Abstract
Pulsed Reduced Dose Rate (PRDR) is a method of irradiation designed to minimize radiation-related toxicities in patients undergoing reirradiation for loco-regional reoccurrence of glioblastoma. PRDR delivers a standard 2 Gy fraction delivered on a conventional medical linear accelerator using conventional 3D conformal beam arrangements. To reduce the likelihood of normal tissue complications, radiation is delivered over ten 0.2 Gy sub-fractions with a 3 minute time interval between subfractions to give a maximal time averaged dose rate of 4 Gy/hr. However, a TomoTherapy unit has a fixed output rate of 8 Gy/min. If the dose per fraction is conventionally planned at less than 0.6 Gy/fraction, the result is a clinically unacceptable treatment plan. The method described in this paper involves a virtual grid style blocking scheme, where half of the beam angles are directionally blocked using 15 equally spaced segments surrounding the center of the image set. Ten patients treated using conventional PRDR with an average PTV volume of 353.3 ml were retrospectively re-planned using five techniques (standard 2 Gy fraction, 2 Gy in ten 0.2 Gy fractions without grid blocking, two grid patterns, and a combination plan incorporating both grids) and analyzed with conformation numbers (CN), homogeneity indexes (HI), and dose volumes to normal tissues. Plans were optimized using equal constraints and machine parameters. The grid method allowed for clinically acceptable treatment plans at 0.2 Gy with a treatment time ≤ 3min per subfraction. The average HI was slightly poorer for the combination plan versus the standard 2 Gy fraction plan (0.064 versus 0.027) and the CN was similar over all techniques (0.72 – 0.73) employed. Normal tissue dose volumes for each patient were also similar for each technique. Initial ion chamber measurements agree with predicted values for a 0.2 Gy subfraction. PRDR is deliverable on a TomoTherapy system using our virtual directional blocking method. Results can be slightly improved through the use of two grids alternated on a daily basis. The dose to normal structures for individual patients was similar for all methods.
Keywords: Reduced Dose Rate, TomoTherapy, quality assurance, reirradiation, grade 4 glioma
INTRODUCTION
Pulsed Reduced Dose Rate (PRDR) is a retreatment technique in which the daily therapy dose of 2 Gy is delivered using ten 0.2 Gy sub-fractions with a minimum 3 minute interval between subfraction deliveries to yield a maximal time averaged dose rate of 4 Gy/hr. In a recent review of 86 recurrent grade 4 glioma patients retreated with PRDR, Adkison and colleagues (1) have found that the median time from 1st radiotherapy (RT) to PRDR was 14 months. Patients receiving PRDR within 14 months of 1st RT (n=43) had a median survival of 21 weeks; while those treated ≥ 14 months post RT had a median survival of 28 weeks (n=43) (p=0.004 and HR= 1.82 with a 95% CI ranging from 1.25 to 3.10). These data compare favorably to historical data sets, as only 16% of patients were treated at 1st relapse (with 46% at 2nd relapse, 32% at 3rd/4th relapse and 4% at 4th/5th relapse). In their study PRDR was delivered using a standard linear accelerator with 3D conformal delivery techniques using a two or three beam arrangement to treat recurrent grade 4 gliomas. It was found that cumulative doses in excess of 100 Gy were well tolerated. Moreover, age at initial diagnosis, KPS at time of retreatment, and initial tumor grade were also found to be prognostic for survival following retreatment on multivariate analysis. Given these initial promising results an expanded prospective study is planned to further define toxicity and efficacy.
Due to the varying location of grade 4 glioma recurrence, the PTV can be in close proximity to several organs at risk (OAR), such as the optic chiasm, optic nerves, and brainstem. For these patients, a more conformal method of delivery may be beneficial to reduce the dose to the OAR. (2,3) While in the above mentioned trial (1) no adverse events were reported, it is a stated goal of palliative RT to cause no further harm to the patient, and hence the risk of inducing normal tissue complications through retreatment should be minimized to the largest extend possible. The reduction of risk for adverse effects is of particular importance in the retreatment of tumors due to local failure.
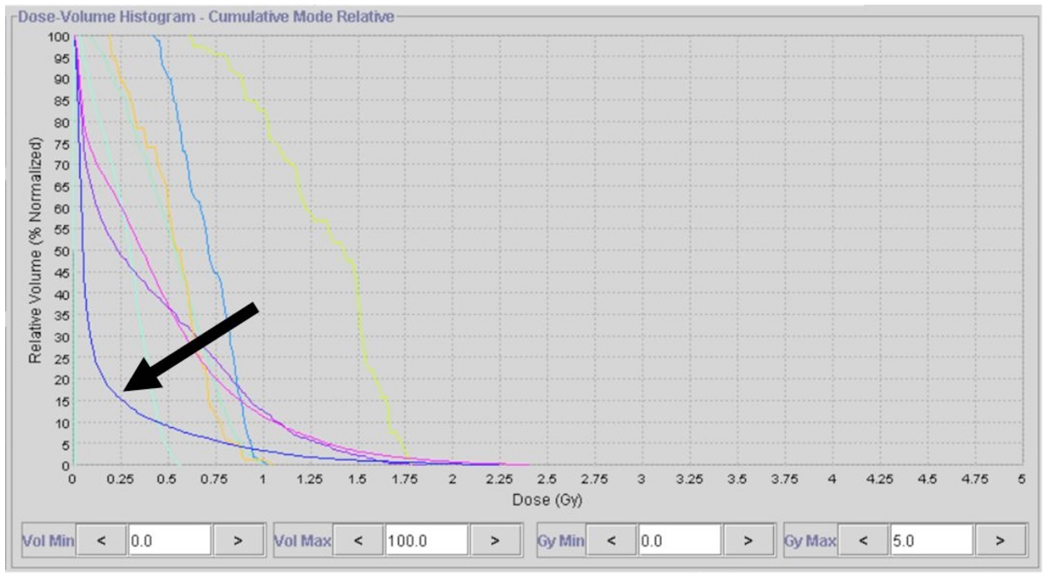
Since TomoTherapy uses a fixed dose rate output and moreover modulates the beam using a binary MLC reduced dose rate radiotherapy is difficult to produce. Using the Tomotherapy planning station, a user can reduce the dose per fraction to less than 0.6 Gy/fraction in a standard grade 4 glioma treatment plan, but as a consequence of this, the DHV for the PTV becomes clinically unacceptable. (Figure 1)
Figure 1.
DVH of a conventionally planned 2-fraction plan, delivering 2 Gy per fraction, after conversion to 0.2 Gy fractions. Note that the PTV DVH (Arrow) is lower than most of the critical structures. This is due to the short leaf open times (LOTs) for individual projections, which are forced to fully closed when below a threshold limit.
The dramatic decrease in PTV dose homogeneity when the final dose is computed for small doses is due to the extremely short leaf open times (LOTs) for individual beam segments. Many of these LOTs have a value shorter than the minimum that is mechanically achievable (~20 ms) and so are forced fully closed when below the threshold limit. (4) Due to the desired low dose per projection in a typical PRDR plan, a large percentage of these beam segments are forced to be completely closed because of this effect, and the target and normal structure dose volume histograms can be adversely affected.
The purpose of this study was to develop a technique that would allow the delivery of an acceptable grade 4 glioma PRDR treatment plan that would limit the dose to 0.2 Gy per fraction using standard Tomotherapy treatment planning tools, however the technique described here could also be employed for other tumor types in the body. This was achieved through the use of a directionally blocked virtual grid designed to block half of the beam projections. The blocking scheme allows for the dose per projection to be zero over the directionally blocked segments while achieving a dose per projection above the minimum threshold in the unblocked areas, thereby allowing for beam modulation at low doses.
METHODS
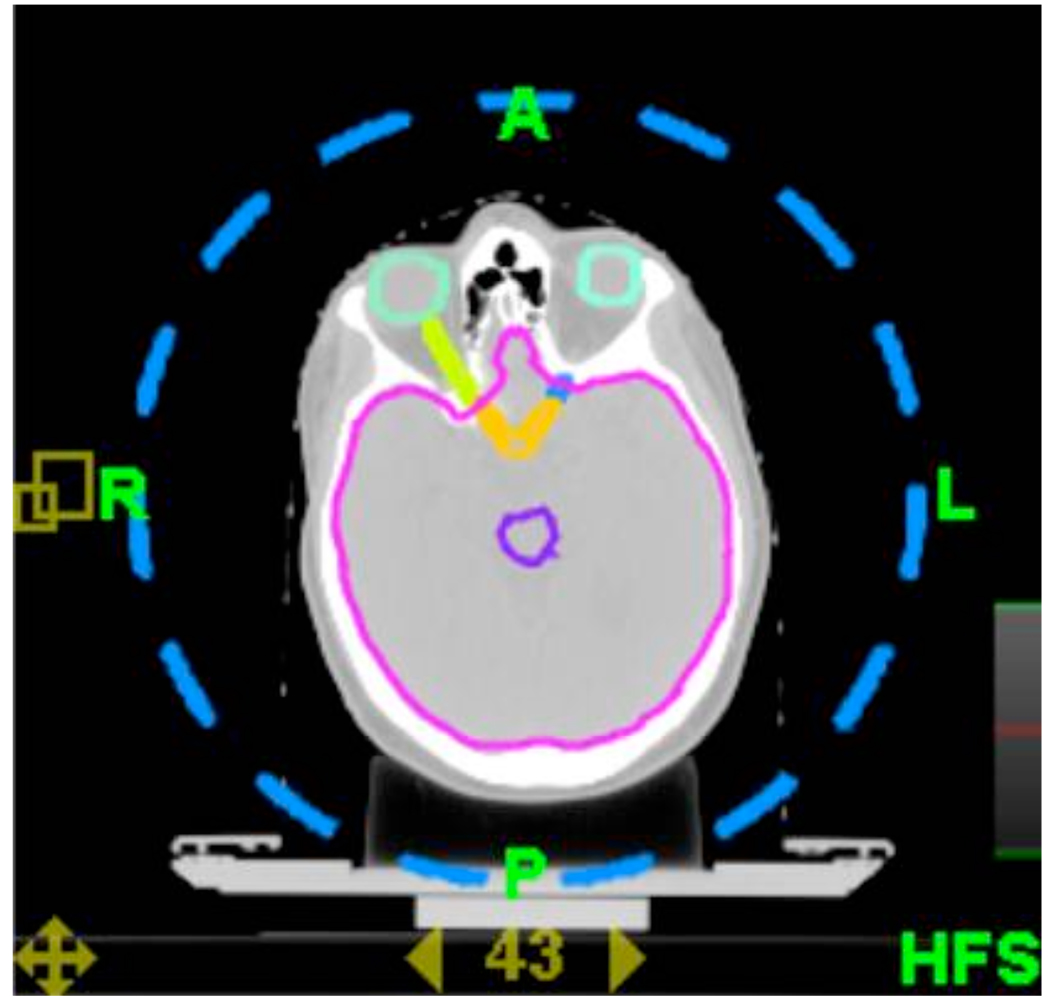
A virtual cylindrical grid was contoured using a 13.5 cm radius from the central point of the CT image set. (Figure 2) This was done to eliminate any contour overlap conflict between the patient anatomy and the virtual grid. The arc length of the grid contour was equal to the arc length left open in all cases. Due to the fact that the image set center is aligned with the TomoTherapy isocenter, the grid is aligned to the treatment beam. Initially, grid styles of 15, 18, and 24 spaces were tested on a single patient to determine their relative benefits. The 15-space technique was marginally better in dose homogeneity than the 18-space technique, with the 24-space technique demonstrating the poorest DVH. The 15-space technique was then applied to all patients. Two separate grids rotated with respect to each other such the directionally blocked areas in either grid were open in the other were created as standards and realigned to individual patient plans using Pinnacle® v8.1t (Philips Medical Systems, Fitchburg, WI). This was then exported to the TomoTherapy planning system v2.1 (Tomotherapy, Middleton, WI).
Figure 2.
Example of the virtual grid applied to the patient. The grid extends the length of the head in the superior-inferior direction and is directionally blocked in the treatment planning system.
The center of the grid was aligned to the center of the image set to allow for equal blocking across all beam projection angles. While this grid could be centered around the PTV, there is the possibility of additional artifacts if the grid is not aligned with the physical machine isocenter. To accurately align the grid contours to the patient image, a cylindrical structure was created at the image set center and used to align the reference contour dataset to the patient image.
In order to allow the clinical testing of this technique on a RANDO head phantom (Phantom Laboratory (Salem, NY) the following planning parameters were employed for all techniques: 0.43 pitch, 2.5cm field size, maximum modulation factor of 3, a total prescription dose of 4 Gy to 98% of tumor volume (2 Gy per fraction for 2 fractions), and OAR within the field range (eyes, brainstem, optic nerves and chiasm) were set to a 2 Gy max dose with a 50% 1 Gy dose limit. The total prescription dose of 4 Gy was chosen to ensure that all plans could be delivered at least twice, (i.e, 2 Gy in 2 fractions, 0.2 Gy in 20 fractions) and is not representative of the clinically used retreatment dose schedules which are either 50Gy (25 fractions of 2 Gy) or 54 Gy (27 fractions of 2 Gy). Additionally, a directional blocking constraint was placed on the grid, which limited the number of projections that could be delivered. These objectives were not necessarily met in all plans. All plans were converted to full dose after 100 iterations.
Ten (10) patients with various grade 4 glioma locations and sizes previously retreated using Linac-based PRDR (PTV mean: 353.3 ml, Range 225.5–571.3 ml) were planned using five techniques: a standard 2 Gy fraction, a standard 2 Gy fraction delivered in 0.2 Gy sub-fractions, complementary plans using the two complementary 15-space grids, and a combination plan created by adding the dose datasets from both grid styles together using the plan summation tool in TomoTherapy research planning software. PTV volumes were previously defined using CT, T1-weighted 3D-SPGR and T2-FLAIR MRI images.
Metrics for quantifying the plan quality included the conformation number (CN), as defined by van’t Riet et al (5), and the homogeneity index (HI), as defined in ICRU Report 62 (6) as well as the dose to the whole brain volume.
| (1) |
| (2) |
Where Vpres is the total volume receiving a dose greater than or equal to the prescription dose, VT,pres is the volume within the target receiving a dose equal to or greater than the prescription dose, VT is the PTV volume, and Dx is the dose delivered to X % of the target volume.
Radiochromic film was used to verify the dose distribution in a RANDO head phantom (Phantom Laboratory (Salem, NY). Contours were imported to the RANDO head phantom from a separate patient and aligned based on bony anatomy to the phantom. A Tomotherapy plan was then created based on the head phantom CT image and virtual grid. The film was trimmed to fit the shape of the head in the transverse plane and exposed to ten 0.2 Gy fractions. The phantom was immobilized via a thermoplastic head frame to improve reproducibility. 24 hours post irradiation, film was scanned and analyzed. Film was compared to dose planes from the predicted treatment plan via ImageJ and MATLAB (Mathworks, Natick, MA).
In addition to the radiochromic verification, two patients were randomly selected and their grid-based plan was verified using a delivery quality assurance (DQA) setup with a standard cylindrical “cheese” phantom. After an initial MVCT alignment to verify phantom location, EDR film placed in the coronal plane was exposed to ten 0.2 Gy fractions. Film response to dose was calibrated from 0 to 4 Gy using a standard Linear accelerator. Additionally, a calibrated ion chamber measurement was used to measure a point dose within the PTV. As the optical density vs. dose curve for EDR film is more sensitive at the 2 Gy level than at the 0.2 Gy level, a full 2 Gy treatment (10 subfractions of 0.2 Gy) was delivered and scaled down to 0.2 Gy using an average of each fraction’s ion chamber measurement. 24 hours post irradiation, film was developed and analyzed. The scaled film was then compared to the dose plane generated for an individual 0.2 Gy dose fraction in the DQA software.
RESULTS
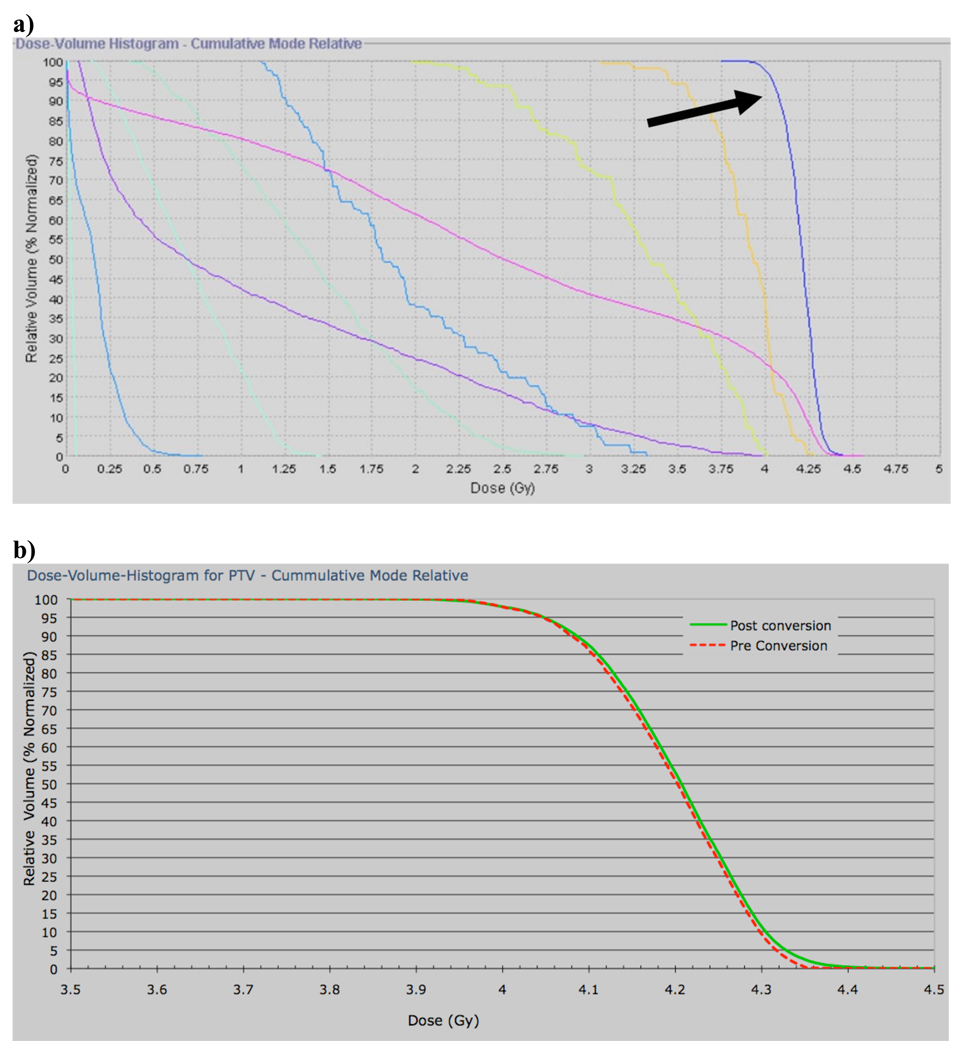
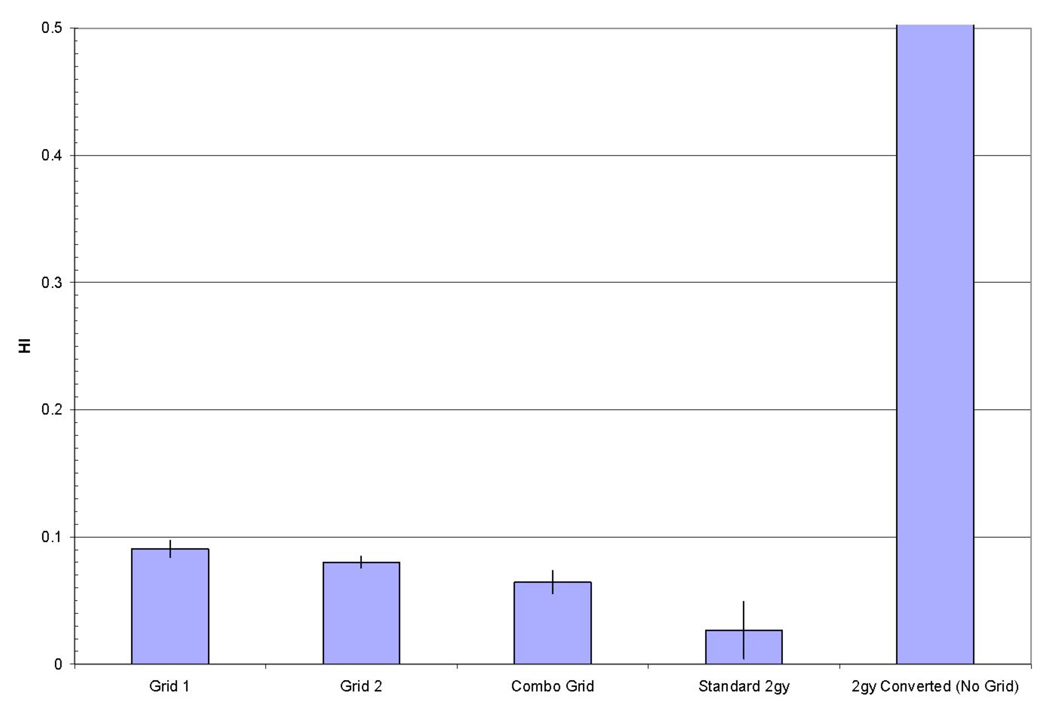
Figure 3a demonstrates a typical DVH for all structures with the application of a grid, while Figure 3b shows a close-up view of the pre- and post conversion DHV for the PTV. As one can see from Figure 3b there are only slight differences between these dose volume histograms with the DVH for the converted plan having a higher maximum dose and a more pronounced high dose tail, resulting in decreased dose homogeneity within the PTV. Figure 4 shows the HI for all methods used. As one can see from Figure 4 the homogeneity index for the grid 1 plan is 3.34 times that of the standard 2Gy plan and that for the grid 2 plan is 2.96 times that of the standard 2 Gy plan, while the HI for the complementary grid plan is the lowest with being 2.37 times as large as the HI for the standard 2 Gy plan. The HI for the standard 2 Gy plan was 0.027. However, for all grid plans the decrease HI is still within the clinically acceptable range since it is less than 0.1, with the complementary grid plan giving the lowest HI of 0.064, and hence yielding the steepest PTV DHV for the three grid based plans. Moreover, note that PTV DVH homogeneity for the standard 2 Gy fraction plan in which no grid was employed and delivered in 0.2 Gy subfractions was clearly unacceptable for all patients (cf. Figure 4).
Figure 3.
a) DVH of a 2-fraction grid-based plan after final conversion to 0.2 Gy fractions. The PTV DVH (arrow) achieves the prescribed dose. b) PTV DVH of a representative 2-fraction grid-based plan pre- (dashed red) and post- (solid green) final conversion to 0.2 Gy fractions. Computation of the final dose resulted in a similar DVH to the initially predicted values.
Figure 4.
Average homogeneity index of all patients. All grid-based techniques demonstrate a uniformity of coverage similar to a standard 2 Gy plan. The 0.2 Gy fractionated plan without any grid applied is much higher than the other techniques and had a value of 9±3.04.
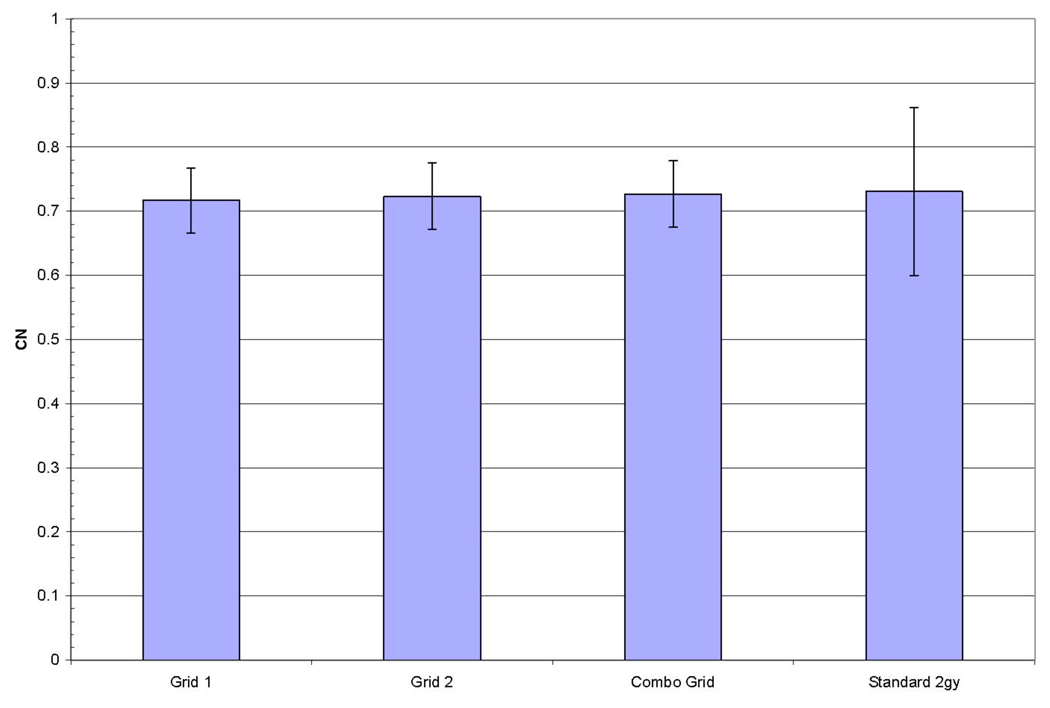
Figure 5 shows the conformation number (CN) for all methods used. CN was nearly equivalent for the three grid based techniques when compared to the standard treatment plan delivered in a single 2 Gy fraction. Note, that for the standard treatment plan being broken up into ten 0.2 Gy subfractions no acceptable CN could be achieved for the PTV for any patient.
Figure 5.
Average conformality number for all patients. All grid techniques were equivalent to a standard plan delivered in a single 2 Gy fraction.
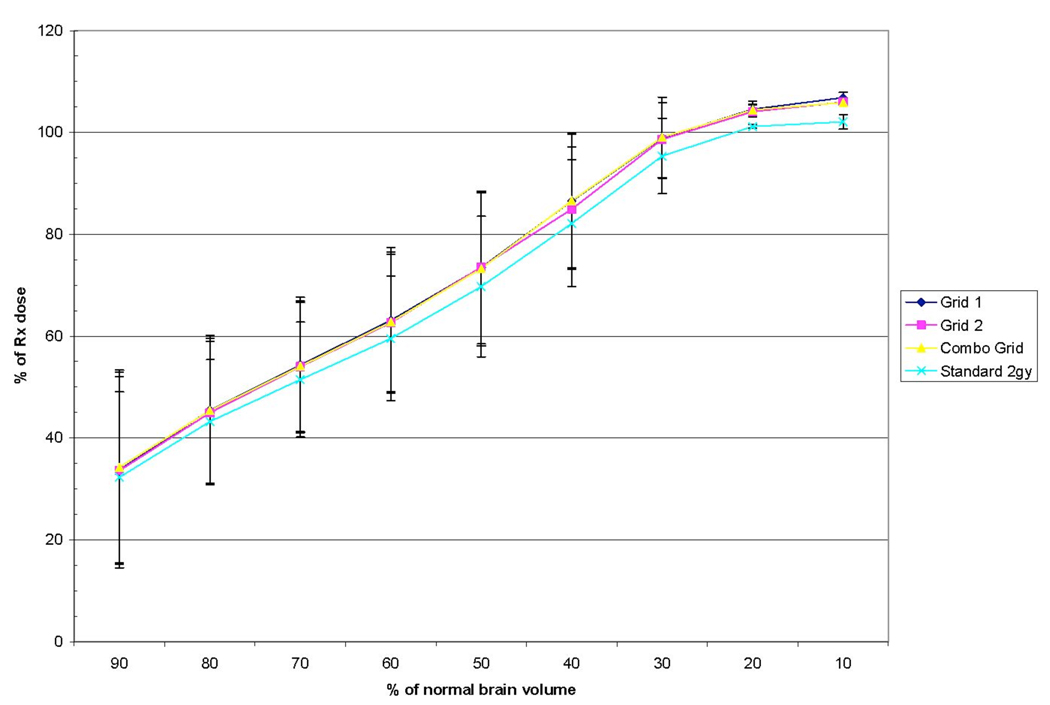
Normal brain irradiation volumes are shown in Figure 6. The three grid based techniques had almost identical volumes for all dose levels. The standard 2 Gy technique appeared to be slightly lower on average than the grid based techniques (i.e. the two complementary grids and the combination of the two), but this difference was minimal. Other normal tissues were difficult to compare between plans due to widely varying locations of tumors and frequent overlapping of tumor volumes and typical avoidance structures on some patients. Nevertheless, no significant deviation between plans for individual patients was seen.
Figure 6.
Average percentage of prescribed tumor dose delivered to normal brain tissue. The standard 2 Gy plan delivered a marginally lower dose on average than grid based techniques.
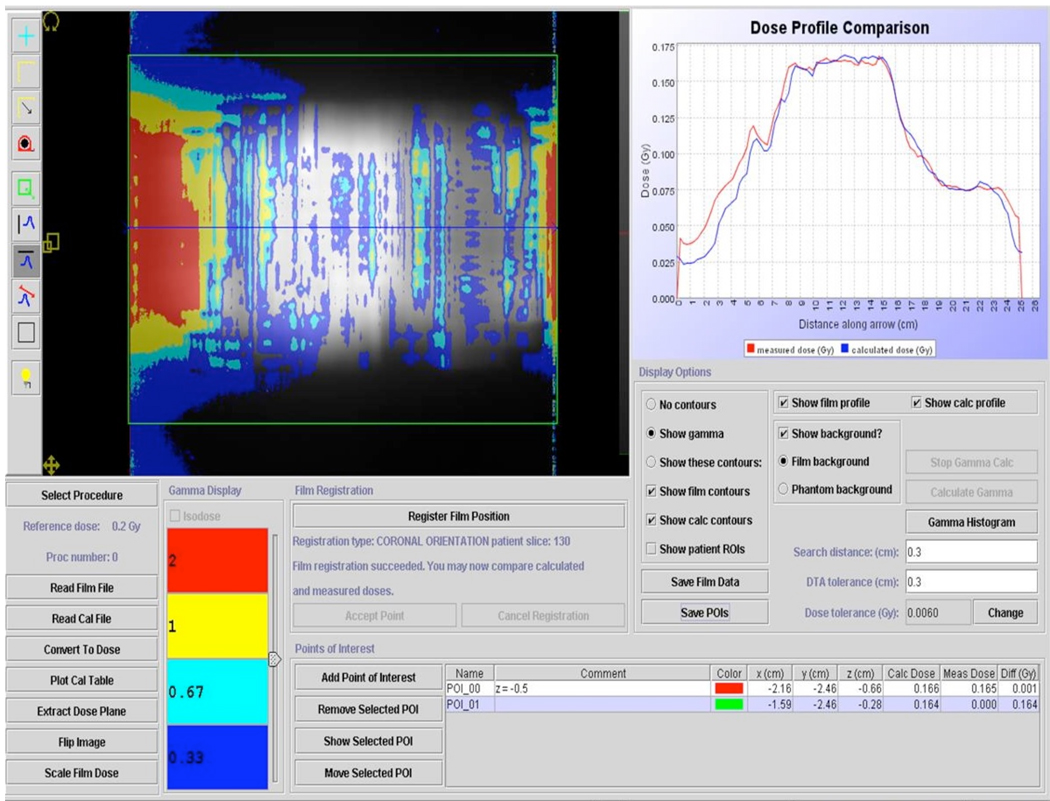
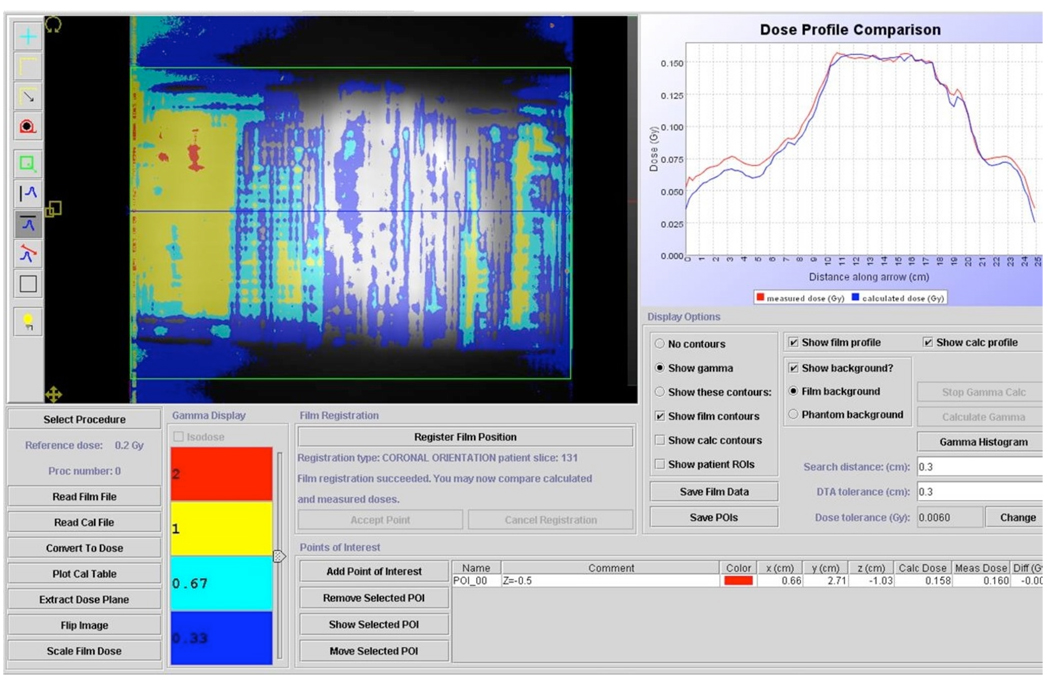
The results of the DQA measurements for the two randomly selected patients are shown in Figures 7 and 8. The plans for both patients had excellent agreement in the high dose target region, however there was a slightly higher than expected dose in the gradient region on the right side of the coronal plane for both patients. Averaged Ion Chamber readings (based on 10 measurements per patient) agreed to within 0.6 % and 1.2 % with the predicted values for patient 1 and 2, respectively.
Figure 7.
Patient-specific DQA for one of the patient’s grid based plans. EDR film was treated with ten fractions of 0.2 Gy and scaled by the average of ion chamber readings for all fractions to allow for absolute dose comparison. Agreement in the high dose region was very good, with some areas in the dose falloff region receiving higher than expected dose.
Figure 8.
Patient-specific DQA for a second patient’s grid based plan. Agreement in the high dose region was very good, with some areas in the dose falloff region receiving higher than expected dose.
Radiochromic film used in a RANDO phantom demonstrated a near uniform relative dose across the entire transverse plane on both films after a grid based 2 Gy (10 subfractions of 0.2 Gy) treatment (cf. Figure 9). Some areas in the gradient region surrounding the PTV were 0.2 Gy above the predicted dose when an absolute dose conversion was applied. Heterogeneity within the PTV was accurately predicted by the treatment planning system. The higher than expected dose in some regions may be an effect of the planning/delivery technique and should be considered when prescribing to patients for recurrent grade 4 glioma.
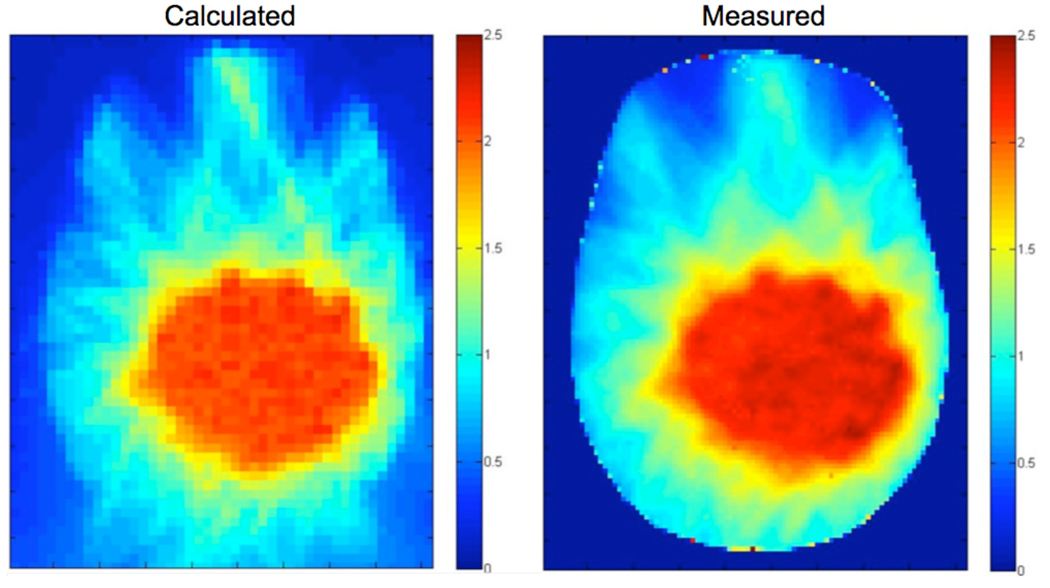
Figure 9.
(Left) Planar dose map generated by Tomotherapy TPS in RANDO phantom and (Right) measured radiochromic film. (Units are Gy and based on a 2 Gy total delivery treated using 10 subfractions of 0.2 Gy each). Agreement between planned and delivered dose is good, with dose heterogeneities within the tumor volume accurately calculated.
The total planning time for a single plan for each patient was on the order of one hour from grid alignment in Pinnacle3 to TomoTherapy final dose. Delivery time for all patients’ 0.2 Gy subfractions was approximately 180 seconds, which, factoring in reloading of the plan and patient repositioning, means that a daily 2 Gy fraction would take approximately 45 minutes to deliver. A pitch of 0.86 would reduce the sub-fraction time to 120 seconds, but target coverage may suffer.
DISCUSSION
Our results demonstrate that doses on the order of 0.2 Gy per subfraction are deliverable on a TomoTherapy unit using a virtual grid and standard treatment planning software. By increasing the LOT for some of the projections, we circumvent the physical constraints caused by leaf travel time and a deliverable 0.2 Gy subfraction can be treated accurately.
It is important to note that while most of the projections are in a linear dose response region of the LOT, there will be many projections in the periphery that may be in a non-linear response region. The LOT at the threshold may not be modeled correctly, which could be the cause for the mismatch of delivered and predicted dose in the low dose regions. Additionally, while there were some measured differences in the low dose region of the TomoTherapy “cheese” phantom, the low dose regions had excellent agreement in the RANDO phantom, which is a more accurate surrogate for the human head. Due to these concerns, patient specific QA should be performed to verify accurate delivery for a given TomoTherapy-based PRDR plan.
Under the TomoTherapy-based PRDR technique proposed in this study, there is no three-minute interval between subfractions. The lack of a time interval between subfractions differs from a Linac-based PRDR, as does the overall time-averaged dose rate (4 Gy/hr vs 2.67 Gy/hr). The decreased time-averaged dose rate for Tomotherapy is caused by the time it takes to load the next subfraction and initiating its treatment. However, the time-averaged dose rate for Tomotherapy PRDR is below our maximally clinically used time-averaged dose rate that we have employed in Linac based PRDR. This reduced time averaged dose rate of 2.76 Gy/hr is unlikely to lead to different radiobiological effects than those expected from the maximum time averaged dose rate of 4 Gy/hr in vivo for both tumors and normal tissues, since both time averaged dose rates fall well within the intermediate dose rate range. However, this intermediate dose rate region is not well explored for grade 4 gliomas, and hence the true effect of this difference is unknown at this time.
CONCLUSIONS
The PRDR method was designed to reduce normal tissue complications in retreatment of recurrent grade 4 glioma. While this has shown promise, there is the possibility of Tomotherapy delivering a more conformal treatment than the 3D conformal 2 or 3 field beam arrangements that are currently employed for PRDR. The fixed dose rate of TomoTherapy and MLC speed currently limits its ability to deliver low doses accurately. If a virtual grid is applied, this limitation can be overcome and low dose fraction delivery is possible with standard planning tools.
Acknowledgement
We would like to acknowledge the technical assistance of Dr. Leah Schubert and Dr. David Westerly throughout this project. This work was support in part by a grant from the National Cancer Institute T32-CA09206.
References
- 1.Adkison JB, Tomé WA, Songwon S, Richards GM, Robins HI, Rasmussen K, Welsh JS, Mahler PA, Howard SP. Reirradiation of Large Volume Recurrent Glioma with Pulsed Reduced Dose-Rate Radiation Therapy. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2009.11.058. (in press) [DOI] [PubMed] [Google Scholar]
- 2.Chan MF, Schupak K, Burman C, Chui CS, Ling CC. Comparison of intensity-modulated radiotherapy with three-dimensional conformal radiation therapy planning for glioblastoma multiforme. Medical Dosimetry. 2003;28:261–265. doi: 10.1016/j.meddos.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Cao D, Holmes TW, Afghan MKN, Shepard DM. Comparison of Plan Quality Provided by Intensity-Modulated Arc Therapy and Helical Tomotherapy. International Journal of Radiation Oncology, Biology, Physics. 2007;69:240–250. doi: 10.1016/j.ijrobp.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 4.Fenwick JD, Tomé WA, Jaradat HA, Hui SK, James JA, Balog JP, DeSouza CN, Lucas DB, Olivera GH, Mackie TR. Quality assurance of a helical tomotherapy machine. Phys Med Biol. 2004;49:2933–2953. doi: 10.1088/0031-9155/49/13/012. [DOI] [PubMed] [Google Scholar]
- 5.van't Riet A, Mak AC, Moerland MA, Elders LH, van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37:731–736. doi: 10.1016/s0360-3016(96)00601-3. [DOI] [PubMed] [Google Scholar]
- 6.ICRU Report 62: Prescribing, Recording and Reporting Photon Beam Therapy (Supplement to ICRU Report 50) Bethesda, MD: International Commission on Radiation Units and Measurements; 1999:1–52.