Abstract
Demodex mites, class Arachnida and subclass Acarina, are elongated mites with clear cephalothorax and abdomens, the former with four pairs of legs. There are more than 100 species of Demodex mite, many of which are obligatory commensals of the pilosebaceous unit of mammals including cats, dogs, sheep, cattle, pigs, goats, deer, bats, hamsters, rats and mice. Among them, Demodex canis, which is found ubiquitously in dogs, is the most documented and investigated. In excessive numbers D. canis causes the inflammatory disease termed demodicosis (demodectic mange, follicular mange or red mange), which is more common in purebred dogs and has a hereditary predisposition in breeding kennels1. Two distinct Demodex species have been confirmed as the most common ectoparasite in man. The larger Demodex folliculorum, about 0.3–0.4 mm long, is primarily found as a cluster in the hair follicle (Figure 1a), while the smaller Demodex brevis, about 0.2–0.3 mm long with a spindle shape and stubby legs, resides solitarily in the sebaceous gland (Figure 1b). These two species are also ubiquitously found in all human races without gender preference. The pathogenic role of Demodex mites in veterinary medicine is not as greatly disputed as in human diseases. In this article, we review the key literature and our joint research experience regarding the pathogenic potential of these two mites in causing inflammatory diseases of human skin and eye. We hope that the evidence summarized herein will invite readers to take a different look at the life of Demodex mites in several common human diseases.
Keywords: Demodex, eye disease, eyelash, hair follicle, mite, skin disease
The life cycle of the Demodex mite is approximately 14–18 days from an egg to the larval stage of protonymph to deutonymph and finally to the adult stage (see Figure 1)2. Because all adult mites have a limited life cycle, their ability to expand in numbers in a human host depends on successful copulation by adult male and female mites in the opening of the hair follicle (near the skin surface). Afterwards, the gravid female moves to the sebaceous gland to deposit eggs, each of which gives rise to a larva and then a protonymph in the sebaceous canal. A protonymph is brought to the opening of the hair follicle and matures into a deutonymph, which crawls on to the skin surface, then re-enters a hair follicle to become an adult. Therefore, during a life cycle, if adults can successfully copulate and produce the next generation, the extent of Demodex infestation will gradually increase in the host over time.
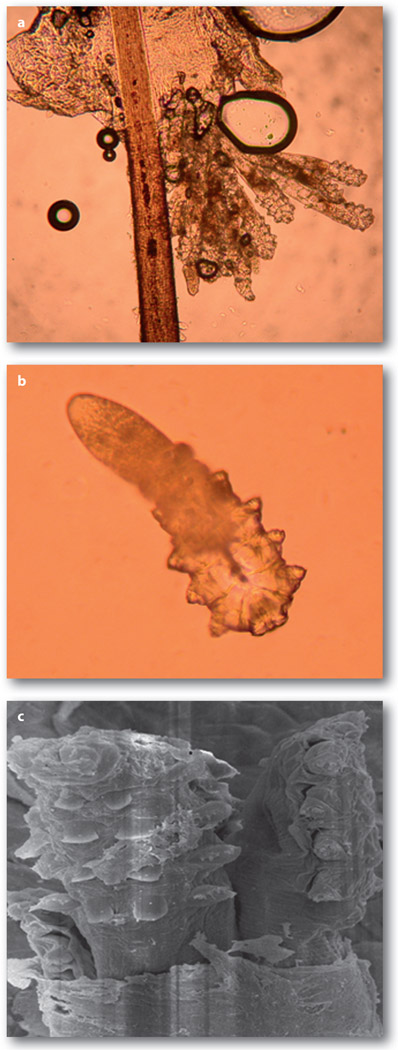
Figure 1.
Microscopic features of adult D. folliculorum(a) and D. brevis (b). D. folliculorum tends to gather as a group in the follicle area of the hair or lash (a). In contrast, D. brevis tends to be in solitude and reside in the sebaceous gland. As a result, D. brevis is not readily detected during sampling of the lash. Under scanning electron microscopy, several D. folliculorum mites are together next to the hair follicle
Scanning electron microscopy reveals the special piercing mouthparts of D. folliculorum as a sharp offensive weapon capable of destroying adipose tissue. Although it has also been proposed that mites feed on follicular and glandular epithelial cells, sebum is thought to be the mite’s main food source. As a result, both Demodex species often coexist at the same skin area and gather in the face, cheeks, forehead, nose and external ear tract, where active sebum excretion creates favourable habitats and breeding conditions. Because these mites are susceptible to desiccation, their lifespan is limited outside the living body, and direct contact is required for transmission of mites from one individual to another. Consequently, an effective regimen in eradicating Demodex infestation should include killing as well as prevention of their copulation and transmission.
Implication of Demodex mites in human diseases
Although Demodex mites have been implicated as a cause of many human skin disorders, their pathogenic role has long been debated3. Such a concern has been raised in part because some Demodex mites can be found in the skin of asymptomatic individuals. Most researchers attribute some skin diseases to Demodex only when their numbers are elevated. To quantify the extent of Demodex infestation in the skin, a surface biopsy has been standardized as the main method4. After cleaning the patient’s skin and a glass slide with ether to improve adherence, a spot of cyanoacrylate adhesive (superglue) is applied on the skin surface of interest before being overlaid with a slide. After approximately 1 minute on the skin, the slide is gently removed and covered by one drop of immersion oil before being mounted with a coverslip. The density of D. folliculorum is measured by counting the number of mites on the slide in a pre-marked surface area of 1 cm2 at a magnification of ×40 and ×100 under a microscope. Because hairs together with the superficial horny layer are sampled with minimal sebaceous glands, D. folliculorum is primarily found. Using this method, Demodex infestation is thought to be non-existent in healthy children of less than one decade of life, increasing in an age-dependent manner, and found probably 100% in elderly skin, unless some eradicating measures are taken. It remains unclear whether such an age-dependent increase is due to cumulative colonization or an increase in sebum levels with age. This quantitative biopsy method has also been used for monitoring the efficacy of various therapies in eradicating D. folliculorum in the skin.
Since 1932, Demodex infestation has been implied as a causative role in rosacea, a chronic inflammatory dermatosis of the convexities of the central face characterized by the presence of multiple small dome-shaped erythematous papules and papulopustules arising on a background of fixed inflammatory erythema. It has been proposed that the pathogenic potential increases if a mite density is higher than five per cm2 1. Several studies have shown a higher mite density on the faces of rosacea patients than on those of age- and sex-matched non-rosacea controls4. It is intriguing that Demodex numbers increase when the outside temperature elevates in spring and summer, coinciding with the time when rosacea is exacerbated. Demodex mites have also been implicated as a cause of other skin diseases such as pityriasis folliculorum (typically found in older women who do not use soap but apply large amounts of makeup), perioral dermatitis (a facial rash typically occurring around the mouth), scabies-like eruptions, facial pigmentation, eruptions of the bald scalp, Demodex folliculitis (an inflammatory reaction in the superficial aspect of the hair follicle), demodicosis gravis (dermal granulomas formed because of mite remnants phagocytosed by foreign-body giant cells) and even basal cell carcinoma. Furthermore, the above skin demodicosis is prone to develop in patients whose local or systemic immune status is compromised by topical or systemic administration of steroids or other immunosuppressive agents or by diseases such as leukaemia and HIV.
Implication of Demodex mites in human eye diseases
Contiguous to the skin, the eye can also be infested by Demodex mites. In the eye, D. folliculorum is found in the lash follicle, whereas D. brevis burrows deep into the lash sebaceous gland and the meibomian gland, the latter of which produces meibum as the superficial layer of the preocular tear film. Because the eye is surrounded by such protruding body parts as the nose, the brow and the cheek, the eyelid is not as accessible as the face to daily cleansing hygiene. Therefore, once Demodex mite infestation establishes in the face, it is likely to spread and flourish in the eyelid. As a matter of fact, the first disorder that was associated with Demodex, dated as early as 1899, was blepharitis (inflammation of the lid), a disease that has continued to be a subject of investigation ever since5. However, as in the skin, the pathogenic potential of these mites in blepharitis had been questioned because a low number of Demodex was found in the lashes of asymptomatic individuals.
Cylindrical dandruff (CD) in eye lashes (Figure 2a) is a common finding in some blepharitis patients. We speculated that the controversy of whether CD can be regarded pathognomonic for Demodex infestation originated from errors of the previously published method in sampling and counting mites. Besides errors in randomly epilating eyelashes and mounting the slide using a drop of oil, the most important one was that Demodex embedded in compact and opaque CD could not be fully counted unless 100% alcohol6 or a drop of fluorescein7 is added to stimulate them to migrate out (Figure 3). Using the modified method, we have confirmed that CD in eye lashes is indeed a reliable clinical sign indicative of Demodex infestation of the eyelash6. Furthermore, we observed that Demodex infestation can still be detected in 50% of patients despite daily lid scrub with Baby shampoo (a routine regimen) for more than 1 year. This result together with those published earlier strongly suggests that the conventional lid scrub with baby shampoo may not eradicate Demodex, rendering it difficult to resolve whether Demodex is pathogenic or not.
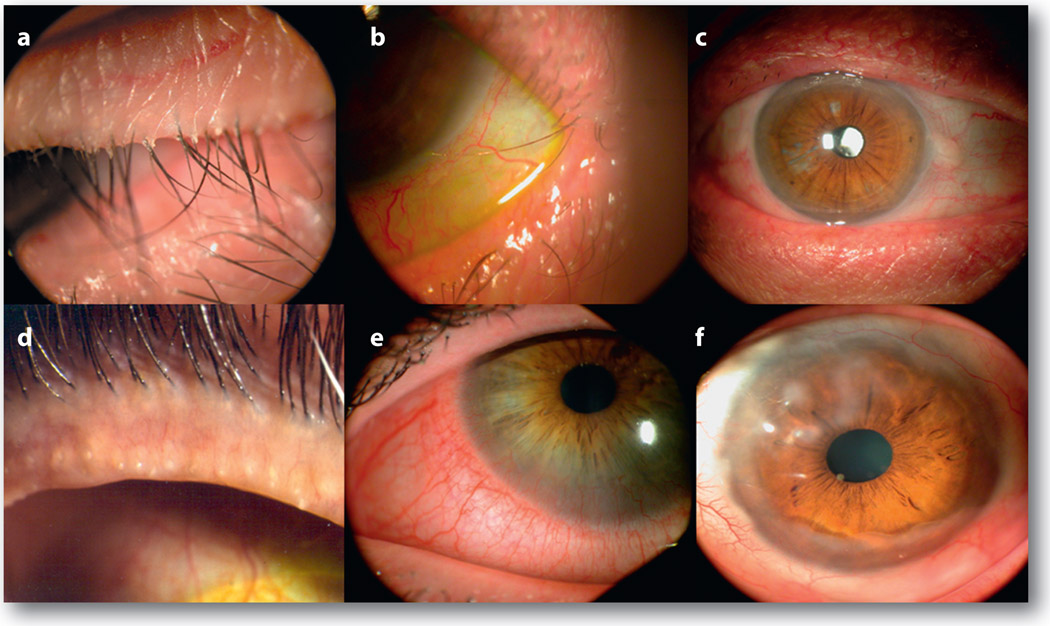
Figure 2.
Clinical features of Demodex infestation in the eye. The most common finding is CD on the skin surface where lashes emit (a). As mites reside close to the follicle, the orientation of lashes become disorganized (a), can turn in to touch the ocular surface (trichiasis) (b), and in the extreme cases results in the loss of lashes (madarosis) (c). Besides lashes, meibomian glands can be involved to manifest plugging of the orifices (d). The inflammation derived from lashes or meibomian glands of the lid margin can be spill over to the conjunctiva (e) and cause a number of corneal lesions, including nodular scar (f)
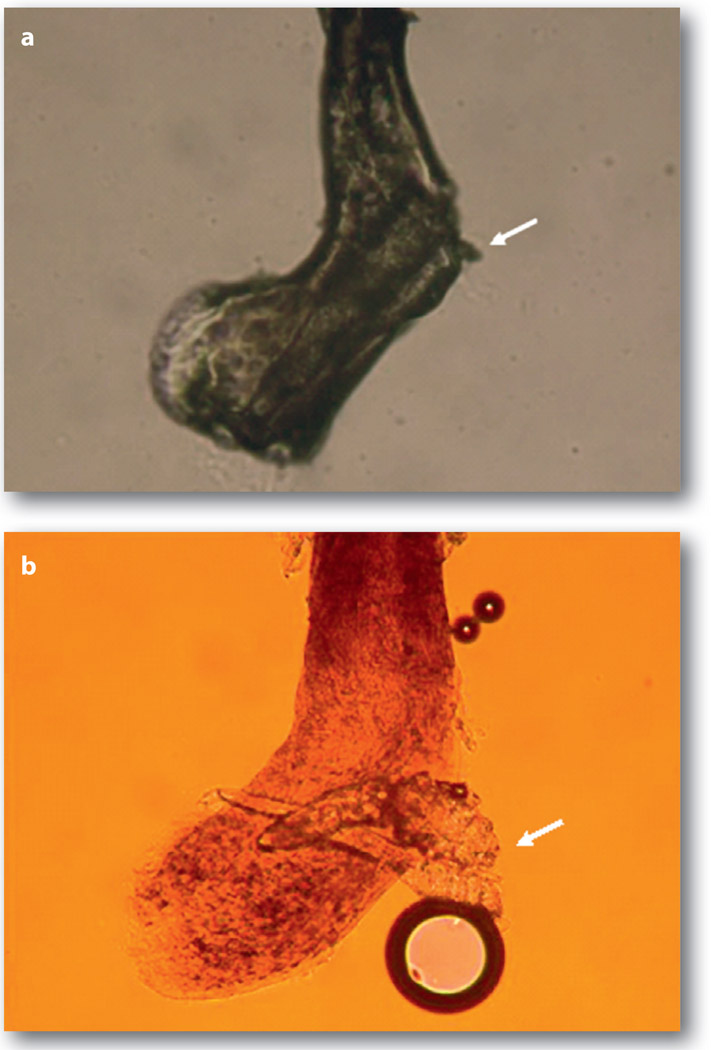
Figure 3.
Unmasking embedded mites in CD by fluorescein solution. A representative lash with CD (a), in which embedded mites are revealed only after application of a drop of an aqueous solution containing fluorescein (b). An arrow marks the hidden mites (a) that are revealed by fluorescein drops. Note that this procedure of dissolving CD frequently generates air bubbles, and the change of colour is due to the presence of fluorescein
Hence, we believe that one way of determining the pathogenic potential of Demodex infestation in blepharitis and other ocular diseases is to identify a treatment that can kill Demodex with a good safety profile. Using an in vitro microscopic observation for a period of 150 minutes, we found that adult D. folliculorum is resistant to a wide range of common antiseptic solutions including 75% alcohol and 10% povidone–iodine, but can dose-dependently be killed by tea tree oil (TTO)8. TTO, a natural essential oil steam-distilled from the leaf of the tea tree Melaleuca alternifolia, has long been used as an aboriginal traditional medicine in Australia for wounds and cutaneous infection. Unlike Bbaby shampoo, lid scrub with TTO not only cleanses CD from the lash root, but also stimulates embedded mites to migrate out to the skin (Figure 4). As a result, weekly lid scrub with 50% TTO and daily lid scrub with tea tree shampoo is effective in eradicating ocular Demodex infestation in vivo, as is evident by bringing the Demodex count down to zero in 4 weeks in a majority of patients9,10. Because refractory ocular surface inflammation resolved after significant reduction of mite counts achieved by this new lid scrub regimen, we concluded that ocular Demodex infestation in lashes can cause the lashes to become mal-aligned (Figure 2a), turn in to touch the cornea (trichiasis) (Figure 2b) and fall off (madarosis) (Figure 2c). Furthermore, its infestation in meibomian glands can cause meibomian gland dysfunction (Figure 2d), leading to disturbance and deficiency of the lipid tear film and blurry vision9. To our surprise, inflammation derived from lashes and meibomian glands (in the context of blepharitis) may spread over to the conjunctiva, resulting in conjunctivitis (Figure 2e) and even to the cornea, resulting in various blinding corneal lesions (Figure 2f)9,10. The tendency of inflammation spread to the conjunctiva and cornea depends on not only the severity of the host inflammatory response, but also the distance from the site of mite infestation. In this regard, the intriguing finding that the rate of detecting D. brevis is much higher in patients who developed corneal lesions10, is consistent with the notion that D. brevis primarily residing in meibomian glands is easier to reach the conjunctiva and the cornea. Future studies are needed to determine whether the pathogenicity of D. brevis differs from that of D. folliculorum. Unlike what has been reported for skin demodicosis, we recently discovered that ocular Demodex infestation could be detected in immune non-compromised paediatric patients inflicted with refractory blepharoconjunctivitis (Liang L, Safran S, Gao Y, Sheha H, Li J, and Tseng SCG, unpublished work). Therefore future studies are also needed to determine whether eyelash sampling is more sensitive than skin surface biopsy in detecting demodicosis in paediatric patients.
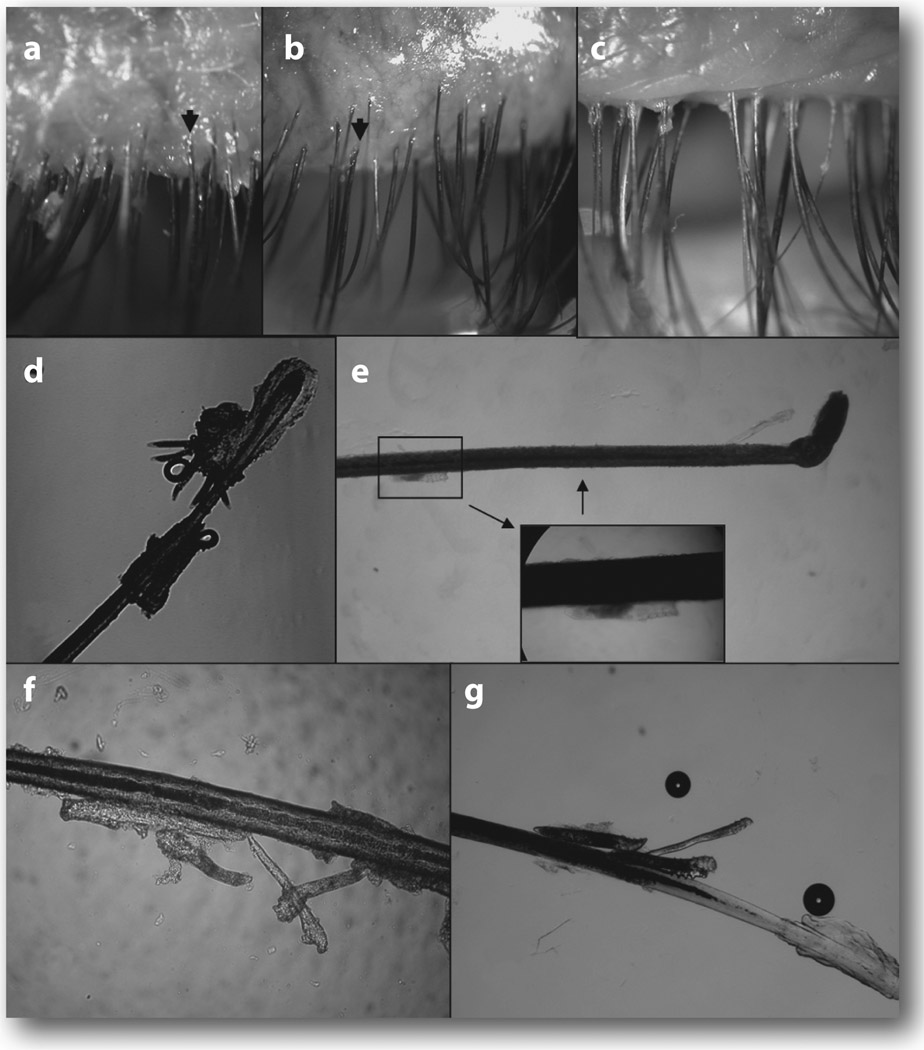
Figure 4.
Migration of Demodex by lid scrub with TTO. In this eye with diffuse CD found in the lashes before treatment (a), lash to be epilated (marked by arrow) showed a fragment of CD attached to the lash and abundant Demodex embedded close to the lash follicle (d). After lid scrub in the orifice with 50% TTO, the lashes became clean and totally free of CD, but tails of Demodex were protruding from the lash roots (b, arrow). At 3 minutes after lid scrub, free Demodex was found on the trunk close to the skin surface, i.e. away from the lash follicle in the epilated lash (e). Rotating these lashes (shown in b) before epilation allowed us to detect a group of Demodex migrating along the lash trunk (f and g). If no lid scrub was carried out at home for one week, CD returned to the lashes 1 week later (c). With permission from Br. J. Ophthalmol.8
Pathogenic mechanism of Demodex mites
Because a high number of Demodex mites have been observed in the skin and eyelashes of patients with the aforementioned diseases, several pathogenic mechanisms have been postulated to support the notion that mites alone are able to inflict significant damage to the habitat that they live in. Mechanically, the mites may block the hair follicles and sebaceous ducts to induce epithelial hyperplasia and hyperkeratinization. The chitinous exoskeleton of the mites may act as a foreign body and cause granulomatous reaction. Debris or wastes generated by mites may elicit inflammatory responses via a delayed hypersensitivity reaction or an innate immune response11.
Nevertheless, one cannot rule out the pathogenic role of microbial agents that may be associated with Demodex infestation. The notion that microbes are involved in pathogenesis of mite-infested diseases has long been suggested because the skin inflammation in rosacea can be markedly improved by topical metronidazole or oral antibiotics including tetracycline, i.e. treatments that do not kill mites. Because TTO also may exert antibacterial, antifungal and anti-inflammatory actions, we cannot attribute its therapeutic benefit in treating the above eye diseases solely to its effect of killing mites. In fact, it has been postulated that the mites may act as vectors to bring in common skin bacterial flora12. Scanning electron microscopy showed bacteria on the mite skin surface. Staphylococcus albus was shown to be transported by these mites from follicle to follicle. Superantigens produced by streptococci and staphylococci that are implicated in a number of diseases may play a role in the induction of rosacea.
To reconcile the apparently disparate findings of increased numbers of D. folliculorum mites, perifollicular inflammation and response of papulopustular rosacea to selective antibiotic therapy in some patients, we have recently isolated Bacillus oleronius inside Demodex mites from one patient with papulopustular rosacea13. We discovered further that this bacterium produced antigens capable of stimulating proliferation of peripheral blood mononuclear cells in patients with rosacea in a significantly higher frequency than in control subjects13. The pooled serum from six patients with papulopustular rosacea exhibited positive immunoreactivity to two pro-inflammatory 62-kDa and 83-kDa proteins produced by this bacterium13. Lately, our collaborative and prospective study of 59 patients disclosed further a strong correlation among positive serum immunoreactivity to these two bacillus proteins, ocular Demodex infestation, facial rosacea and blepharitis (Li J, O’Reilly N, Sheha H, Katz R, Raju VK, Kavanagh K, and Tseng SCG, unpublished work). If such a strong correlation signifies a causative relationship, a new pathogenic paradigm emerges to link both Demodex infestation and microbial infection through symbiotic Bacillus oleronius in causing skin and ocular surface inflammation. Such co-morbidity between mites and Bacillus rests in the symbiosis of the latter in the former, a scenario first reported in the hindgut of a termite. This paradigm explains that the large numbers of dying mites in the follicles or glands may increase the release of the 83-kDa and 62-kDa bacterial antigen load to a critical level to trigger a cascade of host inflammatory responses. Furthermore, it also predicts why such a host immune-inflammatory response might be modified or exaggerated in certain individuals who are allergic or immune-sensitive to mites as alluded to above11. Because ocular surface inflammation is notably reduced by lid scrub with TTO, but not by conventional treatments including lid hygiene with Bbaby shampoo, topical steroids and antibiotics or systemic doxycycline in some rosacea patients that present with corneal lesions10, the mite’s role in stirring up host inflammatory response cannot be ruled out. In short, the co-morbidity based on a symbiotic relationship of B. oleronius in Demodex mites also justifies the consideration of a therapeutic strategy directed to killing the symbiotic bacterium via oral antibiotics such as tetracycline and to killing and preventing mating/reinfestation of Demodex mites, e.g. lid scrub with TTO and general hygiene at the same time. Future investigation into this co-morbidity between mites and microbes may shed new light not only on the understanding of the pathogenesis of this century-old common ailment of the skin and eye, but also other similar unresolved human diseases.
Acknowledgments
Dr Tseng has filed two patents for the use of TTO and its ingredients for treating demodicosis. Studies described in this article are supported in part by a research grant 1R43 EY019586-01 from the National Institutes of Health, National Eye Institute.
Biographies

Noreen Lacey is a graduate of National University of Ireland Maynooth, where she received her BSc in Biology (2004) and PhD (2007). Her PhD research was focused on investigating the factors involved in the induction and persistence of papulopustular rosacea, under the supervision of Dr K. Kavanagh. Results from this study demonstrated a potential role for Demodex mite-related bacterial antigens in the pathogenesis of rosacea. She is currently a postdoctoral researcher at the UCD Clinical Research Centre at the Mater Misericordiae University Hospital, Dublin. Her research interests remain focused on investigating the role of Demodex mites in the biology of human skin. Noreen.lacey@ucd.ie

Kevin Kavanagh is a Senior Lecturer in the Department of Biology at the National University of Ireland Maynooth. His research is focused on understanding the mechanisms employed by microbes to regulate the innate immune response of the host to facilitate their continued persistence. He also has interests in exploring the structural and functional similarities between the immune system of vertebrates and invertebrates. kevin.kavanagh@nuim.ie

Scheffer Tseng was a chaired professor at Bascom Palmer Eye Institute University of Miami School of Medicine until 2002, when he became Chief Scientific Officer leading a research team of 12 people of Bio-Tissue, Inc., a leading tissue engineering company. He has been specialized in ocular surface diseases and surgical reconstruction using stem cells and amniotic membrane, and received research support from NIH, National Eye Institute for over 25 years. For the last 5 years, he has been interested in understanding the pathogenic role of Demodex mites. stseng@ocularsurface.com
Contributor Information
Noreen Lacey, University of Ireland Maynooth.
Kevin Kavanagh, University of Ireland Maynooth.
Scheffer C.G. Tseng, Ocular Surface Center and Ocular Surface Research Education Foundation, Miami
References
- 1.Baima B, Sticherling M. Acta Derm. Venereol. 2002;82:3–6. doi: 10.1080/000155502753600795. [DOI] [PubMed] [Google Scholar]
- 2.Rufli T, Mumcuoglu Y. Dermatologica. 1981;162:1–11. doi: 10.1159/000250228. [DOI] [PubMed] [Google Scholar]
- 3.Pena GP, Andrade Filho JS. Rev. Inst. Med. Trop. Sao Paulo. 2000;42:171–173. doi: 10.1590/s0036-46652000000300012. [DOI] [PubMed] [Google Scholar]
- 4.Forton F, Seys B. Br. J. Dermatol. 1993;128:650–659. doi: 10.1111/j.1365-2133.1993.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 5.Norn MS. Dan. Med. Bull. 1971;18:14–17. [PubMed] [Google Scholar]
- 6.Gao Y-Y, Di Pascuale MA, Li W, et al. Invest. Ophthalmol. Visual Sci. 2005;46:3089–3094. doi: 10.1167/iovs.05-0275. [DOI] [PubMed] [Google Scholar]
- 7.Kheirkhah A, Blanco G, Casas V, Tseng SC. Cornea. 2007;26:697–700. doi: 10.1097/ICO.0b013e31805b7eaf. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y-Y, Di Pascuale MA, Li W, et al. Br. J. Ophthalmol. 2005;89:1468–1473. doi: 10.1136/bjo.2005.072363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y-Y, Di Pascuale MA, Elizondo A, Tseng SC. Cornea. 2007;26:136–143. doi: 10.1097/01.ico.0000244870.62384.79. [DOI] [PubMed] [Google Scholar]
- 10.Kheirkhah A, Casas V, Li W, et al. Am. J. Ophthalmol. 2007;143:743–749. doi: 10.1016/j.ajo.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 11.Bevins CL, Liu FT. Nat. Med. 2007;13:904–906. doi: 10.1038/nm0807-904. [DOI] [PubMed] [Google Scholar]
- 12.Wolf R, Ophir J, Avigad J, et al. Acta Derm. Venereol. 1988;68:535–537. [PubMed] [Google Scholar]
- 13.Lacey N, Delaney S, Kavanagh K, Powell FC. Br. J. Dermatol. 2007;157:474–481. doi: 10.1111/j.1365-2133.2007.08028.x. [DOI] [PubMed] [Google Scholar]