Abstract
Olfactory responses of Drosophila undergo pronounced changes after eclosion. The flies develop attraction to odors to which they are exposed and aversion to other odors. Behavioral adaptation is correlated with changes in the firing pattern of olfactory receptor neurons (ORNs). In this article, we present an information-theoretic analysis of the firing pattern of ORNs. Flies reared in a synthetic odorless medium were transferred after eclosion to three different media: (i) a synthetic medium relatively devoid of odor cues, (ii) synthetic medium infused with a single odorant, and (iii) complex cornmeal medium rich in odors. Recordings were made from an identified sensillum (type II), and the Jensen–Shannon divergence (DJS) was used to assess quantitatively the differences between ensemble spike responses to different odors. Analysis shows that prolonged exposure to ethyl acetate and several related esters increases sensitivity to these esters but does not improve the ability of the fly to distinguish between them. Flies exposed to cornmeal display varied sensitivity to these odorants and at the same time develop greater capacity to distinguish between odors. Deprivation of odor experience on an odorless synthetic medium leads to a loss of both sensitivity and acuity. Rich olfactory experience thus helps to shape the ORNs response and enhances its discriminative power. The experiments presented here demonstrate an experience-dependent adaptation at the level of the receptor neuron.
Keywords: imaginal conditioning, sensory adaptation, odor imprinting, Jensen–Shannon divergence, chemo receptor tuning
Olfaction in the fruit fly, Drosophila melanogaster, is crucial for a variety of behaviors, including associative learning (1, 2), courtship (3), foraging (4), and flight (5, 6). Odorants are detected by approximately 1,300 olfactory receptor neurons (ORNs), which are housed in sensilla on the third antennal segment and individually express one of approximately 50 functional odor receptors in adults (7–9). All ORNs expressing the same olfactory receptor project to one of approximately 50 glomeruli in the antennal lobe, where they synapse with a set of projection neurons (PNs) (10, 11). The activity of ORNs, either excitation or inhibition, provides behaviorally relevant information about odorants such as their identity, concentration, and source. The information transduced by an ORN is processed in the antennal lobe (12, 13) and sent via PNs to the mushroom bodies, which are believed to be centers for olfactory learning and memory (14, 15).
Experience-dependent modifiability (i.e., plasticity) of olfactory representation at the level of the central nervous system is well known (2, 16, 17). Relatively little attention has been paid to long-term changes in sensory neuron activity resulting from odor experiences. It was previously shown that exposure of newly born imago to a particular set of odorants alters their responses to these odors (18, 19). The flies develop an attraction to the chemicals to which they are exposed and an aversion to other chemicals. We have called this phenomenon imaginal conditioning. The term refers to effects of odor experiences after eclosion and involves experience-dependent plasticity at the level of ORN, an uncommon instance of odor imprinting on receptor neurons (19). Here we present an analysis, based on information theory, of the changes in single-unit response patterns to some esters as a result of conditioning.
The effects of conditioning were examined by recording the spiking activity of ORNs. We employed a synthetic medium (19) devoid of complex odor cues present in normal cornmeal medium (20) that could be infused with specific odorants. The larvae were raised on synthetic medium, and the emerging flies were transferred to bottles containing the conditioning medium with specified odors. The effect of the odor environment was examined by recording the pattern of spiking activity from a well characterized sensillum, type II (ab2). This large basiconic sensillum contains two neurons, easily distinguishable by their spike patterns, that express the olfactory receptors Or59b and Or85a, respectively (9, 19, 21). In search of a robust quantitative measure sensitive to the differences in ORN firing patterns resulting from different odor experiences, we used the Jensen–Shannon divergence (DJS), a metric widely used in information theory (22, 23) as a quantitative measure of dissimilarity between pairs of spike patterns. The DJS contrasts the differences in the probability of spike occurrence along a response time course, rather than the average firing rate or the total spike count. We show that odor experience changes sensitivity and acuity in distinguishing between different odors.
Results
Imaginal Conditioning Modulates the Sensitivity of ORNs.
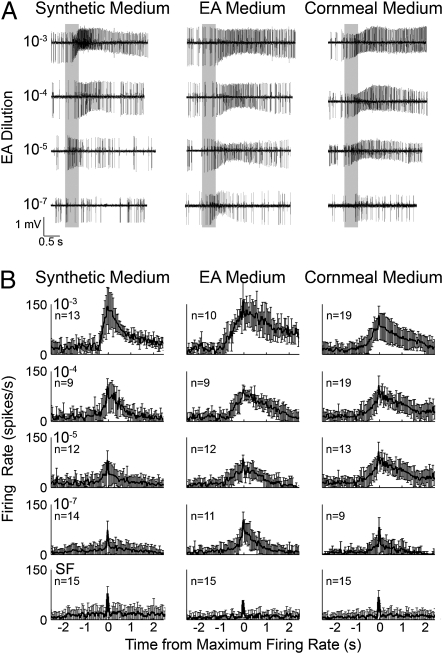
Sensilla basiconica in Drosophila mediate the detection of a variety of general odors (21) and have been classified into seven types. Type I houses four different neurons (i.e., ORNs) and type II contains two neurons (IIa and IIb). This study analyses the effect of imaginal conditioning on firing dynamics of IIa expressing the functional receptor Or59b, responding to a number of odorants. Fig. 1A shows representative traces of type IIa responses to different dilutions of ethyl acetate (EA) in flies conditioned on three different media. Newly eclosed adults raised on odorless synthetic medium were reared on synthetic medium, EA-supplemented synthetic medium, or cornmeal medium. To compare differences in rise and decay kinetics in the ORN firing patterns, we grouped the traces into 50-ms bins and aligned them by the bin with the highest spike number and then compared them with spontaneous firing (SF) treated in the same manner (Fig. 1B). The overall temporal pattern of firing under the three conditions appeared to be similar. A rapid increase followed by a gradual decline in firing. However, closer examination of the response characteristics reveals marked differences under different conditions based on DJS.
Fig. 1.
Responses to EA in type II (ab2) sensilla. (A) Representative traces of extracellular single-unit recordings of type II responses to a 500-ms presentation of EA (gray bar) at dilutions of 10−3, 10−4, 10−5, and 10−7 in flies raised on synthetic, synthetic + 10−4 EA, or cornmeal medium. (B) Mean and SD firing frequencies of type IIa ORNs are shown in response to EA (from 10−3 to 10−7 dilutions), or of SF in 50-ms bins aligned by the bin with the maximum firing rate (n = 9–19 traces).
To better describe the changes in response patterns arising from imaginal odor conditioning, we compared several methods applicable to the analysis of response patterns. In addition to previously used measures such as the maximum spiking rate (24) and total spike count (9, 19) within the time course of the response (Fig. 2 A and B), we also explored the “total information gain” as measured by DJS, which has been used in time series analysis of discrete events (25) and appeared to be suitable for quantifying differences between spike trains (Fig. 2). This statistical treatment is a symmetrical version of the Kullback–Leibler divergence (26), and is robust in quantifying distinction between two series of discrete events based on differences in probability distribution of event occurrence within each sequential time interval. We first compared odor responses to SF within a fixed time period to assess the robustness of the responses to an odor. Later, by using the same procedure, we compared the responses to different dilutions of an odor as well as responses to different odors.
Fig. 2.
Quantitative descriptions of type IIA ORN sensitivity to EA after imaginal odor conditioning. Three ways to quantify conditioning effects on odor responses to EA dilutions are by the maximum spike rate (A), total spike count over the response (B), and total information gain based on ΣDJS (C), between the EA response ensembles against SF ensembles (in a 5-s window centered around the time of maximum spiking). The same data sets shown in Fig 1B are compared using these methods. The cumulative information gain was calculated by the bin-by-bin sum of DJS between the EA responses against SF. The values at the end of this 5-s window yield the total information gain for the respective pairs of spike train samples. Error bars indicate SD of observations. (D) Cumulative plots of both the spike count and the information gain along the 5-s window can be used to describe the temporal characteristics of ORN response ensembles. Plotted are examples (i) in which information gain was marginally more effective than spike counts (cornmeal medium conditioning effect on responses to EA at 10−3) and (ii) in a case in which the cumulative information gain produced a drastically improved distinction (EA conditioning effect on responses to EA at 10−5 dilution). (E) Cumulative plots of ΣDJS of response ensembles to EA dilutions (against SF) provide a measure of the ability to differentiate between an odor response and SF over the course of the 5-s window.
After collecting a number of spike trains in response to a particular odor, we determined the spike occurrence within each sequential time bin for the responses to obtain the probability distribution of the spike counts (i = 0, 1, 2…) within the bin (50 ms). This was repeated for the responses to each odor. In a pair-wise comparison of odor responses, if two spike count distributions in a particular time bin overlap completely, the DJS is 0 (Eq. 1), signifying that the spike count for this bin does not contribute to the differentiation between the odor stimuli. If, on the contrary, the distributions do not overlap at all, the DJS between them is one bit, implying complete certainty of the odor identity corresponding to each spike train.
The DJS for each time bin along the odor response time course was accumulated as the cumulative information gain (ΣDJS), and total information gain at the end of the 5-s response was compared with the corresponding total spike count (Fig. 2C vs. Fig. 2B). As used previously for other information theoretic measures (26), the robustness of the total information gain was assessed by using nonparametric bootstrap resampling (27, 28). We found in most cases that the differences between pairs of odor response ensembles over time were more clearly illustrated in their ΣDJS measures, as resampling of the response ensembles produced relatively small variation in the ΣDJS in comparison with the SD of the other methods relying on simple spike counts. Although the value of ΣDJS is dependent on the bin size and number of bins in the response, the characteristic shape of the ΣDJS values between two ensemble responses are qualitatively similar over a practical range around the adopted 50-ms bin size (12.5–200 ms; Fig. S1).
The cumulative spike count and the cumulative information gain measured by the ΣDJS along the response time course both quantify dynamic features of the odor responses (Fig. 2D); however, there are differences between these measures—particularly when processing the responses to highly dilute odors (compare ii vs. i in Fig. 2D). The cumulative spike count simply reflects the amount of spiking, whereas the DJS determines the distinction in spiking distribution in a small bin. Thus, the ΣDJS provides a measure of the magnitude and timing of differences between two response ensembles in the context of information theory.
Flies raised in any of the three conditions were sensitive to EA at high concentration, although EA medium and cornmeal medium–raised flies showed greater divergence from SF (Fig. 2E). As the EA odor stimulus became more dilute, the sensitivity of flies raised on the odorless synthetic medium dropped first—barely diverging from SF at 10−5 dilution. Sensory adaptation after acute exposure to a stimulus would normally be expected to reduce the magnitude of subsequent responses. Surprisingly, long-term exposure in EA media led to the greatest sensitivity to EA-robust responses to EA at 10−7, not seen in other two groups. Conversely, depriving odor experience with synthetic medium led to the least sensitivity to EA stimuli, barely responding at 10−5. Cornmeal medium–raised flies showed a mean level of sensitivity between the other two conditions (Fig. 2E).
Conditioning Does Not Change the SF Pattern of IIa ORNs.
Histograms of SF frequency of flies from the three conditioning schemes fit Poisson distributions (Fig. S2), and showed no significant differences among them (χ2 test, P > 0.05). This suggests that the mechanism altering the coding of IIa as a result of conditioning has little effect on the “system noise,” the basal level of transduction and excitability of the receptor cell that generates SF.
ORN Concentration Discrimination Is Modified by Imaginal Conditioning.
Another important feature of olfactory perception is the ability to detect minute differences in the concentration of an odor. Greater acuity in detecting differences in odor dilutions indicates a greater capacity of the ORN to code precisely the concentration of an odor. To determine the dynamic range of the dilution-response relationships (shown in Fig. 2C for the three conditioning protocols), we examined the ratio of the information gain (against SF) of the most divergent dilution (10−3) to the average information gain for all dilutions. Both synthetic and EA medium raised flies had similarly large ratios (1.91 and 2.04, respectively) compared with cornmeal medium–raised flies (1.47), suggesting that the former groups could code differences in EA concentration over a greater range.
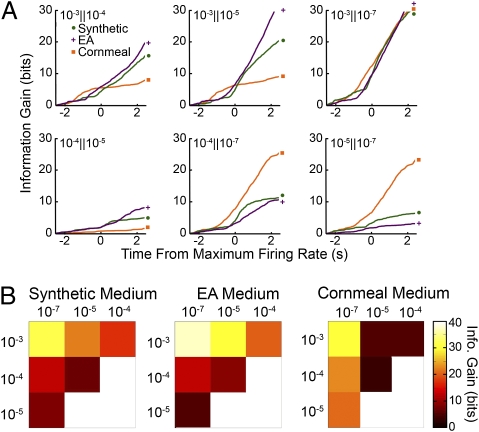
To confirm the conclusions drawn from the dilution-response relationships (Fig. 2C), we directly assessed the pair-wise information gain (ΣDJS) between different dilutions of EA (Fig. 3A). Importantly, direct pair-wise assessment between EA dilutions revealed similar cumulative information gain in both magnitude and pattern when comparing synthetic and EA medium–raised flies, even though the information gain against SF was much greater for EA medium–raised flies (Fig. 2C). Another feature illustrated in Fig. 3A is that in cornmeal medium–raised flies, substantial distinction of 10−3 dilution from other dilutions occurs before the peak firing rate (t = 0 s), implying that the initial phase of the odor response contributes substantially to their dilution distinction, despite the more limited total information gain for 10−3 against 10−4 or 10−5. The total information gain between EA dilutions (endpoints of the cumulative information gain in Fig. 3A) are shown in Fig. 3B. It is evident that the complex odor experience of cornmeal medium rearing led to a characteristically different dilution distinction profile, whereas the other two conditioning schemes led to similar profiles. Also in cornmeal medium-raised flies, there is diminished dilution acuity between higher concentrations of EA, accompanied with higher dilution acuity between lower concentrations, in contrast to the results on synthetic medium and single odor exposure on EA medium.
Fig. 3.
Imaginal conditioning effects on concentration discrimination based on type IIA ORN responses. (A) Cumulative plots of information gain between responses to different dilutions of EA (10−3 to 10−7) demonstrate that the ability to differentiate between the dilutions at different time points along the response is modified by imaginal odor conditioning. (B) Matrices of the total information gain show the pair-wise ability to differentiate among EA dilutions for the three conditioning protocols. The color intensity represents the total information gain between the respective EA dilutions. Table S1 shows the values used.
Cross Conditioning: Modification of the Sensitivity to Other Esters.
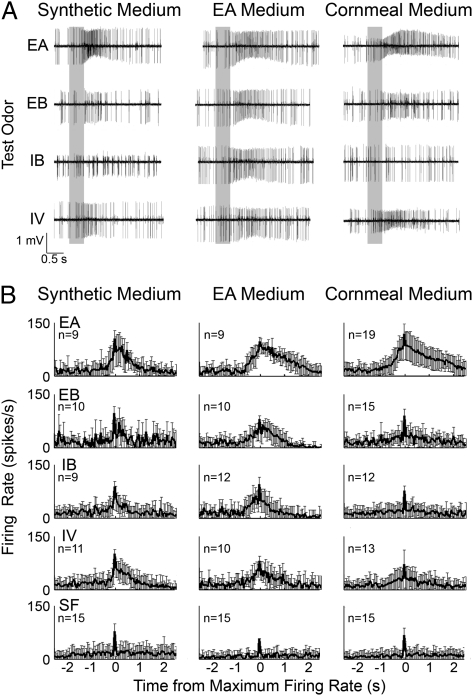
We also characterized the responses to three other esters—ethyl butyrate (EB), isoamyl butyrate (IB), and isoamyl isovalerate (IV)—that can excite IIa ORNs in terms of the ΣDJS measure. Fig. 4 shows a comparison of responses to different odors at the same dilution (10−4) processed in the same manner as our analysis of EA dilution responses. In Fig. 5, the information gain, based on the ΣDJS, is presented in the same manner as our earlier analysis of EA dilution responses. Conditioning with EA increased the sensitivity to all other esters studied compared with synthetic or cornmeal medium (ΣDJS >10 bits over 5 s). However, EA conditioning did not increase the ability to distinguish between other esters.
Fig. 4.
Cross-conditioning effects on type IIA responses to other esters. (A) Representative traces of four acetate esters—EA, EB, IB, and IV—at 10−4 dilution in flies raised on synthetic, EA, or cornmeal media (gray bar indicates 500-ms odor presentation). (B) The firing frequency ensemble of the responses to odors, represented in the same manner as Fig 1 (mean ± SD; n = 9–19 traces).
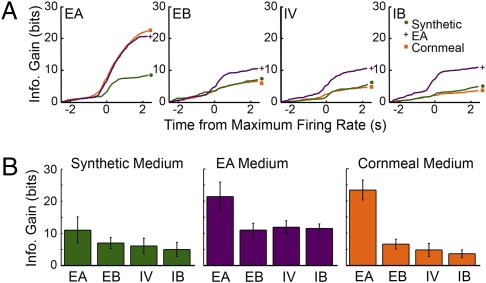
Fig. 5.
Information gain measures of the cross conditioning effect on type IIA ORNs ester response sensitivity. (A) Cumulative plots of the information gain of odor responses against SF, based on the data shown in Fig. 4, and plotted in the same manner as in Fig. 2E and Fig. 3A, show that single odor (EA) conditioning increased the sensitivity not only to EA, but also to the esters IB, EB, and IV, compared with flies raised on synthetic medium. In contrast, the enriched odor experience of cornmeal medium–raised flies selectively tuned responses to EA. (B) Bar plots of the total information gain (in the 5-s response window) display the increased sensitivity to different esters in EA medium–raised flies and increased tuning of EA-specific responses in cornmeal medium–raised flies compared with synthetic medium–raised flies (error bars indicate SD).
We also examined the effect of conditioning on synthetic media infused with EB and IV on IIa coding (Fig. S3). As with EA conditioning, EB and IV conditioning led to enhanced response to EB and IV, respectively. Each ester used for conditioning increased sensitivity to all esters compared with odor-deficient synthetic medium. Exposure to a single ester increases sensitivity to all esters activating the Or59b receptor, although not necessarily to the same extent.
Enriched odor experiences on cornmeal medium result in a very different IIa sensitivity scheme. Although EA sensitivity remains high (ΣDJS of approximately 22 bits over 5.0 s), sensitivity to other esters was lowest compared with the responses in flies raised on synthetic and EA supplemented media (ΣDJS <8 bits over 5.0 s; Fig. 5). Accordingly, the ratio of information gain from SF of the best separated odor (EA) and the average information gain for all esters tested in cornmeal medium–raised flies (2.12), is much greater than those for odor-deprived (1.23) and single odor–exposed flies (1.54 for EA medium rearing). We therefore infer that enriched odor experiences are important for a sharper tuning of IIa ORN responses to EA among esters.
Coding Space Map of Odor Discrimination Under Different Conditioning Schemes.
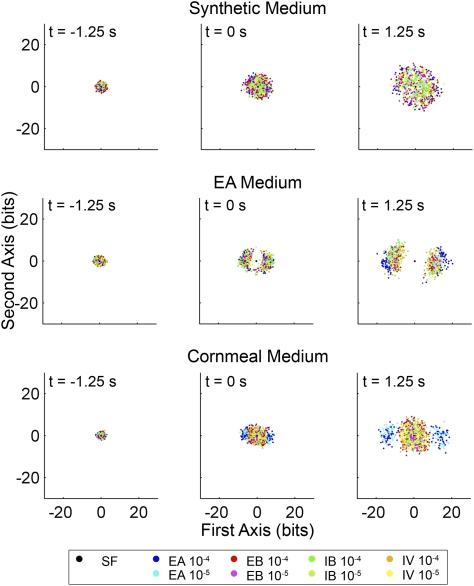
So far we have presented only pair-wise discrimination between the response to a particular odor and SF or the response to another odor. We seek to present a global map of the relationships among odor responses following our conditioning schemes. Because DJS provides a distance metric for pair-wise comparisons for each conditioning regime, we constructed a matrix of distances, mapping the relationships among all responses to different odors and concentrations (Table S2). Using the nonlinear ISOMAP algorithm (29), we were able to reduce the dimensionality of the data such that, in a 2D plot, the overall relationship of the ΣDJS for the different esters at two dilutions (10−4 and 10−5) can be clearly visualized. In this algorithm, the first axis of the plot can be thought of as the axis most sensitive in distinguishing the odor responses and the second axis as being the second best. In Fig. 6, the nine dimensions required to describe the spike ensembles collected under different conditions (four odors at two dilutions and SF) are transformed into 2D maps of best distinction and are displayed at three time points during the spike train ensembles (1.25 s before, at the time of peak firing rate, and 1.25 s afterward). For the sake of clarity, we overlay 200 resampled matrices for each of the nine dimensions reduced using the ISOMAP algorithm (Fig. S4).
Fig. 6.
ISOMAP visualization of the dynamics of the type IIA ester response space. The ISOMAP algorithm generates a map of separation of the responses to individual esters. Shown are the results for three time points: 1.25 s before, at the time of maximum spiking, and 1.25 s afterward. Each color-coded point signifies the location of a particular odor response ensemble relative to the others (200 bootstrapped response ensembles for each pair-wise comparison). The color coding (boxed) specifies the stimulating odor and dilution. Separation of color-coded clusters indicates distinction of a particular stimulus from other stimuli in the odor response space. The ISOMAPs displayed here are derived from the same data presented in Table S2, a subset of which has been used in Fig. 5. These plots clearly demonstrate that odor deprivation leads to poor separation of odor responses—from SF (at the origin) as well as from each other—in odor space. EA enrichment results in clear distinction from SF, but little separation between esters. The complex odor environment of cornmeal leads to clear separation in odor space of EA from the other esters tested.
The ΣDJS based on IIa ORN responses transformed by the ISOMAP algorithm elucidate the conditioning effects on odor discrimination. As shown in Fig. 6, the plots show that synthetic medium–raised flies show the least separation of odor responses from one another and from SF, consistent with the idea that odor deprivation affects both the sensitivity to odors and identification of odors. In contrast, odor sensitivity is boosted by single-ester exposure, as EA medium–raised flies separate SF from responses to different esters the most. However EA medium–raised flies are not effective in separating a particular acetate ester from the others as they are all clustered together. Enriched odor experience with cornmeal medium conditioning tunes the IIa code for EA particularly well among the tested odors, i.e., the corresponding points are well segregated from the points corresponding to other esters, making IIa ORNs effective detectors of EA.
Discussion
The effects of sensory experience on the function of the central nervous system have been studied in many animals. We have extended the concept of experience-dependent plasticity of information processing to primary sensory neurons by examining the effect of long-term odor deprivation or odor exposure on ORN coding. The key observations discussed in this article are: (i) odor deprivation reduces odor sensitivity, (ii) single-odor exposure increases odor sensitivity broadly to a set of related odors, and (iii) complex odor enrichment tunes ORN sensitivity to a particular odor. These findings are paralleled by studies showing that olfactory sensilla undergo significant morphological changes that result from odor deprivation in Caenorhabditis elegans (30), and odor imprinting in zebrafish is correlated with changes in gene expression in their ORNs (31). In addition to plasticity resulting from imprinting or conditioning, other studies in Diptera indicate that odor coding may display circadian variation (32, 33), and depend on age, sex, and hunger level (34). Furthermore, short-term priming by certain ketones and aldehydes has been shown to temporarily decrease CO2 sensitivity (35) in type Ic ORNs; prior exposure to CO2 does not affect odor sensitivity (36). Taken together, these findings strongly suggest that environmental factors play an important role in odor coding in ORNs.
The manner in which sensory neurons encode biologically relevant information is not fully understood. In addition to the firing rate, spike timing and synchrony may also contain functionally relevant information (37, 38). To approach this issue, we have used a measure that is independent of coding scheme. By aligning responses by their peak magnitude and finding bin-by-bin DJS, we can quantify cumulative differences between the responses as well as different phases of response time courses for rates of divergence between spike train ensembles. For example, the spike rate within a particular time window in a previous study (19) did not reveal the striking differences caused by EA conditioning (Fig. 5). Indeed, as shown in Fig. 4B, the maximum firing rate is not greatly different following EA conditioning, whereas the persistence of spiking is much greater in the response to EA, resulting in a drastically greater ΣDJS. This information-theoretic approach may also be extended to analyzing the dynamic role of PNs and local interneurons in processing and transmitting information to higher-order structures. Functional imaging and electrophysiological studies have provided useful insights into information processing in the antennal lobe and mushroom body (39–41, 42). Although local interneurons modulate PNs activity, ORNs are the primary drivers of this activity (43). Thus, odor experience–based plasticity in ORN coding is likely to influence PN tuning and enables the animal to identify an odor and assess its concentration in different environmental contexts.
How do odor experiences modulate ORN coding? Odors first bind to odor binding proteins that activate odor receptors on the cell membrane of the ORN (44). These receptors directly affect membrane excitability (45, 46) in addition to possibly modulating second messenger systems (46). Because imaginal conditioning is a slow process, of the order of hours to days, we must also consider the possibility of changes in receptor protein expression and trafficking as contributors to coding plasticity. Imaginal conditioning may also modify the odor binding proteins and/or the odor receptor complexes, resulting in the changes in sensitivity as well as specificity. In addition, modulation or expression of ion channels may also change the excitability of the ORNs. Any combination of these steps in signal transduction might be modified by imaginal conditioning. Regardless of mechanisms involved, experience-dependent tuning of ORN coding emphasizes the important roles of sensory neurons in information processing.
Methods
Flies, Odors, and Conditioning.
WT Drosophila (CSBz) was from NCBS stock center. The odorants—EA, EB, IB, and IV—were of 99.9% purity (Sigma-Aldrich). Flies were allowed to lay eggs on fresh odorless synthetic medium for 24 h. The eggs were transferred to fresh synthetic medium. Newly eclosed flies were collected within 12 h and placed in fresh synthetic medium containing the desired odor at 10−4 dilution. Recordings were made from female flies after 3 days of conditioning. For details on synthetic media preparation, see SI Methods.
Electrophysiology.
Single unit recordings from ORNs were performed as described earlier (19). Odorants were diluted in liquid paraffin oil (SD Fine Chemicals) and 2 mL of the desired dilution was placed in a custom built olfactometer that delivered a 500-ms odor stimulus through a constant airflow of 9 mL/min.
Statistical Analysis.
The data were analyzed using a series of custom-written programs in Matlab R2009b (MathWorks). Spikes of type II sensilla were sorted by amplitude in each trace, and the spike count within each 50-ms bin was recorded (Fig. S1). Between nine and 19 responses from three to six ORNs to an odor at a particular dilution were recorded to facilitate study of rising and decaying phases of the response, aligned by their maximum firing frequency, i.e., with the bin with the most spikes. The response time course was sampled between 2.5 s before and after the peak firing rate. Bin-by-bin response ensembles were constructed based on the distribution of spike counts for each bin. To create the SF ensembles, 15 traces, each 10 s long, were binned and aligned in the same manner as the odor responses. We denote the probability of observing i spikes (i = 0, 1, 2…) in the τth bin of the response ensemble p as pτ(i). The DJS between the τth bin of two spike train ensembles, each with spike probability distributions of pτ and qτ, respectively, is:
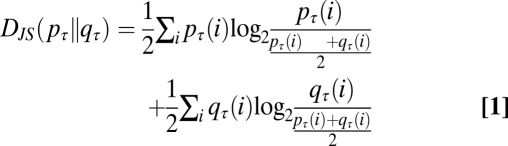 |
The cumulative divergence, ΣDJS was found by accumulating, over the 5-s time course, the pair-wise bin divergence as follows:
We assessed odor sensitivity by finding the ΣDJS between the odor's response ensemble and the SF ensemble. Response ensembles were also compared with each other in a pair-wise manner to determine the ability to discriminate between the ensembles.
By using the data shown in Figs. 1 and 4, the following bootstrap resampling procedure was used to assess the robustness of the total information gain measure between two response ensembles (or between a response ensemble and SF): (i) 1,000 resampled ensemble pairs were created by drawing, with replacement from each respective original ensembles, a set of traces; (ii) the ΣDJS over the entire response was calculated for each of the 1,000 resampled response ensembles pairs; and (iii) the mean and SD of the 1,000 resampled ΣDJS values was found.
ISOMAP visualization was done using code adapted from Tenenbaum et al. (29) applied to sets of 1,000 matrices of ΣDJS values between odor response ensembles at three different time points along the odor responses for each conditioning scheme. Each of the 1,000 matrices (9 × 9) in a set were created by resampling the ΣDJS between all pairs of response ensembles at the particular time point as previously described. The ISOMAP algorithm, using the four nearest neighbors among the nine odor-dilution combinations used, was applied for each bootstrapped matrix. The resulting points for each matrix were normalized by the SF point and overlaid upon each other (for Fig. 6, 200 maps were used).
Supplementary Material
Acknowledgments
We thank Sunil Prabhakar for setting up the single unit rig and members of the laboratories of O.S. and C-F.W. for stimulating discussion during the progress of our work. We also thank Prof. Jing Wang, Prof. Mani Ramaswami, and Prof. V. Nanjundiah for their comments on the manuscript. This research was supported by Defense Research and Development Organization (Delhi, India) Grant ERIP/ER/0102086/M/01 (to O.S.), an Iowa Center for Research by Undergraduates award (to A.I.), and National Institutes of Health Training Grant 5 T32 NS007421-11.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003856107/-/DCSupplemental.
References
- 1.Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 3.Gailey DA, Lacaillade RC, Hall JC. Chemosensory elements of courtship in normal and mutant, olfaction-deficient Drosophila melanogaster. Behav Genet. 1986;16:375–405. doi: 10.1007/BF01071319. [DOI] [PubMed] [Google Scholar]
- 4.Shaver SA, Varnam CJ, Hilliker AJ, Sokolowski MB. The foraging gene affects adult but not larval olfactory-related behavior in Drosophila melanogaster. Behav Brain Res. 1998;95:23–29. doi: 10.1016/s0166-4328(97)00206-4. [DOI] [PubMed] [Google Scholar]
- 5.Frye MA, Dickinson MH. Motor output reflects the linear superposition of visual and olfactory inputs in Drosophila. J Exp Biol. 2004;207:123–131. doi: 10.1242/jeb.00725. [DOI] [PubMed] [Google Scholar]
- 6.Trimarchi JR, Schneiderman AM. Different neural pathways coordinate Drosophila flight initiations evoked by visual and olfactory stimuli. J Exp Biol. 1995;198:1099–1104. doi: 10.1242/jeb.198.5.1099. [DOI] [PubMed] [Google Scholar]
- 7.Shanbhag S, Müller B, Steinbrecht R. Atlas of olfactory organs of Drosophila melanogaster 1. Types, external organization, innervations and distribution of olfactory sensilla. Int J Insect Morphol Embryol A. 1999;28:377–397. [Google Scholar]
- 8.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 10.Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- 11.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 12.Root CM, et al. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis RL. Mushroom bodies and Drosophila learning. Neuron. 1993;11:1–14. doi: 10.1016/0896-6273(93)90266-t. [DOI] [PubMed] [Google Scholar]
- 15.Heisenberg M. Mushroom body memoir: From maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 16.Devaud JM, Acebes A, Ferrús A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J Neurosci. 2001;21:6274–6282. doi: 10.1523/JNEUROSCI.21-16-06274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szyszka P, Galkin A, Menzel R. Associative and non-associative plasticity in kenyon cells of the honeybee mushroom body. Front Syst Neurosci. 2008;2:3. doi: 10.3389/neuro.06.003.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorpe W. Further studies on pre-imaginal olfactory conditioning in insects. Proc R Soc Lond B Biol Sci. 1939;127:424–433. [Google Scholar]
- 19.Chakraborty TS, Goswami SP, Siddiqi O. Sensory correlates of imaginal conditioning in Drosophila melanogaster. J Neurogenet. 2009;23:210–219. doi: 10.1080/01677060802491559. [DOI] [PubMed] [Google Scholar]
- 20.Lewis EB. A new standard food medium. Drosoph Inf Serv. 1960;34:117–118. [Google Scholar]
- 21.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 22.Lin J. Divergence measures based on the Shannon entropy. IEEE Trans Inf Theory. 1991;37:145–151. [Google Scholar]
- 23.Yao Y. Information-theoretic measures for knowledge discovery and data mining in Entropy Measures, Maximum Entropy Principle and Emerging Applications. In: Karmeshu , editor. Heidelberg: Springer; 2003. pp. 115–136. [Google Scholar]
- 24.Rospars J-P, Lánský P, Duchamp-Viret P, Duchamp A. Spiking frequency versus odorant concentration in olfactory receptor neurons. Biosystems. 2000;58:133–141. doi: 10.1016/s0303-2647(00)00116-7. [DOI] [PubMed] [Google Scholar]
- 25.Grosse I, et al. Analysis of symbolic sequences using the Jensen-Shannon divergence. Phys Rev E Stat Nonlin Soft Matter Phys. 2002;65:41905–41921. doi: 10.1103/PhysRevE.65.041905. [DOI] [PubMed] [Google Scholar]
- 26.Kullback S, Leibler R. On information and sufficiency. Ann Math Stat. 1951;22:79–86. [Google Scholar]
- 27.Shlens J, Kennel MB, Abarbanel HD, Chichilnisky EJ. Estimating information rates with confidence intervals in neural spike trains. Neural Comput. 2007;19:1683–1719. doi: 10.1162/neco.2007.19.7.1683. [DOI] [PubMed] [Google Scholar]
- 28.Efron B. Bootstrap methods: Another look at the jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 29.Tenenbaum JB, de Silva V, Langford JC. A global geometric framework for nonlinear dimensionality reduction. Science. 2000;290:2319–2323. doi: 10.1126/science.290.5500.2319. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay S, Lu Y, Shaham S, Sengupta P. Sensory signaling-dependent remodeling of olfactory cilia architecture in C. elegans. Dev Cell. 2008;14:762–774. doi: 10.1016/j.devcel.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harden MV, Newton LA, Lloyd RC, Whitlock KE. Olfactory imprinting is correlated with changes in gene expression in the olfactory epithelia of the zebrafish. J Neurobiol. 2006;66:1452–1466. doi: 10.1002/neu.20328. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan P, Chatterjee A, Tanoue S, Hardin PE. Spike amplitude of single-unit responses in antennal sensillae is controlled by the Drosophila circadian clock. Curr Biol. 2008;18:803–807. doi: 10.1016/j.cub.2008.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Den Otter CJ, Tchicaya T, Schutte AM. Effects of age, sex and hunger on the antennal olfactory sensitivity of tsetse flies. Physiol Entomol. 1991;16:173–182. [Google Scholar]
- 35.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 36.Sachse S, et al. Activity-dependent plasticity in an olfactory circuit. Neuron. 2007;56:838–850. doi: 10.1016/j.neuron.2007.10.035. [DOI] [PubMed] [Google Scholar]
- 37.Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- 38.Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- 39.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 40.Jefferis G, et al. Comprehensive maps of Drosophila higher olfactory centers: Spatially segregated fruit and pheromone representation. Cell. 2006;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cassenaer S, Laurent G. Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature. 2007;448:709–713. doi: 10.1038/nature05973. [DOI] [PubMed] [Google Scholar]
- 43.Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. Propagation of olfactory information in Drosophila. Proc Natl Acad Sci USA. 2007;104:11826–11831. doi: 10.1073/pnas.0704523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galindo K, Smith DP. A large family of divergent Drosophila odorant-binding proteins expressed in gustatory and olfactory sensilla. Genetics. 2001;159:1059–1072. doi: 10.1093/genetics/159.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato K, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 46.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.