofluorescence images (26). In this study, autofluorescence images at 405 nm excitation were acquired with a color CCD camera. Using linear discriminant analysis, a classifier was developed to distinguish neoplastic and normal regions of interest based on the ratio of red-to-green pixel intensities, resulting in 100% sensitivity and 91.4% specificity in a validation set. Objective analysis techniques can provide a repeatable way to determine the threshold for demarcating suspicious lesions, while also lessening the need for extensive user training. In primary care settings, optical imaging devices could offer health care providers effective screening tools, which can assist in identification of cancer at the earliest stages. Indeed, maintaining and expanding access to early screening is becoming increasingly important given the growing shift of poor and underinsured people towards suburban and rural areas, away from major metropolitan areas where most healthcare services are concentrated (27).
Guiding Treatment through Real-Time Biopsy and Histology
Optical imaging technologies can also be used to help clinicians in low-resource areas to diagnose and treat early malignancies. When a suspicious lesion is identified, the current practice in most developed countries is to perform an invasive biopsy to obtain tissue for histopathologic analysis. The ability to perform a biopsy is crucial for accurate diagnosis, but requires considerable physical infrastructure, personnel, and economic resources. High-resolution optical imaging can provide an alternative means of evaluating cellular morphology when histo- and cytopathology facilities are unavailable. Even in well-equipped centers, the ability of optical imaging to guide the clinician to sample the most appropriate sites may significantly reduce cost and improve the diagnostic value of biopsies obtained from the patient (28).
Although results from current optical imaging technologies are promising, there are still several hurdles to overcome before these devices can be translated into low-resource clinical settings. First, better needs assessment studies are required to more effectively delineate design requirements and guide the development of appropriate technology for use in developing countries. Such considerations include developing robust battery-powered devices for sites where electric power may be unreliable, designing devices which require minimal user expertise for settings where the availability of highly trained practitioners may be limited, and developing low-cost image contrast agents which can be stored safely for long periods in environments with potentially extreme temperatures and humidity levels. Quality control of devices is also important in the developing world, to ensure that instruments can remain calibrated and functional over time in settings without trained technicians. And finally, researchers and technology developers must overcome the relative lack of funding sources that enable non-profit and for-profit institutions to develop devices for low-resource settings and to carry out necessary field studies and translation of technology in the absence of traditional market incentives.
Cancer is projected to become the world's leading cause of death by 2010, with the burden of disease shifting further towards medically underserved populations in industrialized countries and the developing world (10). New approaches are required across the spectrum of cancer management, in prevention, diagnosis, treatment, education and care. If developed and tested appropriately, optical imaging technologies can play an important role in several aspects, from providing objective diagnostic screening at the community healthcare level, to enabling pathology guidance in the clinical setting. Importantly, by delivering these technical capabilities within cost-effective platforms, the impact on public health can be magnified through expanding patient access to previously unreachable healthcare systems.
Acknowledgments
Supported by National Institutes of Health Grants: R01 EB007594, R01 CA103830, and R01 CA124319.
References
- 1.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massoud TF, Gambhir SS. Integrating noninvasive molecular imaging into molecular medicine: an evolving paradigm. Trends Mol Med. 2007;13:183–191. doi: 10.1016/j.molmed.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Barton MB, Frommer M, Shafiq J. Role of radiotherapy in cancer control in low-income and middle-income countries. Lancet Oncol. 2006;7:584–595. doi: 10.1016/S1470-2045(06)70759-8. [DOI] [PubMed] [Google Scholar]
- 4.Tunstall-Pedoe H. Preventing Chronic Diseases. A Vital Investment: WHO Global Report. Int J Epidemiol. 2006;35:1107. [Google Scholar]
- 5.Jones LA, Chilton JA, Hajek RA, Iammarino NK, Laufman L. Between and within: international perspectives on cancer and health disparities. J Clin Oncol. 2006;24:2204–2208. doi: 10.1200/JCO.2005.05.1813. [DOI] [PubMed] [Google Scholar]
- 6.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 7.Denavas-Walt C, Proctor B, Lee C. Income, poverty and health insurance coverage in the United States: 2006. US Census Bureau. 2007 [Google Scholar]
- 8.Contag CH. In vivo pathology: seeing with molecular specificity and cellular resolution in the living body. Annu Rev Pathol. 2007;2:277–305. doi: 10.1146/annurev.pathol.2.010506.091930. [DOI] [PubMed] [Google Scholar]
- 9.Pierce MC, Javier DJ, Richards-Kortum R. Optical contrast agents and imaging systems for detection and diagnosis of cancer. Int J Cancer. 2008;123:1979–1990. doi: 10.1002/ijc.23858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle P, Levin C. World cancer report. World Health Organization; 2008. [Google Scholar]
- 11.Curvers WL, Singh R, Song LM, et al. Endoscopic tri-modal imaging for detection of early neoplasia in Barrett's oesophagus: a multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system. Gut. 2008;57:167–172. doi: 10.1136/gut.2007.134213. [DOI] [PubMed] [Google Scholar]
- 12.McWilliams A, MacAulay C, Gazdar AF, Lam S. Innovative molecular and imaging approaches for the detection of lung cancer and its precursor lesions. Oncogene. 2002;21:6949–6959. doi: 10.1038/sj.onc.1205831. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda N, Honda H, Hayashi A, et al. Early detection of bronchial lesions using newly developed videoendoscopy-based autofluorescence bronchoscopy. Lung Cancer. 2006;52:21–27. doi: 10.1016/j.lungcan.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Brown JQ, Wilke LG, Geradts J, Kennedy SA, Palmer GM, Ramanujam N. Quantitative optical spectroscopy: A robust tool for direct measurement of breast cancer oxygenation and total hemoglobin content in vivo. Cancer Research. 2009;69:2919–2926. doi: 10.1158/0008-5472.CAN-08-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686–695. doi: 10.1016/j.gie.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 16.Tanbakuchi A, Rouse A, Udovich J, Hatch K, Gmitro F. A clinical confocal microlaparoscope for real-time in vivo optical biopsies. J Biomed Opt. 2009;14:044030. doi: 10.1117/1.3207139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gareau DS, Li Y, Huang B, Eastman Z, Nehal KS, Rajadhyaksha M. Confocal mosaicing microscopy in Mohs skin excisions: feasibility of rapid surgical pathology. J Biomed Opt. 2008;13:054001. doi: 10.1117/1.2981828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lam S, Standish B, Baldwin C, et al. In vivo optical coherence tomography imaging of preinvasive bronchial lesions. Clin Cancer Res. 2008;14:2006–2011. doi: 10.1158/1078-0432.CCR-07-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walmer DK, Merisier D, Littman E, et al. Portable colposcopy in low-resource settings. J Acquir Immune Defic Syndr. 2004;37 3:167–170. [PubMed] [Google Scholar]
- 20.Winkler JL, Tsu VD, Bishop A, Scott R, Sellors JW. Confirmation of cervical neoplasia using a hand-held, lighted magnification device. Int J Gynaecol Obstet. 2003;81:35–40. doi: 10.1016/s0020-7292(02)00413-7. [DOI] [PubMed] [Google Scholar]
- 21.Lane PM, Gilhuly T, Whitehead P, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J Biomed Opt. 2006;11:024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 22.Rahman M, Chaturvedi P, Gillenwater AM, Richards-Kortum R. Low-cost, multimodal, portable screening system for early detection of oral cancer. J Biomed Opt. 2008;13:030502. doi: 10.1117/1.2907455. [DOI] [PubMed] [Google Scholar]
- 23.Muldoon TJ, Pierce MC, Nida DL, Williams MD, Gillenwater A, Richards-Kortum R. Subcellular-resolution molecular imaging within living tissue by fiber microendoscopy. Opt Express. 2007;15:16413–16423. doi: 10.1364/oe.15.016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle P. The globalisation of cancer. Lancet. 2006;368:629–630. doi: 10.1016/S0140-6736(06)69225-8. [DOI] [PubMed] [Google Scholar]
- 25.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 26.Roblyer D, Kurachi C, Stepanek V, et al. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prev Res. 2009;2:423–431. doi: 10.1158/1940-6207.CAPR-08-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuehn BM. Poverty shift may burden health system. Jama. 2007;297:1047–1048. doi: 10.1001/jama.297.10.1047. [DOI] [PubMed] [Google Scholar]
- 28.Thekkek N, Richards-Kortum R. Optical imaging for cervical cancer detection: solutions for a continuing global problem. Nat Rev Cancer. 2008;8:725–731. doi: 10.1038/nrc2462. [DOI] [PMC free article] [PubMed] [Google Scholar]


