Abstract
Twenty-four patients with proven benign and low-grade brain neoplasms each performed two iterations of four fMRI paradigms: language (word generation), primary and association auditory (text listening), upper limb fine motor control (alternating-limb bilateral finger tapping), and primary visual perception (reversing checkerboard). Activation clusters with varying thresholds were generated for each scan and used to calculate reproducibility parameters: Difference in the Center of Mass (COM) location, Rsize, and Roverlap. The average difference in the COM, Rsize, and Roverlap values ranged from 1.70±0.53 mm – 10.60±3.21 mm, 0.6±0.04 – 0.90±0.05 and 0.23±0.12 – 1±0.16 respectively for all tasks. These values are within the range of, or higher than, previously published reports on fMRI test-retest precision. FMRI is indicated to be a noninvasive tool with acceptable reproducibility measures for assessing the localizations of multiple language and sensorimotor functions in patients scheduled for radiotherapy treatment.
Keywords: fMRI, reproducibility, benign and low-grade brain neoplasms, Blood Oxygen Level Dependent
Introduction
The accuracy of fMRI for measuring activation in patients with brain neoplasms has been of special interest due to its ability to detect dynamic changes in Blood Oxygen Level Dependent (BOLD) (1,2) brain activity and its application in the clinical setting (3, 4, 5, 6). The BOLD effect is based on a hemodynamic response secondary to neuronal activity. This hemodynamic basis of fMRI raises concerns for accuracy and reproducibility, especially when the fMRI mapping is intended for treatment planning purposes. The BOLD signal is a reflection of the capillaries and veins deoxygenated hemoglobin content. Signal changes from draining veins have larger BOLD effects because they carry less of the deoxygenated blood. Draining from a large cortical region can cause BOLD displacement from the activation volume resulting in signal changes in these regions (7). This phenomenon is known as the large-vessel effect. FMRI for treatment planning typically involves the application of multiple sensory and cognitive paradigms. Using fMRI aided radiation therapy treatment planning allows one to reduce radiation dose to eloquent areas in proximity to the tumor (8, 9). The accuracy of fMRI mapping has been compared to electrocortical stimulation (ECS) for localization of sensorimotor (10, 11) and language (12, 13) functions. In general, these studies have established the spatial accuracy of fMRI with close correspondence to ECS. Other studies have examined both accuracy and reproducibility of fMRI for the neurocognitve functions of language (14, 15), motor function (16, 17), and vision (18).
Previous studies have employed various parameters to measure reproducibility such as: the center of mass, the number of activated voxels (Rsize), and the reproducibility of the location of activated voxels (Roverlap) for visual activation patterns (19, 20). However, the reproducibility of a battery of multiple sensory and language-related fMRI activations during the same imaging session for multiple brain regions for patients having benign or low-grade brain neoplasms has not been reported. In this study we have examined the test re-test reliability of fMRI including multiple task activation and brain regions within subjects that have been previously diagnosed with benign and biopsy proven low-grade neoplasms. We have specifically focused on the reproducibility of fMRI maps measured by three previously established parameters: evaluating the difference in the center of mass (COM) location, Rsize, and Roverlap.
Materials and Methods
Data Acquisition
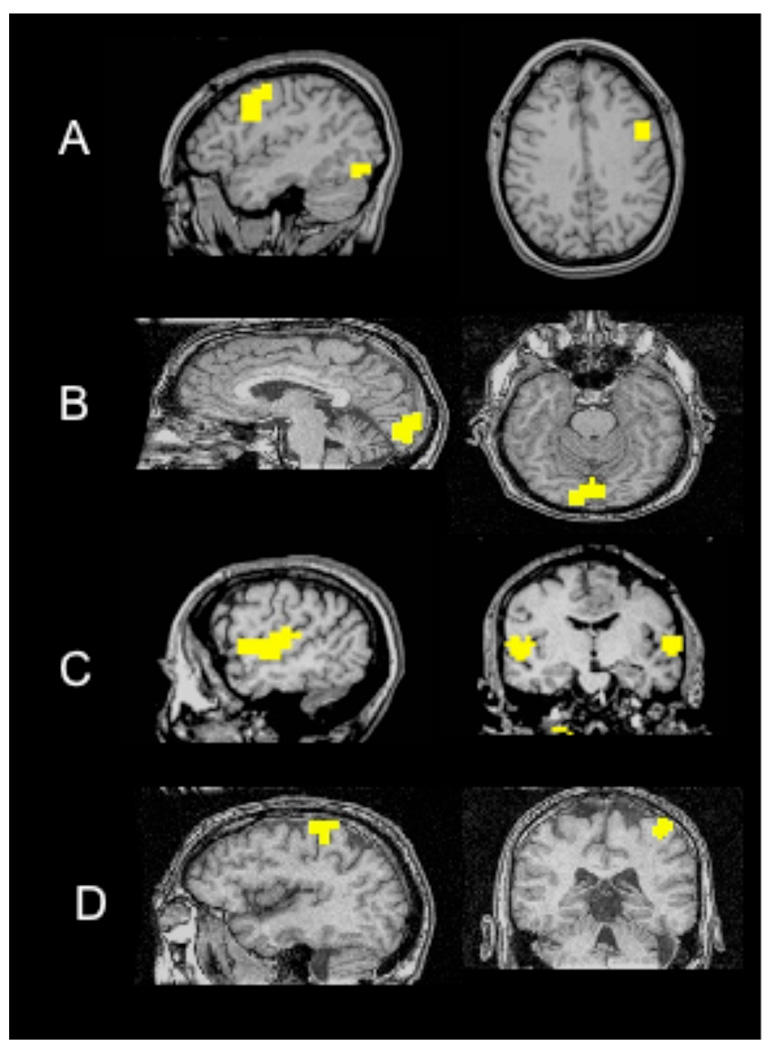
FMRI activation maps were generated for 24 subjects (males 14, females 10; median age 52±16.62) with previously diagnosed benign and biopsy proven low-grade brain neoplasms using a GE Signa 1.5T magnetic resonance imaging (MRI) scanner. The imaging protocol included a 3DSPGR T1 brain volume, T2 coronal anatomical, and 8 BOLD-weighted functional scans. The 3DSPGR T1 gradient echo sequence (TR/TE = 21/7 ms, α = 40°; field of view (FOV) = 24 cm2, matrix (MA) = 256 X 256, slice thickness varied between 1.2–1.4 mm) consisted of 124 slices. For the functional scans, the imaging protocol consisted of acquiring 22–24 coronal slices to detect brain activity for specific task from a single shot gradient echo EPI sequence (TR/TE = 2000/40 ms; α = 85°; FOV = 24cm2, MA = 64 X 64, slice thickness = 6 mm, interslice gap = 1 mm). Each patient performed 4 functional tasks, with each task performed twice for a total of 8 functional scans. The second iteration of each functional task was performed in opposite order to the first iteration. The fMRI tasks were block paradigms with each task block bookended and interleaved by a non-task, or “rest” block of equal duration. The task attempted to localize one of the following: expressive language function in region of lateral inferior/middle frontal gyri (word generation paradigm); visual perception in primary and association visual cortices of bilateral occipital pole (reversing checkerboard paradigm), primary and association auditory responses in bilateral superior temporal gyri (text listening); and upper limb fine motor control in bilateral primary sensorimotor cortices (alternating-limb-bilateral finger tapping paradigm). See Figure 1. for typical regions of activation associated with each fMRI task.
Figure 1.
Activation of anatomical regions from a single subject performing the following functional task for A) AWG (lateral inferior/middle frontal gyri), B) 8Hz Checkerboard (occipital lobe), C) Text Listening (bilateral superior temporal gyri), and D) Finger Tapping (primary sensorimotor cortices, Right hand).
fMRI task descriptions
Antonym word generation
The Antonym word generation (AWG) task consisted of a series of single words projected on a screen at the foot of the table. In an effort to minimize head motion that occurs during overt pronunciation, the patient was instructed to think silently of a word that means the opposite of the word that appears on the screen. Words appeared at a rate of 2 seconds per word. The words appeared for 20 seconds while performing the task followed by 20 seconds of a blank screen. This repeated a total of 5 times for a scan duration of 3 minutes and 44 seconds. This task was practiced with each patient prior to the scan to confirm their performance ability. After each scan the patient was asked to assess their performance during the fMRI acquisition. All patients reported at least moderate task compliance.
Text Listening
The patient was instructed to listen to the auditory narration of a text passage with eyes closed. The passage was read for 20 seconds followed by a 20 second rest. This task was repeated 4 times with a scan time of 3 minutes and 4 seconds.
Alternating-Limb Finger Motor
Before the patient entered the scan room, it was verified that the patient could perform this task. The subject was instructed to sequentially oppose each of the fingers of one hand to the thumb with eyes closed. The task began with the right hand tapping for 16 seconds, left hand tapping for 16 seconds followed by a 16 second rest. The task was repeated 4 times with a scan time of 3 minutes and 32 seconds. The second iteration of this task began with the left hand tapping first for 16 seconds, right hand tapping for 16 seconds, and rest for 16 seconds.
8 Hz Checkerboard
Black and white checkerboard reversing squares flashed on the screen for a 20 second duration, followed by a 20 second blank screen. This visual stimulus was repeated 4 times with a scan time of 3 minutes and 4 seconds. A cross hair fixation was centered on the screen for the focal point of the patient. The patient was instructed to visually center their gaze on the screen during the entire scan.
Post Processing
Post processing was performed using fMRI-specific software AFNI (21). 3D motion correction, spatial smoothing with a 9mm Gaussian filter, and linear coregistration was performed between the 3D anatomical images and all EPI fMRI datasets for each subject. A binary-valued reference BOLD response template was created for each task with the same on-off timing as the stimulus. This block reference template was smoothed and temporally delayed to account for variance and lag in the hemodynamic response. The subject’s BOLD response was verified to match the template in expected areas by viewing the voxel signals near the appropriate anatomy. The voxelwise correlations were used to derive an uncorrected t-value based on the fit of each timecourse to the reference template.
A subjective threshold was chosen per subject and scan based upon observed measures of selectivity to minimize suprathreshold artifact voxels and sensitivity and spatial extent to ROI fMRI response activations. This method of subjectively varying the statistical threshold for each subject and fMRI scan accounts for individual variables in BOLD task responses, and is the strategy for thresholding all clinical fMRI scans at our institution and common clinical practice for evaluation of individual fMRI pretreatment mappings. With over 12 years of expertise in clinical fMRI thresholding, a sole investigator (C.M.) was responsible for selecting all the threshold levels. To minimize the effect of a single threshold and explore the effect of varying threshold values on the repeatability calculations, two additional thresholds were applied to each dataset. The two additional thresholds were calculated values ± 20% of each selected threshold. Thus, a total of 3 threshold values were applied to each fMRI dataset. The full range of threshold values, including ± 20%, for each task were as follows: AWG t>4.8 to t>20.4, Alternating Limb Finger Motor Right Hand (Finger TapR) t>3.2 to t>25, Alternating Limb Finger Motor Left Hand (Finger TapL) t>3.6 to t>26.4, 8Hz Checkerboard (8 HzChecker) t>3.2 to t>15.6, Text Listening right hemisphere (TextR) t>2.8 to t>18, Text Listening left hemisphere (TextL) t>2.8 to t>25.8.
AFNI’s 3dclust command was used to determine the activation clusters for each threshold. Specifically the voxels were constrained to connect to their 3D nearest neighbors and the clusters contained 10 or more voxels. For each activation cluster spatially identified as task-relevant, 3 measures of reproducibility were calculated using the parameters that Rombouts et al. applied to fMRI of the visual cortex (19): 1) The difference in the COM location, 2) measuring the number of reproducible activated voxels, Rsize, and 3) measuring the common regions of activation between the two iterations for the same task, Roverlap.
The linear distance between the COM for identified clusters between two task iterations was calculated. Voxels that exceeded the significance threshold were used in determining the difference in the center of mass per cluster. fMRI tasks which are based on a contrast between conditional states will often demonstrate suprathreholds voxel clusters in multiple regions. An example is the AWG task: conditional contrast between task and “rest” conditions can be expected to show activity in language-related regions and also visual responses secondary to the presentation of visual task cues. For purposes of this study, ROIs of singular task-related functional regions were specified, and repeatability calculations were based solely on these primary regions of activated relevant cortex. Ideally, the COM differential should have a value approaching zero representing activation within the same area.
The measure of Rsize represents the repeatability of suprathreshold cluster size across task iteration. Rsize = 2* Vsmallest /(V1t +V2t) [1] where V1t is the averaged size of the activated volume for threshold, t, in scan1 and V2t is the averaged size of the activated volume for threshold, t, in the corresponding scan 2. Vsmallest is the smallest activated volume size of the two scans at a given threshold.
The Roverlap measure represents the spatial concurrence of task-related clusters for each paired task iteration. Roverlap = 2* Voverlap / (VI1+ VI2) [2]. VI1 is the number of voxels activated in iteration 1 and VI2 is the number of voxels activated in iteration 2. Voverlap is the number of overlapped voxels contained in both VI1 and VI2.
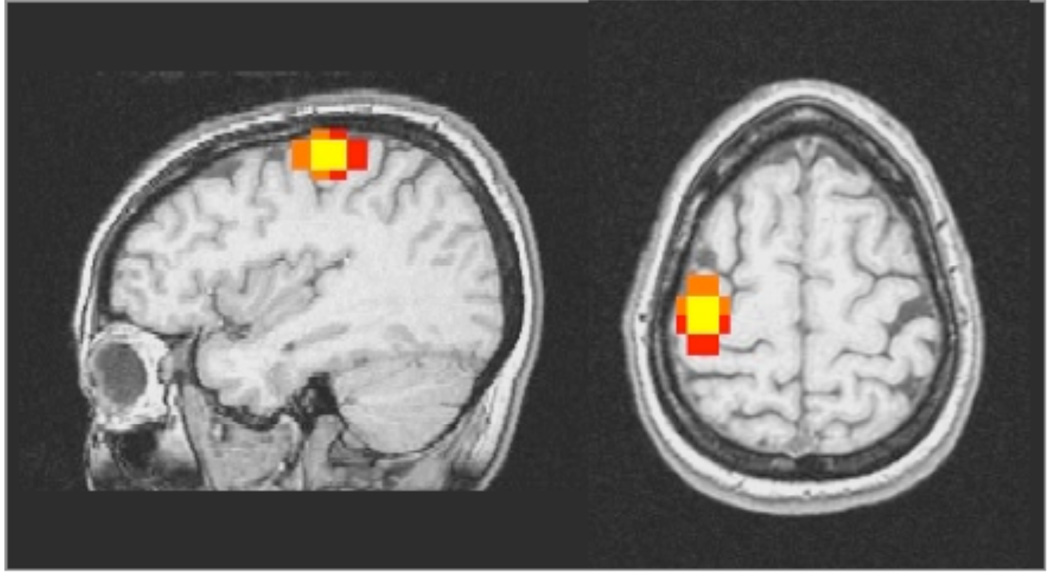
Ideally the value of both Rsize and Roverlap should be very close to 1 representing well matched size of activated clusters and excellent overlap between the activation regions for the varying thresholds for each task iteration. Each of the calculations for the difference in the COM, Rsize and Roverlap were derived for each repeated pair of fMRI scans at each of 3 thresholds. Figure 2 shows an example of the difference in activated voxels from repetitions of a finger motor task. The voxels that are activated in both iterations of the task are yellow. The orange represents activated voxels from the first iteration, and red represents voxels activated in the second iteration.
Fig. 2.
Single subject overlap region of finger motor task with left hand shown in the sagittal and axial slices. Orange equals voxels activated in the first iteration, red equals voxels activated in the second iteration, and yellow equals overlapped activated voxels from both iterations.
Results
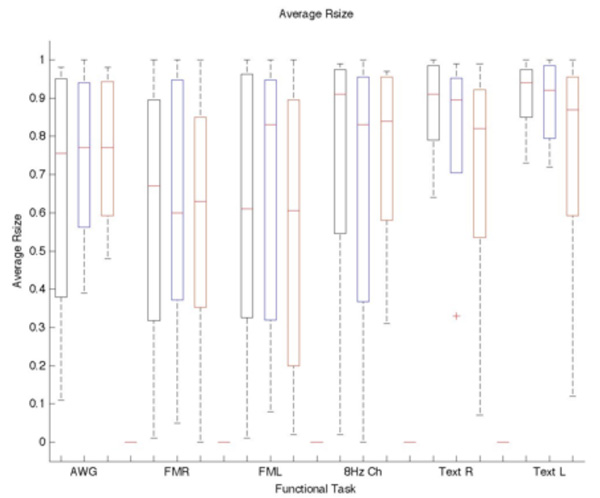
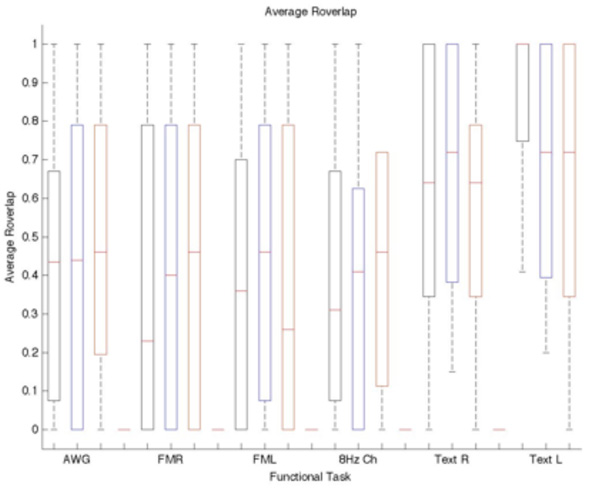
All subjects were able to successfully perform the entire fMRI protocol of four repeated task scans. Individual task thresholds including the ±20% ranged from t>2.8 to t>26.4 to accommodate the widely varying levels of task performance and BOLD response across the cohort of 24 subjects and 184 individual task iterations. The displacement between the COM (cf. Fig. 3), Rsize (cf. Fig. 4), and Roverlap (cf. Fig 5) were calculated to measure the reproducibility of fMRI to define avoidance regions for radiotherapy treatment planning. Rsize and Roverlap varied between 0.0 (worst) and 1.0 (best) (20). The average median distance of the COM difference for each task and threshold is displayed in Table I. The average median Rsize and Roverlap values for each task and selected threshold are listed respectively: AWG 0.77±0.01, 0.44±0.01; Finger TapR 0.6±0.04, 0.4±0.12; Finger TapL 0.83±0.13, 0.46±0.10; 8 HzChecker 0.83±0.04, 0.41±0.08; TextR 0.90±0.05, 0.72±0.05; and TextL 0.92±0.04, 0.72±0.16. R and L correspond to the right and left cortex. The averaged Rsize values ranged from 0.55±0.31 – 0.91±0.07 with the text listening task having the highest repeatability for Rsize, cf. Fig 4. The finger-tapping task showed the greatest variability in cluster size and overlapped voxels between both scan iterations. The averaged Roverlap values ranged from 0.34 ±0.35 – 0.88±0.21 for all tasks (cf. Fig 5).
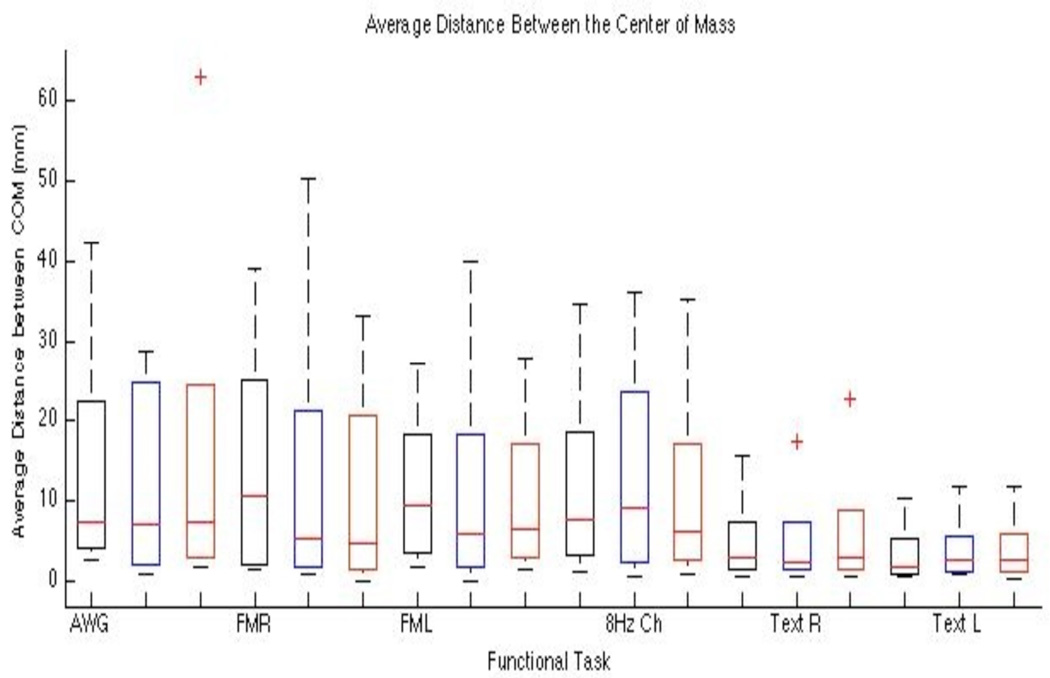
Figure 3.
Box plot of the average distance between the Center of Mass vs Threshold and Task. Blue indicates the selected standard threshold and black and red are the −20% and + 20% of the standard. The COM is not significantly affected by varying the threshold. Each bar represents the lower (25%) and upper (75%) quartile. The red horizontal line represents the median value for each threshold and task. The minimum and maximum values are represented by the brackets at both ends of each bar. The outliers are shown beyond the brackets.
Figure 4.
Box plot of the average Rsize vs Threshold and Task. Blue indicates the selected standard threshold and black and red are the −20% and + 20% of the standard. The Rsize is not significantly affected by varying the threshold. Each bar represents the lower (25%) and upper (75%) quartile. The red horizontal line represents the median value for each threshold and task. The minimum and maximum values are represented by the brackets at both ends of each bar. The outliers are shown beyond the brackets.
Figure 5.
Box plot of the average Roverlap vs Threshold and Task. Red indicates the selected standard threshold and black and red are the −20% and + 20% of the standard. The Roverlap is not significantly affected by varying the threshold. Each bar represents the lower (25%) and upper (75%) quartile. The red horizontal line represents the median value for each threshold and task. The minimum and maximum values are represented by the brackets at both ends of each bar.
Table I.
Average Center of Mass Distance (mm)
| Median Thresh 1 |
Median Thresh 2 |
Median Thresh 3 |
STD | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| AWG | 7.25 | 7.20 | 7.40 | 0.10 | 0.80 | 63.00 |
| Finger TapR | 10.60 | 5.30 | 4.80 | 3.21 | 0.00 | 50.20 |
| Finger TapL | 9.40 | 5.80 | 6.35 | 1.94 | 0.10 | 39.80 |
| 8 HzChecker | 7.60 | 9.10 | 6.30 | 1.40 | 0.60 | 36.10 |
| TextR | 2.80 | 2.35 | 2.90 | 0.29 | 0.50 | 22.80 |
| TextL | 1.70 | 2.55 | 2.65 | 0.52 | 0.40 | 11.70 |
Discussion
A measure of test re-test reliability of fMRI including multiple task activation within subjects for multiple brain regions of benign and biopsy proven low-grade neoplasms has been performed. Brain activity was detected for all scanning sessions for the BOLD weighted functional scans: language (word generation task), primary and association auditory (text listening), upper limb fine motor control (alternating-limb bilateral finger tapping), and primary visual perception (8 Hz reversing checkerboard).
Reproducibility varied among the tasks used for activation. The reversing checkerboard visual stimulus and antonym word generation tasks had the least amount of overlapped clusters across thresholds. It is possible that the variance for the antonym word generation task could be due to its higher cognitive nature. The active performance of a cognitive task would be expected to involve a strategic recruitment of neurons that is relatively variable compared to the other 3 tasks applied in this study. Both text listening and reversing checkerboard tasks involve a more passive response to stimulus presentation, while the finger tapping task is expected to mostly involve the repetitive activation of primary sensorimotor neurons. The relatively lower reproducibility measures for the visual stimulus were not expected, but might be attributed to the stimulus presentation equipment design. The rear projection screen and mirror viewing system employed in this study subtend a low field-of-view from inside the magnet bore. This low degree of viewing angle represents a lowered control of the visual field and might have contributed to the visual response variability. It is also noted that the reversing checkerboard stimulus generally yielded the lowest threshold values, further indicating a possible weakness to this experimental equipment design.
Reproducibility was analyzed based on the distance between the COM activation, the size of activation, and the overlapped region of activation. Previous published results by Rombouts et al had similar findings for the checkerboard task with the mean Rsize = 0.83±0.16 and mean Roverlap = 0.31±0.11. Of 4 tasks and 3 reproducibility parameters measured, activation for the Text Listening Task had the highest reproducibility for each parameter across thresholds. Performing task within the same imaging session may have minimized motion and increased reproducibility.
It is known that laterality indexing can be sensitive to selected threshold levels (22). In this study comparing fMRI results of single subject data across multiple paradigms, we chose to subjectively vary the threshold in a manner which would account for the variance among the individual subjects’ task performance and BOLD response. This method is akin to the manner in which clinical fMRI thresholding is commonly applied to individual subjects for pretreatment mapping. The wide range of selected thresholds (t > 2.8 to t > 26.4) provide insight into the variability of fMRI responses across individuals and tasks. To further address the issue of threshold sensitivity, additional comparisons were calculated by adjusting each subjectively selected threshold by ± 20%. The results that were obtained in this study inherently provide a measure of validation for the selective thresholding that was applied. In particular, the measures for Rsize indicate a consistency for each ROI fMRI activation comparison across the two task iterations. Additionally, the data indication that the wide range of applied thresholding did not significantly alter the reproducibility measures provides an indication that the thresholds were within a suitably neutral range. While it is possible to apply a single threshold to maintain a consistency of statistical confidence, and a standard threshold is necessary when assessing averaged group results, the variability in individual subject fMRI task performance across multiple paradigms is more accurately assessed for clinical evaluation by selective thresholding.
Conclusion
Reproducibility of activation in repeated tasks indicates the reliability of fMRI procedures for mapping multiple brain functions. Application of 3 varying thresholds (selected standard ±20%) did not significantly alter the reproducibility measures. fMRI is indicated to be a viable tool for localizing activation with reproducible confidence across 4 different tasks in patients with low-grade brain neoplasms. The usefulness of fMRI for radiotherapy has been validated as a presurgery evaluation tool. The data presented is within range of prior presurgical evaluation studies. Investigation of this technique should be considered for the definition of conformal avoidance regions in patients undergoing radiotherapy.
Acknowledgements
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) 1F31NS5297-01 and National Institute of Health R01-CA109656. The authors extend their thanks to the reviewers whose insightful commentary was instrumental toward improving this written report.
Abbreviations
- BOLD
Blood Oxygen Level Dependent
- COM
Center of Mass
- EPI
Echo Planar Imaging
- fMRI
functional Magnetic Resonance Imaging
- Roverlap
reproducibility of the location of activated voxels
- Rsize
reproducibility of the number of activated voxels
References
- 1.Ogawa S, Lee T, Kay A, Tank D. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim S, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Detre JA. Clinical applicability of functional MRI. J Magn Reson Imaging. 2006;23:808–815. doi: 10.1002/jmri.20585. [DOI] [PubMed] [Google Scholar]
- 4.Jack CR, Thompson RM, Butts RK, Sharbrough FW, Kelly PJ, Hanson DP, Riederer SJ, Ehman RL, Hangiandreou NJ, Cascino GD. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology. 1994;190:85–92. doi: 10.1148/radiology.190.1.8259434. [DOI] [PubMed] [Google Scholar]
- 5.FitzGerlad DB, Cosgrove GR, Ronner S, Jing H, Buchbinder BR, Belliveau JW, Rosen BR, Benson RR. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR AM JNeuroradiol. 1997;18:1529–1539. [PMC free article] [PubMed] [Google Scholar]
- 6.Pouratian N, Bookheimer SY, Rex DE, Martin NA, Toga AW. Utility of preoperative functional magnetic resonance imaging for identifying language cortices in patients with vascular malformations. Neurosurg Focus. 2002;13:21–32. [PubMed] [Google Scholar]
- 7.Disbrow EA, Slutsky DA, Roberts TP, Krubitzer LA. Functional MRI at 1.5 tesla: a comparison of the blood oxygenation level-dependent signal and electrophysiology. Proc Natl Acad Sci USA. 2000;97:9718–9723. doi: 10.1073/pnas.170205497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, Schulder M, Narra V, Kalnin AJ, Cathcart C, Jacobs A, Lange G, Holodny AI. Functional magnetic resonance imaging aided radiation treatment planning. Med Phys. 2000;27:1563–1572. doi: 10.1118/1.599022. [DOI] [PubMed] [Google Scholar]
- 9.Atlas SW, Howard RS, Maldjian J, Alsop D, Detre JA, Listerud J, D’Esposito M, Judy K, Zager E, Stecker M. Functional magnetic resonance imaging of regional brain activity in patients with intracerebral gliomas: findings and implications for clinical management. Neurosurgery. 1996;38:329–338. doi: 10.1097/00006123-199602000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Roessier K, Donat M, Lanzenberger R, Novak K, Geissler A, Gartus A, Tahamtan AR, Milakara D, Czech T, Barth M, Knosp E, Beisteiner R. Evaluation of preoperative high magnetic field motor functional MRI (3 tesla) in glioma patients by navigated electrocortical stimulation and postoperative outcome. J Neurol Neurosurg Psychiatry. 2005;76:1152–1157. doi: 10.1136/jnnp.2004.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suess O, Suess S, Brock M, Kombos T. Intraoperative electrocortical stimulation of Brodman area 4;a 10-year analysis of 255 cases. Head & Face Medicine. 2006;2:1–13. doi: 10.1186/1746-160X-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bookheimer S. Pre-surgical language mapping with functional magnetic resonance imaging. Neuropshychology Review. 2007;17:145–155. doi: 10.1007/s11065-007-9026-x. [DOI] [PubMed] [Google Scholar]
- 13.FitzGerlad DB, Cosgrove GR, Ronner S, Jing H, Buchbinder BR, Belliveau JW, Rosen BR, Benson RR. Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR AM JNeuroradiol. 1997;18:1529–1539. [PMC free article] [PubMed] [Google Scholar]
- 14.Casey BJ, Cohen JD, O’Craven K, Davidson RJ, Irwin W, Nelson CA, Noll DC, Hu X, Lowe MJ, Rosen BR, Truwitt CL, Turski PA. Reproducibility of fMRI results across four institutions using a spatial working memory task. Neuroimage. 1998;8:249–261. doi: 10.1006/nimg.1998.0360. [DOI] [PubMed] [Google Scholar]
- 15.Maldjian JA, Laurienti PJ, Driskill L, Burdette JH. Multiple reproducibility indices for evaluation of cognitive functional MR imaging paradigms. AJNR AM JNeuroradiol. 2002;23:1030–1037. [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington GS, Buonocore MH, Farias ST. Intrasubject reproducibility of functional MR imaging activation in language tasks. AJNR AM JNeuroradiol. 2006;27:938–944. [PMC free article] [PubMed] [Google Scholar]
- 17.Havel P, Braun B, Rau S, Tonn JC, Fesl G, Cruckmann H, Ilmberger J. Reproducibility of activation in four motor paradigms: an fMRI study. J Neurol. 2006;253:471–476. doi: 10.1007/s00415-005-0028-4. [DOI] [PubMed] [Google Scholar]
- 18.Vlieger EJ, Lavini C, Majoie CB, Heeten GJ. Reproducibility of functional MR imaging results using two different MR systems. AJNR AM JNeuroradiol. 2003;24:652–657. [PMC free article] [PubMed] [Google Scholar]
- 19.Rombouts SARB, Barkhof F, Hoogeneraad FGC, Sprenger M, Valk J, Scheltens P. Test-retest analysis with functional MR of the activated area in the human visual cortex. AJNR. 1997;18:1317–1322. [PMC free article] [PubMed] [Google Scholar]
- 20.Rombouts SARB, Barhof F, Hoogenraad FGC, Sprenger M, Scheltens P. Within-subject reproducibility of visual activation patterns with functional magnetic resonance imaging using multislice echo planar imaging. MRI. 1998;16:105–113. doi: 10.1016/s0730-725x(97)00253-1. [DOI] [PubMed] [Google Scholar]
- 21.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 22.Ruff IM, Petrovich, Brennan NM, Peck KK, Hou BL, Tabar V, Brennan CW, Holodny AI. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery. AJNR. 2008;29:528–535. doi: 10.3174/ajnr.A0841. [DOI] [PMC free article] [PubMed] [Google Scholar]