Abstract
Adjuvant effects on innate as well as adaptive immunity may be critical for inducing protection against mucosal HIV and simian immunodeficiency virus (SIV) exposure. We therefore studied effects of Toll-like receptor agonists and IL-15 as mucosal adjuvants on both innate and adaptive immunity in a peptide/poxvirus HIV/SIV mucosal vaccine in macaques, and made three critical observations regarding both innate and adaptive correlates of protection: (i) adjuvant-alone without vaccine antigen impacted the intrarectal SIVmac251 challenge outcome, correlating with surprisingly long-lived APOBEC3G (A3G)-mediated innate immunity; in addition, even among animals receiving vaccine with adjuvants, viral load correlated inversely with A3G levels; (ii) a surprising threshold-like effect existed for vaccine-induced adaptive immunity control of viral load, and only antigen-specific polyfunctional CD8+ T cells correlated with protection, not tetramer+ T cells, demonstrating the importance of T-cell quality; (iii) synergy was observed between Toll-like receptor agonists and IL-15 for driving adaptive responses through the up-regulation of IL-15Rα, which can present IL-15 in trans, as well as for driving the innate A3G response. Thus, strategic use of molecular adjuvants can provide better mucosal protection through induction of both innate and adaptive immunity.
Keywords: APOBEC3G, HIV vaccine, Toll-like receptor agonist, IL-15, Polyfunctional T cells
As simian immunodeficiency virus (SIV) is restricted to mucosal tissue shortly after rectal or vaginal challenge, a strong preexisting mucosal immune response might prevent or slow viral transmission (1–3). Previous studies from our laboratory showed that mucosal immunization of macaques with an HIV/SIV peptide vaccine was more effective against SIV/HIV challenge than systemic vaccination (4) and that a peptide-prime/poxviral boost approach that induced a strong cytotoxic T lymphocyte (CTL) mucosal response could impact dissemination of intrarectally administered pathogenic SIV/HIV-ku2 (5). An improved regimen might actually abort the local mucosal infection before dissemination. Certain molecular adjuvants, such as Toll-like receptor (TLR) agonists and cytokines, can improve the quantity and quality of antigen-specific T-cell responses (6, 7). Studies in mice showed that TLR2, -3, and -9 agonists activate and mature dendritic cells (DCs) to enhance immune responses (8, 9) and our laboratory discovered a synergistic adjuvant effect of a combination of these agonists (9, 10). We and others have also found that IL-15 is a strong cytokine adjuvant, as it promotes the homeostatic expansion of CD8+ memory T cells, and the induction of higher avidity, longer-lived T cells (11–14). Combinations of these adjuvants might therefore improve the magnitude and quality of vaccine-induced responses.
Although most interest in molecular adjuvants has been to improve adaptive immunity, here we also investigated their ability to induce protective innate immunity. We found APOBEC3G (A3G) expression levels were correlated inversely with viral load. A3G is a potent host antiretroviral factor that can restrict HIV-1 infection through multiple mechanisms (15, 16). Recent studies showed that A3G mRNA expression was elevated in both HIV-1-infected long-term nonprogressors and HIV-exposed seronegative individuals and also correlated with a slow progression in long-term nonprogressors (17, 18). In addition, mucosal immunization led to persistent expression of A3G in macaques (19). PolyI:C, one component of our vaccine adjuvant, was reported to inhibit HIV amplification in DCs via A3G induction (20). In the current macaque study, we demonstrated an innate mechanism by which adjuvants may contribute to viral control, and identified candidate adaptive immune correlates of protection.
Results
To investigate the immunogenicity and protective efficacy of TLR agonists and IL-15 adjuvanted SIV vaccine, we intracolorectally immunized 20 Indian rhesus macaques using a peptide prime/modified vaccinia virus Ankara (MVA) boost protocol with or without a triple combination of TLR2/6, -3, and -9 agonists [macrophage activating lipoprotein-2 (MALP-2), Poly I:C, and D-type CpG, respectively] and IL-15 (Fig. 1A). Macaques were primed three times with peptides (5, 21) and boosted twice with MVA expressing SIV proteins (Table S1) (22), in all cases with the adjuvants. A fifth group, given TLR agonists and IL-15 without vaccine, was included during immunization as an adjuvant-only control. Seven weeks after the last boost (3 weeks after surgical biopsy to allow recovery), all animals were intrarectally challenged with 10 infectious doses of SIVmac251, a batch and dose that has infected 100% (30/30) of naive animals in previous studies of our collaborators (22–25) and others (7). Indeed, even a 5-fold lower dose infected 11 of 11 animals (26).
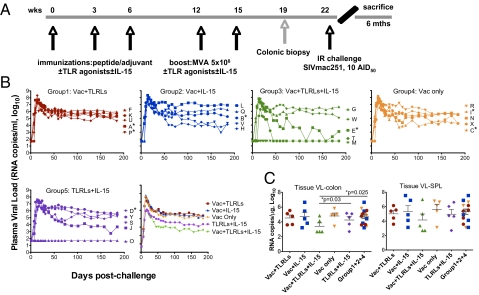
Fig. 1.
Protective efficacy of TLR agonists and IL-15 adjuvanted peptide-primed/rMVA-boosted vaccine regimens. (A) Schedule of intracolorectal immunization protocol. (B) Levels of viral RNA in plasma of the individual animals (denoted by single letters), as well as geometric means of the groups with different immunization protocols following intrarectal challenge with 10 ID50 SIVmac251. *, Mamu-B*17+ animals. (C) VL in the colon and spleen were measured at the time of necropsy. Comparisons among groups were performed by two-sided Wilcoxon's rank-sum tests. Bars show mean and SEM of Log10.
We did not observe any obvious adverse effects during the study. We monitored plasma SIV RNA levels (Fig. 1B) and CD4+ T-cell count to evaluate regimen efficacy. Because we monitored the macaques for only 6 months postchallenge, none of the animals showed a significant drop in CD4+ T-cell count. Macaques receiving vaccine without adjuvant (group 4) showed high peak and set-point plasma viral loads (VL) (7.7, and 5.3 logs), similar to those of macaques receiving vaccine adjuvanted with either TLR agonists (group 1) (7.6 and 5.6 logs) or IL-15 (group 2) (7.9 and 5.5 logs) alone. In contrast, macaques that received vaccine with both TLR agonists and IL-15 (group 3) showed improved protection: one animal (M) escaped infection, two others (T and E) rapidly cleared virus from the blood, and the remaining two had infections similar to those in other groups. As a whole, group 3 had lower peak and set-point plasma VL (6.2 and 3.8 logs, P < 0.02 compared to groups 1, 2, and 4 by a two-tailed t test) as well as a significantly lower tissue VL in the colon than all of the other vaccinated groups (P < 0.05) (Fig. 1C), the latter suggesting that vaccination controlled viral infection in the local mucosal tissues. Even in the adjuvant-only group 5, one animal (O) was protected and another (J) showed some decline in VL over time, in contrast to all (30/30) naive historical controls (7, 22–25) and animals in groups 1, 2, and 4. This finding motivated a search for possible correlates of protection in both innate and adaptive immunity.
We assessed a variety of adaptive immune parameters before challenge. Consistent with our previous studies (5), humoral responses did not appear to play a role. Even 1 week postchallenge, only 3 of 25 animals had significant antibody titers to gp120 (F, L, and V, Table S2), and none of them showed protection. Four weeks after the last immunization, all vaccinated monkeys generated similarly high levels of virus-specific tetramer+ CD8+ T cells in the colon, with strong responses to all six vaccine epitopes tested, and no evidence for antigenic competition was observed (Fig. 2A and Fig. S1). The frequency of tetramer-positive central and effector memory T cells did not correlate with set-point plasma VLs (Fig. 2B), although the frequency of tetramer-positive cells in the colon significantly exceeded those in the secondary lymphoid tissue (P < 0.0001) (Fig. 2C), indicating a stronger local immune response induced by this mucosal vaccine regimen.
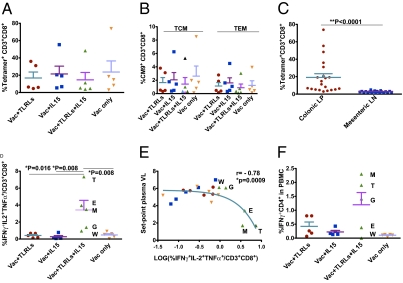
Fig. 2.
Cellular immune responses before viral challenge. (A) The sum of CD3+CD8+ Mamu-A*01-tetramer+ cell frequencies (for six epitopes) were measured in the colonic lamina propria at week 19. (B) Within the CM9+ CD3+CD8+ cell population, central memory T lymphocyte (TCM: CD28+CD95+) and effector memory T lymphocyte (TEM: CD28-CD95+) responses were assessed by eight-color intracellular cytokine staining (ICS) assays. (C) Comparison of CD3+CD8+ tetramer+ cell frequency in different compartments was performed by two-tailed Wilcoxon's matched-pairs rank tests. (D and F) Frequencies of CD8+ T cell response (triple positive for IFNγ, IL-2, and TNFα) and CD4+ T cell response (IFNγ+) in PBMCs were measured at week 19 and comparisons among the groups were performed by two-tailed Wilcoxon's rank sum tests. (E) Correlation between prechallenge CD8+ triple-functional T-cell response frequency and set-point plasma VL was evaluated by three-decay dose-dependent inhibition curve with R value shown, and the P value was calculated by Fisher's exact test for the 2 × 2 comparison log VL < or > 4 and log % polyfunctional T cells < or > 0.3 (log of 2). The animals in different groups were color-coded: group 1 in red, group 2 in blue, group 3 in green, and group 4 in orange. Data show mean and SEM.
To determine whether different adjuvants induce qualitatively—rather than quantitatively—different virus-specific T-cell responses, we evaluated the functionality of the immune responses by intracellular cytokine staining (ICS). Peripheral blood mononuclear cells (PBMCs) were stimulated with six CTL-epitopes and two T-helper epitope clusters, and CD4+ /CD8+ T cells were stained for intracellular IFNγ, IL-2, or TNF. Only animals in group 3 had a higher frequency of antigen-specific polyfunctional CD8+ T cells (P = 0.008–0.016) (Fig. 2D), as well as single-cytokine-producing cells (Fig. S2 A–C), compared with all other vaccine groups (P < 0.02 for IFNγ and ≤ 0.032 for TNFα). Furthermore, triple-cytokine-producing cells actually produced more IL-2 and TNFα, but less IFNγ, than single-cytokine-producing cells, as determined by geometric mean fluorescent intensity (MFI) (Fig. S2D). Surprisingly, we found that a nonlinear fit model (three-decay dose-dependent inhibition model similar to a classic inhibitor titration curve), instead of a linear correlation observed previously (7, 27), was more appropriate to describe the relationship between triple- or single-cytokine-producing antigen-specific CD8+T cells and set-point plasma VL (Fig. 2E and Fig. S2 E–G). The difference between this nonlinear fit model and the linear ones is the existence of a T-cell-response threshold-like effect for detecting a substantially reduced VL (P = 0.0009). Only when T-cell immunity is in the range above ≈2% (log ~0.3) polyfunctional specific T cells is a significant VL reduction observed, whereas below this range (on the relatively flat part of the curve), there is no correlation between T-cell responses and VL. In view of the known greater resistance of Mamu-B*17 macaques (28), it is notable that this correlation remained significant (R = −0.766, P = 0.011), even when all Mamu-B*17+ animals were excluded from the analysis. Even for the five animals in group 3, plasma VL was inversely correlated with polyfunctional T cells (P < 0.03) (Fig. S2H). For the antigen-specific IFNγ+ CD4+ T cells, the highest frequencies were seen in the best-protected group (as well as the lowest one), and a trend toward inverse correlation with set-point plasma VL was observed (Fig. 2F and Fig. S2 I and J).
To our surprise, some of the animals that received only adjuvant were partially protected (Fig. 1B): one of five adjuvant-only animals did not get infected, one showed lower set-point VL, and the overall peak and set-point VL in this group was low (6.2 logs and 4.5 logs, respectively). As these animals did not receive any vaccine antigen to mediate adaptive immunity, these data suggest the hypothesis that innate immunity was involved. In an attempt to identify innate factors, we first examined A3G and TRIM5α, which are innate restriction factors for HIV/SIV, and found that A3G (Fig. 3A) but not TRIM5α (Fig. S3) was up-regulated in the protected groups. To determine whether A3G was unique or merely representative of many innate responses contributing to protection, we further assessed natural killer (NK) cells, type I IFN, IFN regulatory factors, IFN-induced MxA (29), CXCL9, IL-1β, TNFα, and Granzyme B expression levels in the mesenteric lymph nodes (MLN) (Fig. S3), and did not observe significant differences among the groups, whereas type I IFN and TNFα 4 weeks after the last boost were up-regulated in the vaccinated group adjuvanted with TLR agonists alone, which had one of the highest VLs, consistent with the recent finding of a deleterious role played by type I IFN in mediating immune activation (30). In contrast, the combination of TLR agonists and IL-15 resulted in lower type I IFN, suggesting that IL-15 counteracts this effect by a mechanism that remains to be explored. However, none of these factors correlated with set-point plasma VLs (Fig. S4), which indicated that they were not the main mediators for innate protection.
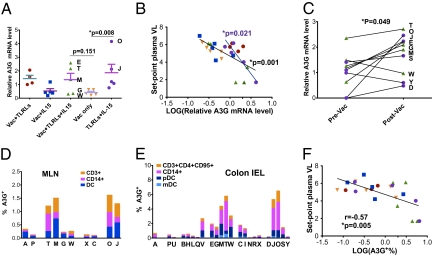
Fig. 3.
A3G expression levels before viral challenge. (A) Relative mRNA expression level of A3G in the MLN at week 19 was measured by quantitative real-time RT-PCR using macaque-specific A3G primers/probe. Comparisons among the groups were performed by two-sided Wilcoxon's rank sum tests. (B) Correlations between set-point plasma VL and the relative A3G mRNA level in the MLN for all of the animals (black line) were evaluated by a two-tailed Spearman's rank test, and for the animals in the adjuvant only group (purple line and purple filled circles) by a linear regression analysis according to the nature of their distributions. The animals in different groups were color-coded: group 1 in red, group 2 in blue, group 3 in green, group 4 in orange, and group 5 in purple. (C) Comparison of relative mRNA expression level of A3G in pre- and postvaccination PBMC samples from animals receiving both TLR agonists and IL-15 with or without Vac (groups 3 and 5) were assessed by Wilcoxon's matched pairs rank test. (D and E) Different A3G+ cell populations were measured by ICS staining in the MLNs (D) and colon IEL (E) of representative animals from different immunization groups (at week 19). (F) Correlations between set-point plasma VL and A3G expression level in the Colon IEL were evaluated by a two-tailed Spearman's rank test, Data show mean and SEM.
Thus, only A3G levels correlated with protection and might be the main innate immunity factor to mediate this protection. The mRNA level of A3G in the MLN was higher in groups that received TLR agonists than the ones without. In addition, in the adjuvant-only group, animal O, which failed to get infected, had the highest A3G level, and animal J, that had a lower set-point VL, also had a relatively high level of A3G (Fig. 3A), as did the protected animals E, T, and M in group 3 that also received both types of adjuvants. A significant inverse correlation was found between plasma VLs and A3G levels (P = 0.0012), and this was particularly accentuated for the adjuvant-only group, for which only innate immunity was involved (Fig. 3B). The correlation remained significant (P = 0.0018), even if all of the Mamu-B*17+ animals were excluded from the analysis. The significant inverse correlation for group 5 alone supports the conclusion that A3G was a major factor determining protection in the group that was administered only the adjuvants. In this context, the combination of TLR agonists and IL-15 (groups 3 and 5) significantly up-regulated expression of A3G from prevaccination levels (Fig. 3C), whereas neither adjuvant alone did (groups 1, 2, or 4) (Fig. S5C). This significant change from before to after vaccination contrasts with the stable levels in healthy individuals (31), suggesting an effect of vaccination. Furthermore, we assessed A3G expression in MLN (Fig. 3D and Fig. S6) and colon intraepithelial lymphocyte (IEL) (Fig. 3E and Fig. S6) by flow cytometry and found that groups 3 and 5 had higher A3G+ cell percentages mostly in monocytes and DCs and only a small proportion (less than one-third) in the CD4+ T-cell compartment. In contrast to T cells (32), monocytes and DCs express primarily the protective low molecular mass (LMM) form of A3G (20, 33, 34). Significant inverse correlations were observed between the A3G expression increase and the set-point plasma (Fig. 3F) and colon tissue VLs (Fig. S7). The increase in A3G was confirmed by a third independent method, Western blot (Fig. S5B).
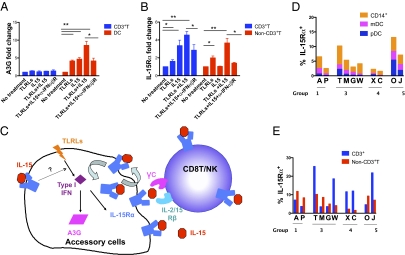
The data demonstrated a synergistic effect of TLR agonists and IL-15 to induce protective immunity against intrarectal SIVmac251 challenge, involving both T-cell adaptive and innate immunity. However, the mechanism by which this synergy is achieved is unknown. To explore the possible mechanisms, we measured A3G levels upon stimulation with TLR agonists and IL-15 in vitro and found that A3G levels were enhanced with the combination of both adjuvants compared with either one alone, especially in DCs, which was consistent with our in vivo data (Fig. 4A). Interestingly, IL-15Rα was also significantly enhanced by treatment with both adjuvants in vitro in T cells and non-T accessory cells (Fig. 4B). IL-15Rα on T cells can directly capture IL-15 and activate T cells (11), whereas IL-15Rα on accessory cells stabilizes and concentrates IL-15, and mediates IL-15 transpresentation, which is believed to be the dominant mechanism by which the IL-15 signal is delivered in vivo (35). The up-regulation of both A3G and IL-15Rα was inhibited by blockade of the IFNαβR (Fig. 4 A and B), implying a mechanism dependent on type I IFNs. Based on these results, we propose a model of TLR agonist and IL-15 interaction (Fig. 4C), in which the induction of IL-15Rα on accessory cells is a key step. TLR agonists, possibly via type I IFN, up-regulate IL-15Rα on accessory cells, which then capture exogenous IL-15 in the vaccine and facilitate the activation of CD8+ T cells/NK cells via a transpresentation mechanism. A higher level of IL-15Rα on antigen-presenting cells (APCs) in the MLNs of monkeys with both adjuvants was indeed observed (Fig. 4 D and E). We also envision (Fig. 4C) that TLR ligands, together with IL-15, act on accessory cells to induce A3G expression, possibly through a transient autocrine type I IFN mechanism, which does not have the deleterious effects noted earlier for prolonged circulating levels of type I IFN.
Fig. 4.
Mechanisms of the synergistic effects of TLR agonists and IL-15 to induce cellular and innate immunity. Healthy macaque PBMCs were treated with TLR agonists or IL-15 for 3 days and A3G+ (A) and IL-15Rα+ (B) cell populations were measured by eight-color ICS/surface assays. Monoclonal antibody blocking human IFNα/β receptor chain 2 (50 μg/mL) was used to pretreat the cells for 30 min before TLR agonists and IL-15 were added. Results are representative of at least three independent experiments. (C) The proposed synergy model for TLR agonists and IL-15 to induce cellular and innate immunity. Upon the stimulation of TLR agonists, accessory cells were induced to express type I IFN, which then up-regulates A3G and IL-15Rα expression. As a consequence, surface IL-15Rα binds IL-15, which was provided with vaccine, and delivers an IL-15 signal to neighboring T cells/NK cells through transpresentation. In addition, the IL-15/IL-15Rα complex on the cell surface contributes to the long survival of CD8 memory T cells via recycling of the complex. IL-15 might synergize with TLR agonists to induce A3G and IL-15Rα. (D and E) Different IL-15Rα+ cell populations (Methods) were measured by surface staining in the MLNs of representative animals from different immunization groups. Data show mean and SEM.
Discussion
This macaque study demonstrated that adjuvant-induced innate immunity, in the form of A3G, was likely responsible for the partial protection against subsequent SIVmac251 challenge. Consistent findings were observed in our previous studies where, of two cohorts of SIV/HIV-infected macaques, one of four in the first cohort (4, 36) and three of four in the second cohort (5) in the adjuvant-only group failed to get infected. We identified that the enhanced A3G expression was widely distributed in DCs, monocyte/macrophage, and CD4+T cells. It worth mention that APCs, such as DCs and CD14+ monocytes, which express mainly the protective LMM form of A3G (20, 33, 34), were the main A3G+ cells in MLN and colon IEL (Fig.3 D and E). DCs play a critical role in mediating HIV transmission in the mucosal tissues. They are at the front line of encountering HIV, and besides being directly infected by HIV, even in the absence of viral replication they can also capture and transfer HIV virus to CD4+ T cells via synapses. Elevated A3G-mediated intrinsic resistance of DCs might offer potential to counteract HIV infection at the mucosal portal of entry (33). In the current study, it was likely that APCs that encounter adjuvant/vaccine in the colorectal mucosa are induced to express A3G, which, upon subsequent challenge, was beneficial for viral control. Because there was an inverse correlation of VL with A3G but not with IFNα/β or any other downstream IFN-induced genes before challenge, we believe the effect of A3G is specific and A3G cannot be just a marker for IFN-induced genes. Consistent with a recent study (19), we found that memory CD4+ T cells were also induced to express A3G, which, if present in the LMM form (32), might mediate protection against virus penetrating the colorectal epithelial barriers.
Nevertheless, it should be noted that in group 3, innate immunity alone cannot explain all of the protection because animal M, with the greatest protection, had lower levels of A3G than animal T in group 3 or even animal J in group 5. Moreover, three of five animals in group 3 reduced VL below 50 copies per milliliter, whereas only one animal in group 5 did so. Therefore, in the group 3 animals, polyfunctional T cells, in addition to A3G, are necessary to explain the protection observed. Interestingly, we observed a nonlinear correlation between the polyfunctional T-cell responses and the set-point plasma VL. We hypothesize that this threshold-like effect might explain the contradictory reports as to whether T-cell responses correlate with protection in SIV-macaque models and humans (27, 37–39), because if responses were all on the plateau phase, no correlation would be found. This threshold effect is also consistent with a proposed hypothesis that such effects may explain the recent failure of the STEP trial (40). However, more studies are needed to verify this interpretation.
Our macaque data clearly show that neither TLR agonists nor IL-15 alone were sufficient to induce the high levels of functional antigen-specific CD8+ T cells correlating with protection. Our interpretation is that although TLR agonists induced IL-15Rα, they failed to induce enough IL-15, and thus exogenous IL-15 was needed. On the other hand, without the induction of IL-15Rα on accessory cells (and perhaps T cells as well), the IL-15 signal would not be delivered properly. To take full advantage of IL-15-mediated T memory cell induction in future HIV vaccines, the incorporation of components such as TLR agonists or CD40 ligand to enhance IL-15Rα expression on accessory/T cells may be critical.
It is important to point out that because of the use of an adjuvant-only control group, the data presented here constitute unique evidence directly correlating adjuvant-induced innate immunity with protection against SIV infection in vivo. This evidence is in agreement with a previous study showing in vitro up-regulation of A3G can prevent cells from HIV/SIV infection in vitro (41). A number of observations have implicated innate immunity in affecting HIV replication and AIDS progression; however, direct experimental evidence was lacking. In this macaque study, we demonstrated that repeated mucosal administration of TLR agonists and IL-15 alone without antigen was sufficient to enhance A3G expression, and this alone was sufficient to account for a certain level of protection against subsequent SIV challenge. This result supports the idea that certain adjuvants, able to induce persistent innate immunity, could play a role in preventing SIV transmission, and may explain some adjuvant-only animals in previous studies (4, 5). Because the outcomes of early SIV infection are mainly dependent on the race between viral replication and dissemination and immune responses against it, the development of rapid or preexisting innate immune responses are essential to allow adaptive immunity sufficient time to mount an effective protective response. Innate immunity, however, is commonly believed to lack memory and be short-lived. In contrast to this dogma, recent findings suggested that NK cells can mediate long-lived, memory-like recall responses independent of B and T cells in a delayed hypersensitivity model (42). We found that A3G can be up-regulated upon stimulation with certain combinations of adjuvants, and most importantly, a high level of A3G expression could be sustained for a surprisingly long time for an innate response after the immunization (measured at 4 weeks but apparently effective even at 7 weeks at the time of challenge). A recent study also found prolonged up-regulation of A3G after a mucosal vaccination, but did not investigate the role of adjuvants-only (19). If these characteristics of A3G can be used for future HIV vaccine design, we might be able to elicit a combined innate and adaptive immune response soon enough to prevent the mucosal transmission of the virus.
In conclusion, our data highlight the importance of both innate and adaptive immunity in effective control of infection by vaccination, and suggest correlates of protection that motivate further study of these parameters. The proposed model provides insights for designing future HIV vaccines.
Methods
Rhesus Macaques, Immunization, and Challenge.
Twenty-five Indian rhesus macaques (Macaca mulatta) were maintained in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International and with approval of the National Cancer Institute Animal Care and Use Committee. All were seronegative for SIV, simian retroviruses 1, 2, and 5, and simian T-cell leukemia/lymphotropic virus type 1 before the study. All animals were Mamu-A*01+, Mamu-B*08−, and seven were Mamu-B*17+ (Table S3). Four groups (five animals per group) were used for immunization. Group 1: Vac+TLRLs; group 2: Vac+IL-15; group 3: Vac+TLRLs+IL-15; and group 4: Vac only. For immunization, each peptide vaccine (Table S1) (5, 21) contained 0.5 mg of each peptide mixed with DOTAP (Roche, 100 μL per dose) with or without a combination of 500 μg per dose of D-type CpG oligodeoxynucleotide, 10 μg per dose of MALP2, and 1 mg per dose of PolyI:C (In Vivogen), or 300 μg per dose of recombinant human IL-15 (Peprotech). Recombinant MVA viruses expressing SIVmac239 Gag, pol, env and Rev, Tat, Nef (5 × 108/immunization for each) (22) were administered intracolorectally with or without adjuvants. Intracolorectal inoculations were performed as previously described, except a 41-cm catheter was used to deliver material into the lower colon (4). A fifth group (five animals) that received only TLR agonists and IL-15 without peptide and MVA-SIV was included to serve as an adjuvant-only control. Macaques were primed at week 0, 3, and 6 with peptides or adjuvants and boosted at week 12 and 15 with MVA-SIV and adjuvants (22). At week 22, all animals received an intrarectal inoculation of 10 ID50 of SIVmac251 [provided by Nancy Miller, National Institute of Allergy and Infectious Diseases (NIAID), National Insitutes of Health], sufficient to infect 100% (30/30) of naive controls in previous studies (7, 22–25). After challenge, SIV RNA levels were monitored by NASBA assays for 6 months by Advanced BioScience Laboratories, Inc with a cutoff value of 50 copies. Set-point plasma VL was calculated by averaging the geometric means of plasma VLs from day 45 to 185 postchallenge. We collected colon lamina propria and IEL as previously described (4).
Immunologic Assays.
We measured SIV-specific T cell responses by ICS assays (43), using the following antibodies: CD3-PE-Cy7, CD8-APC-Cy7, CD28-FITC, CD95-PE-Cy5 (BD Pharmingen); and CD4-qdot605, IFNγ-Alexa700, IL-2-Alexa 647, TNFα-PE (Biolegend). Mamu-A*01 tetramers (CM9, LV10, LA9, QA9, SL8, and KA9) were obtained from the MHC tetramer core facility, NIAID. Assessment of A3G and IL-15Rα expression used: CD3-APC, CD14-APC-Cy7, lineage mixture-FITC, HLA-DR-PE-Cy7, CD11c-PE-Cy5, CD123-PerCP-Cy5.5 (BD Pharmingen), IL-15Rα-PE (R&D Systems), and rabbit anti-A3G pAb IgG (ImmunoDiagnostics), with donkey-anti-rabbit-Alexa680 (In Vitrogen) as secondary antibody. Total DCs were defined as the sum of mDC (Lin-HLA-DR+CD11c+), and pDC (Lin-HLA-DR+CD11c-CD123+). For in vitro studies, healthy macaque PBMCs were treated with MALP2, Poly I:C, and CpG (0.2, 25 and 5 μg/mL) and IL-15 (25 ng/mL) for 3 d and A3G+ and IL-15Rα+ cells were measured by ICS/surface assays. Monoclonal antibody blocking human IFNα/β receptor chain 2 (50 μg/mL, clone# MMHAR-2, PBL IFN Source) was used to pretreat the cells for 30 min before TLR agonists and IL-15 were added.
Real-Time RT-PCR.
Total RNAs from MLNs and PBMCs were isolated, treated with DNase (Ambion) and further purified with RNeasy columns (Qiagen). Next, 400 ng of RNA from each specimen was reverse transcribed and RT-PCR was used with macaque specific A3G Taqman primers/probe (ABI) to measure relative mRNA expression levels by the comparative threshold cycle (Ct) method. The Ct values for A3G were normalized to the geometric mean of GAPDH and β-actin.
Statistical Analyses.
We performed statistical analyses with Prism for Mac, version 5 (Graph Pad) and SAS for Windows version 9.1.3. In all analyses, we used a two-sided significance level of 0.05.
Supplementary Material
Acknowledgments
We thank J. Bacher, J. Medley, J. Dennis, and A. Cisar for veterinary support and surgeries; M. Eckhaus and I. Cabrera for necropsies and pathology support; R. Pal and P. Markham, and S. Orndorff for the viral loads, CD4, and antibody assays; D. Watkins for MHC typing; Nancy Miller for providing pathogenic SIVmac251 challenge stock; the National Institute of Allergy and Infectious Diseases tetramer core facility for providing the tetramers; Lisa Smith for secretarial assistance; and M. Robert-Guroff and J. Lifson for critical reading of the manuscript and helpful suggestions. This work was supported in part by the Intramural Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and the National Institutes of Health Intramural AIDS Targeted Antiretroviral Program.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0911932107/-/DCSupplemental.
References
- 1.Lackner AA, Mohan M, Veazey RS. The gastrointestinal tract and AIDS pathogenesis. Gastroenterology. 2009;136:1965–1978. doi: 10.1053/j.gastro.2008.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z-Q, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley JM, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyakov IM, et al. Mucosal AIDS vaccine reduces disease and viral load in gut reservoir and blood after mucosal infection of macaques. Nat Med. 2001;7:1320–1326. doi: 10.1038/nm1201-1320. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov IM, et al. Impact of vaccine-induced mucosal high-avidity CD8+ CTLs in delay of AIDS viral dissemination from mucosa. Blood. 2006;107:3258–3264. doi: 10.1182/blood-2005-11-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wille-Reece U, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwissa M, et al. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J Exp Med. 2007;204:2733–2746. doi: 10.1084/jem.20071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Q, et al. Toll-like receptor ligands synergize through distinct dendritic cell pathways to induce T cell responses: Implications for vaccines. Proc Natl Acad Sci USA. 2008;105:16260–16265. doi: 10.1073/pnas.0805325105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Q, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh S, et al. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc Natl Acad Sci USA. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer JD, et al. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc Natl Acad Sci USA. 2007;104:18648–18653. doi: 10.1073/pnas.0709198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutzler MA, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8 T cells that are partially independent of CD4 T cell help. J Immunol. 2005;175:112–123. doi: 10.4049/jimmunol.175.1.112. [DOI] [PubMed] [Google Scholar]
- 15.Harris RS, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 16.Chiu YL, et al. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 17.Jin X, et al. APOBEC3G/CEM15 (hA3G) mRNA levels associate inversely with human immunodeficiency virus viremia. J Virol. 2005;79:11513–11516. doi: 10.1128/JVI.79.17.11513-11516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biasin M, et al. Apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G: A possible role in the resistance to HIV of HIV-exposed seronegative individuals. J Infect Dis. 2007;195:960–964. doi: 10.1086/511988. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, et al. Mucosal immunization in macaques upregulates the innate APOBEC 3G anti-viral factor in CD4(+) memory T cells. Vaccine. 2009;27:870–881. doi: 10.1016/j.vaccine.2008.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trapp S, et al. Double-stranded RNA analog poly(I:C) inhibits human immunodeficiency virus amplification in dendritic cells via type I interferon-mediated activation of APOBEC3G. J Virol. 2009;83:884–895. doi: 10.1128/JVI.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzuris JL, et al. Molecular determinants of peptide binding to two common Rhesus macaque major histocompatibility complex class II molecules. J Virol. 2001;75:10958–10968. doi: 10.1128/JVI.75.22.10958-10968.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaccari M, et al. Reduced protection from simian immunodeficiency virus SIVmac251 infection afforded by memory CD8+ T cells induced by vaccination during CD4+ T-cell deficiency. J Virol. 2008;82:9629–9638. doi: 10.1128/JVI.00893-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson LJ, et al. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol. 2004;78:2212–2221. doi: 10.1128/JVI.78.5.2212-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecchinato V, et al. Immune activation driven by CTLA-4 blockade augments viral replication at mucosal sites in simian immunodeficiency virus infection. J Immunol. 2008;180:5439–5447. doi: 10.4049/jimmunol.180.8.5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosati M, et al. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol. 2005;79:8480–8492. doi: 10.1128/JVI.79.13.8480-8492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosati M, et al. DNA vaccination in Rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci USA. 2009;106:15831–15836. doi: 10.1073/pnas.0902628106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, et al. Immune control of an SIV challenge by a T-cell-based vaccine in Rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yant LJ, et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol. 2006;80:5074–5077. doi: 10.1128/JVI.80.10.5074-5077.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abel K, et al. Anatomic site and immune function correlate with relative cytokine mRNA expression levels in lymphoid tissues of normal Rhesus macaques. Cytokine. 2001;16:191–204. doi: 10.1006/cyto.2001.0961. [DOI] [PubMed] [Google Scholar]
- 30.Mandl JN, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 31.Koning FA, et al. Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J Virol. 2009;83:9474–9485. doi: 10.1128/JVI.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreisberg JF, Yonemoto W, Greene WC. Endogenous factors enhance HIV infection of tissue naive CD4 T cells by stimulating high molecular mass APOBEC3G complex formation. J Exp Med. 2006;203:865–870. doi: 10.1084/jem.20051856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pion M, et al. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J Exp Med. 2006;203:2887–2893. doi: 10.1084/jem.20061519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stopak KS, Chiu YL, Kropp J, Grant RM, Greene WC. Distinct patterns of cytokine regulation of APOBEC3G expression and activity in primary lymphocytes, macrophages, and dendritic cells. J Biol Chem. 2007;282:3539–3546. doi: 10.1074/jbc.M610138200. [DOI] [PubMed] [Google Scholar]
- 35.Waldmann TA. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 36.Belyakov IM, Isakov DV, Zhu Q, Dzutsev AH, Berzofsky JA. A novel functional CTL avidity/activity compartmentalization to the site of mucosal immunization contributes to protection of macaques against SHIV viral depletion of mucosal CD4+ T cells. J Immunol. 2007;178:7211–7221. doi: 10.4049/jimmunol.178.11.7211. [DOI] [PubMed] [Google Scholar]
- 37.Kiepiela P, et al. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 38.Schellens IM, et al. Abundance of early functional HIV-specific CD8+ T cells does not predict AIDS-free survival time. PLoS ONE. 2008;3:e2745. doi: 10.1371/journal.pone.0002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDermott AB, et al. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive Rhesus macaques. J Virol. 2005;79:15556–15566. doi: 10.1128/JVI.79.24.15556-15566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McElrath MJ, et al. Step Study Protocol Team. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: A case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pido-Lopez J, et al. Stimulation of cell surface CCR5 and CD40 molecules by their ligands or by HSP70 up-regulates APOBEC3G expression in CD4(+) T cells and dendritic cells. J Immunol. 2007;178:1671–1679. doi: 10.4049/jimmunol.178.3.1671. [DOI] [PubMed] [Google Scholar]
- 42.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 43.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nat Protoc. 2006;1:1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.