Abstract
Mutations or deletions in PARKIN/PARK2, PINK1/PARK6, and DJ-1/PARK7 lead to autosomal recessive parkinsonism. In Drosophila, deletions in parkin and pink1 result in swollen and dysfunctional mitochondria in energy-demanding tissues. The relationship between DJ-1 and mitochondria, however, remains unclear. We now report that Drosophila and mouse mutants in DJ-1 show compromised mitochondrial function with age. Flies deleted for DJ-1 manifest similar defects as pink1 and parkin mutants: male sterility, shortened lifespan, and reduced climbing ability. We further found poorly coupled mitochondria in vitro and reduced ATP levels in fly and mouse DJ-1 mutants. Surprisingly, up-regulation of DJ-1 can ameliorate pink1, but not parkin, mutants in Drosophila; cysteine C104 (analogous to C106 in human) is critical for this rescue, implicating the oxidative functions of DJ-1 in this property. These results suggest that DJ-1 is important for proper mitochondrial function and acts downstream of, or in parallel to, pink1. These findings link DJ-1, pink1, and parkin to mitochondrial integrity and provide the foundation for therapeutics that link bioenergetics and parkinsonism.
Keywords: parkin, Parkinson's disease, oxidative stress, Drosophila
Parkinson’s disease (PD) is the most common movement disorder and the second most common neurodegenerative disease. Clinically, it is characterized by resting tremor, rigidity, bradykinesia, gait abnormality, and slow movement (1, 2). PD patients show severe dopaminergic neuron loss, resulting in a decrease of striatal dopamine levels responsible for the motor features (3, 4). Age is the most potent risk factor for PD, but other contributing factors include exposure to environmental toxins like paraquat and rotenone. Although predominantly idiopathic, genetic mutations account for ≈10% of cases (5). Studies of genes responsible for familial parkinsonism/PD are yielding critical insight into mechanisms shared by sporadic and familial disease.
Mutations in DJ-1/PARK7, PINK1/PARK6, and PARKIN/PARK2 lead to autosomal recessive parkinsonism. Properties of DJ-1 suggest that it may be at a compelling intersection for several risk factors in PD, including genetics, oxidative stress, environmental factors, and age. First, DJ-1 gene mutations lead to early-onset autosomal recessive parkinsonism (6). Second, the DJ-1 protein is sensitive to oxidative stress and may act as a redox-responsive molecular chaperone that can prevent protein misfolding (7). Third, tolerance toward paraquat in animals is mediated, in part, through modifications of DJ-1 protein (8). Finally, age induces the same modifications of the DJ-1 protein as environmental toxins (8). Mice mutant for DJ-1 show dopamine reuptake dysfunction (9) and have increased sensitivity to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) (10). Two DJ-1 orthologs (DJ-1a and DJ-1b) exist in Drosophila, and when deleted, flies have decreased climbing ability (11) and increased sensitivity to H2O2, paraquat, and rotenone (12). Taken together, these data indicate that the DJ-1 protein responds to key risk factors for PD, including age and toxins.
Parkin is an E3 ubiquitin ligase whose mutations account for the majority of autosomal recessive parkinsonism, and Parkin may be important for mitophagy (13, 14). The second most common cause of early-onset parkinsonism is mutations in PINK1, a kinase localized to mitochondria that may be involved in mitochondrial fission (15). In Drosophila, loss of function of either pink1 or parkin leads to male sterility and abnormal wing posture (16–18). Moreover, up-regulation of parkin rescues pink1 mutation, thus placing the two genes in the same genetic pathway, with parkin downstream of pink1 (17, 18). Electron microscopic analysis of pink1 and parkin null mutants shows swollen mitochondria in flight muscle and testes, suggesting that these genes are important for mitochondrial integrity. Interestingly, epidemiological and other studies have linked complex I inhibitors, such as rotenone, paraquat, and 1-methyl-4-phenylpridinium (MPTP metabolite), to parkinsonism in humans (19). Given that DJ-1 is also localized to mitochondria (20–22), and that mutations in DJ-1 lead to parkinsonism, these results raise the question of whether there are links between DJ-1 and mitochondrial function.
Here we demonstrate that flies deleted for DJ-1 share biological defects in common with flies lacking pink1 and parkin. We further show that up-regulation of the fly DJ-1 (DJ-1a or DJ-1b) or of human DJ-1 (hDJ-1) can rescue pink1 deficiency; moreover, rescue is dependent on C104 of DJ-1, implicating the oxidative functions of DJ-1 in the rescue. These studies suggest that DJ-1, pink1, and parkin function in common biological processes that are critical for mitochondrial function, such that compromise of their activity leads to human disease.
Results
DJ-1 Double-Mutant Flies Have a Shortened Lifespan and Reduced Climbing Ability.
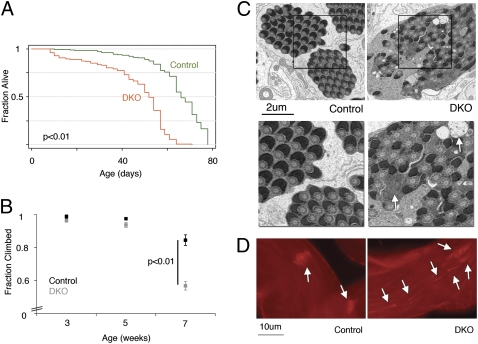
Several functions have been suggested for DJ-1a and DJ-1b in Drosophila, including possible compensatory roles for each other (23). Therefore, to better characterize the effects of DJ-1 loss, we generated flies mutant for both DJ-1a and DJ-1b genes (double knockout, DKO) in a homogenous background (Methods). We observed reduced lifespan of DKO flies compared with controls, and decreased spontaneous movement of the animals over time (median lifespan of 62 days for controls versus 52 days for DKO; P < 0.01) (Fig. 1A, Fig. S1). In testing locomotor activity, we found that by 7 weeks of age, DKO flies showed severe climbing defects compared with controls (only 56% of DKO flies compared with 90% of controls were able to climb; P < 0.01) (Fig. 1B), suggesting an age-dependent decline in motor activity in DKO flies.
Fig. 1.
DKO mutant flies show multiple defects with age. (A) Lifespan of control flies compared with DKO flies. More than 200 flies for each genotype were used (P < 0.001, log–rank test). (B) Climbing ability of control and DKO flies at different ages. The fraction of total flies that climbed out of the required distance in the given time is shown (mean ± SEM; n > 100 flies). P = 0.0004 (t test, two tailed) between control and DKO at 7 weeks. (C) Electron micrographic images of control and DKO spermatogenesis. The mitochondrial derivatives are regular and orderly in the control but are aberrant in DKO (white arrows). (Lower) Higher magnification of squared areas above. (D) Progression of sperm individualization, highlighted with phalloidin, which labels sperm head investment cones (white arrows), is orderly and compact in the control but disorganized in DKO testes (3-day males).
Defects in DKO Spermatogenesis.
During this characterization, we noted that DKO flies seemed to have reduced fertility. To test this directly, we set up single-pair matings of control or DKO male flies (<3 days old) with wild-type virgin females and scored successful matings by the presence of progeny pupal casings after 10 days. This assay revealed that whereas 96% of control male flies were fertile, DKO males had high infertility (26 of 27 control males compared with 0 of 26 DKO males tested were fertile; P = 2.8e-14, Fisher's exact test). In contrast, DJ-1a and DJ-1b single knockout flies were fertile (Table S1). The reverse crosses using virgin DKO mutant females also showed reduced fertility (48.6 ± 3.2 pupal casings per control female compared with 19.5 ± 4.8 for DKO female, mean ± SEM; P = 1.4e-5, Fisher's exact test). The infertility of DKO males prompted further investigation because of the striking similarity to mutants of pink1 and parkin, both of which are male sterile.
To analyze the infertility, we focused on spermatogenesis. DKO sperm appeared morphologically normal but were immotile (Movie S1 and Movie S2). Because dysfunctional mitochondria are the basis of infertility in pink1 and parkin mutants, we assessed the Nebenkern, the structure in the spermatozoa with fused mitochondria, in DKO spermatogenesis. Abnormal vacuoles were present in the Nebenkern of DKO mutants, similar to those observed in pink1 and parkin mutants. We then examined the structure of the mitochondria by electron microscopy and found profound abnormalities in mitochondrial derivatives in DKO sperm compared with control (Fig. 1C). The last step in spermatogenesis involves separation of individual spermatozoa from each other and can be visualized by the position of the investment cones. In contrast to the orderly wave of investment cone movement of control testes, DKO mutant testes showed misoriented and asynchronous progression (Fig. 1D). Taken together, these results suggest a disruption of mitochondrial-dependent events in DKO flies, akin to the defects of pink1 and parkin mutants.
Mitochondrial Respiration and ATP Production Are Defective in DKO Flies.
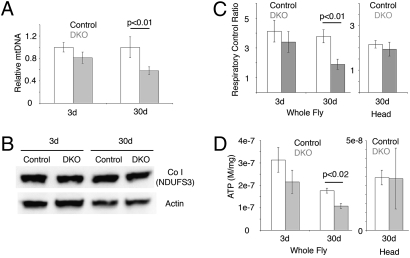
Mitochondrial dysfunction can be rooted in a change in the overall number of mitochondria, mtDNA level or integrity, and/or mitochondrial function. To assess the status of DKO mitochondria, we first determined the ratio of mtDNA to genomic DNA using real-time PCR. Whole-fly analysis revealed similar levels of mtDNA at 3 days but a consistent decrease of ≈40% in DKO at 30 days compared with controls (Fig. 2A). The mtDNA decrease, however, did not reflect a change in the protein levels of complex I subunit NDUFS3 (Fig. 2B). Because of a lack of antibodies against Drosophila mitochondrial subunits, other subunits were not tested. These findings suggest that DKO and control flies have comparable levels of mitochondria but that there is an age-dependent loss of mtDNA in DKO flies.
Fig. 2.
Mitochondrial dysfunction in DKO mutant flies. (A) The relative level of mtDNA in young (3 days) and old (30 days) control (white) and DKO (gray) thoraces (mean ± SEM, three DNA extractions for 3 days, four for 30 days). The ND5 locus was used to measure mtDNA levels, normalized to the nuclear let-7 locus. P = 0.0055 (t test, two tailed). (B) Western immunoblot for level of complex I subunit NDUFS3 (Upper) and actin shows similar levels in control and DKO with age. (C) Mitochondrial RCR of control (white) and DKO (gray). (Left) Whole flies (mean ± SEM, three preparations of mitochondria for 3 days, four for 30 days) (two-tailed t test, P = 0.005 between 30-day control and DKO samples). (Right) Heads (mean ± SEM, seven preparations of mitochondria). Mitochondrial RCR showed significance in aged whole fly and a modest but insignificant decrease in aged heads RCR (two-tailed t test, P = 0.096 between 30-day control and DKO). (D) ATP levels (mole/mg) of control (white) and DKO (gray) flies (mean ± SEM, three sample preparations) (two-tailed t test, P = 0.011 between 30-day control and DKO samples from whole flies).
We then assessed mitochondrial transmembrane potential via tetramethylrhodamine ethylester (TMRE), a membrane-permeate fluorescent probe. When a mitochondrial membrane potential is generated by substrates, TMRE fluorescence decreases owing to sequestration of the probe in the mitochondrial matrix. Conversely, in the presence of carbonyl cyanide m-chlorophenyl hydrazone, an uncoupler, the cross-membrane potential is dissipated and total fluorescence increases. Using this approach, we found that DKO mitochondria were capable of generating and maintaining cross-membrane potential with either complex I or II substrates (Fig. S2).
We next examined mitochondrial respiratory and phosphorylating activities through polarography. Rates of ADP-stimulated and ADP-depleted oxygen consumption (state 3 and 4, respectively) were measured using the complex I substrate pair pyruvate plus malate and substrate II substrate succinate. The respiratory control ratio (RCR; the state 3/state 4 ratio) reflects the degree of coupling between substrate oxidation and ADP phosphorylation and is a way to assess the physical and functional integrity of mitochondria. Mitochondria from 3-day control and DKO flies had comparable RCR. In 30-day flies, state 3 respiratory rates were similar in control and DKO mitochondria; however, state 4 respiration was higher in DKO mitochondria, leading to a significant reduction of RCR in DKO compared with controls (1.9 ± 0.3 compared with 3.8 ± 0.4; P = 0.0005) (Fig. 2C). These results suggest a decline in mitochondrial function in DKO flies as they age. Consistent with the RCR results, we observed a decrease of ≈40% in total ATP in aged DKO flies (Fig. 2D).
To determine whether similar changes could be detected in the brain, we performed the same assays on mitochondria from 30-day DKO and control heads. Mitochondria from DKO mutant heads had lower, although not statistically significant, RCR compared with controls (Fig. 2 C and D). Taken together, these data indicate that mitochondrial function in DKO mutant animals become severely compromised with age.
DJ-1 Mutant Mice Have Mitochondrial Dysfunction.
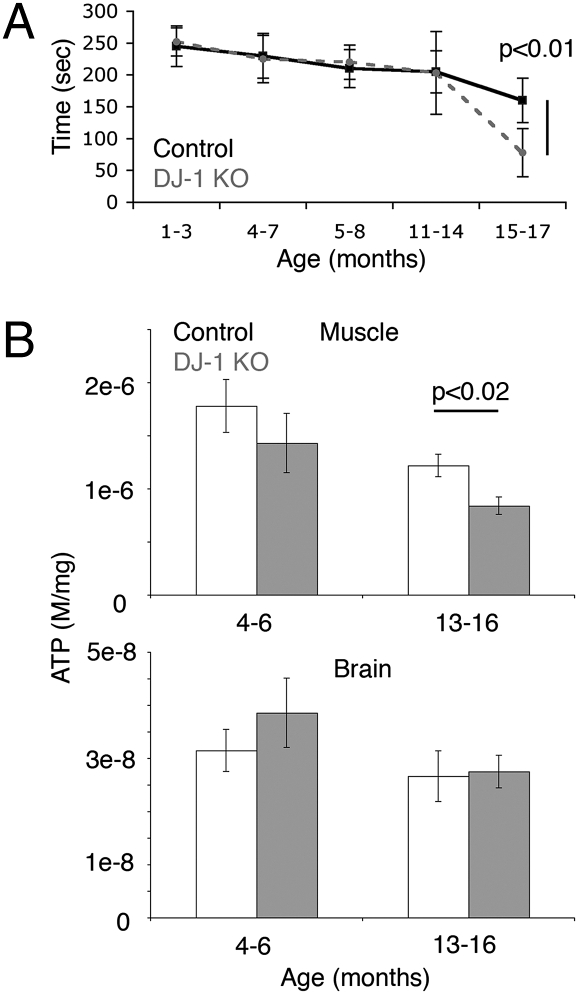
To determine whether mammalian DJ-1 mutants also exhibited mitochondrial defects, we examined a DJ-1 knockout mouse line that was generated by exonic trapping of the murine DJ-1 gene. These studies revealed that DJ-1 mutant mice showed an age-dependent decline by rotorod assay (Fig. 3A). This age-dependent movement impairment parallels the decreased climbing ability of DKO flies (Fig. 1B).
Fig. 3.
DJ-1 KO mice show motor and mitochondrial dysfunction. (A) Rotorod test of control (solid) and DJ-1 KO (dashed) animals of different ages, showing a significant (P < 0.01) difference by 15 months (mean ± SD, n = 6–10 animals per time point). (B, Upper) ATP levels (moles/mg) in hindlimb skeletal muscle (a mixture of soleus and gastrocnemius muscle) of control (white) and DJ-1 null (gray) mice (mean ± SEM, n = 6 animals) (two-tailed t test, P = 0.017 between 14-month control and KO samples). (Lower) ATP levels from brain extracts (mean ± SEM, n = 6 animals).
We then asked whether DJ-1 mutant mice have mitochondrial dysfunction; in these studies, we focused on skeletal muscle, given the motility defects. To assess overall skeletal muscle mitochondrial function, mitochondria were isolated from a mixture of quadriceps and calf muscle, including both fast- and slow-twitch fibers. The aged (16 month) DJ-1 mutant mice had a slightly lower RCR than controls (3.7 ± 0.3 vs. 4.3 ± 0.5, respectively, mean ± SEM). Moreover, we found that ATP levels of skeletal muscle from 13–16-old month DJ-1 knockout mice were lower compared with aged-matched controls (Fig. 3B). Younger mice (4–6 months) showed no significant difference in ATP levels, and we did not observe a change in overall brain ATP levels between control and DJ-1 mutant mice in either young or aged animals (Fig. 3B). These data indicate that age-accentuated mitochondrial defects were observed in both fly and mouse DJ-1 mutants.
DJ-1 Can Rescue pink1 Drosophila Mutants.
Mitochondrial dysfunction has been well documented in the pink1/parkin pathway in Drosophila (17, 18). Given that the effects in DKO flies were similar to those of parkin and pink1 mutants, we tested whether DJ-1 genetically interacted with the pink1/parkin pathway. To do this, we used the GAL4-UAS system to up-regulate DJ-1a, DJ-1b, or hDJ-1 to assess whether DJ-1 can modify pink1 mutant effects. Wings in pink and parkin mutants are held up owing to flight muscle mitochondrial defects, with 6-day pink1 mutants showing ≈30% normal wing posture at 25 °C; this wing posture defect was rescued by either pink1 or parkin expression, as previously shown (Table 1) (17, 18).
Table 1.
Rescue of pink1 loss of function wing posture by DJ-1
| Genotype* | Rescue % (fraction)† |
| pink1B9;;da-GAL4/+ | 29.4 (20/68) |
| pink1B9;UAS-pink1/+;da-GAL4/+ | 64.7 (11/17) |
| pink1B9;UAS-parkinC2;da-GAL4 | 97.6 (41/42) |
| pink1B9;UAS-sDJ-1b/+;da-GAL4/+ | 52.8 (19/36) |
| pink1B9;UAS-DJ-1a;da-GAL4/+ | 57.4 (35/61) |
| pink1B9;UAS-hDJ-1;da-GAL4/+ | 57.9 (22/38) |
*pink1B9 denotes a deletion in pink1 gene, parkinC2 is wild-type parkin, sDJ-1b is wild-type DJ-1b.
†Male fly wing posture was scored in 6-day adult flies at 25 °C, with the number of flies rescued/total scored.
Strikingly, up-regulation of either DJ-1 ortholog rescued the pink1 mutant wing posture to the same degree as up-regulation of pink1 (Table 1). This result was confirmed with independent transgenic lines of DJ-1a, DJ-1b, and hDJ-1. We did not, however, observe rescue with every transgenic line tested; further analysis suggested an important role of expression level of the DJ-1 transgene, with too-high expression of DJ-1 being deleterious (Discussion). C104 of DJ-1b (C106 in hDJ-1) is oxidized in aged animals and in animals treated with oxidative stress and has been found to be critical for DJ-1b function in response to oxidative stress (8). Mutating C104 to Ala in DJ-1b abrogated rescue of the pink1B9 wing postural defect, whereas mutation of C45 to Ala, a second site capable of being oxidized, retained normal function (Table S2). Moreover, DJ-1b C45A mutants were able to ameliorate both structural defects and TUNEL signals seen in the thorax of pink1B9 flies (Table S2). These data suggest that C104 is critical for DJ-1b function to both rescue and protect against oxidative stress in pink1 mutants.
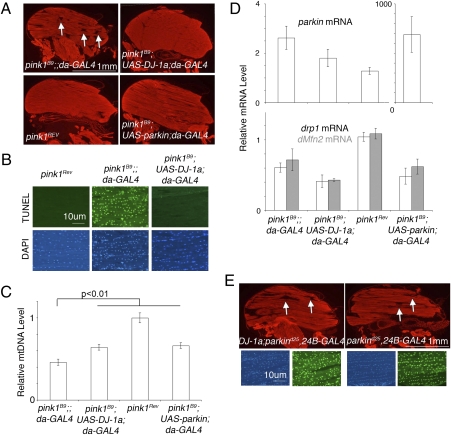
To determine whether rescue by DJ-1 was restricted to the wing posture effect, we examined mtDNA levels and apoptosis in the thorax (17, 18). Consistent with wing posture rescue, up-regulation of DJ-1a also restored thoracic muscle morphology in the pink1 mutant (Fig. 4A), rescued muscle apoptosis (Fig. 4B and Fig. S3) and mtDNA levels (Fig. 4C), comparable to parkin rescue. pink1Rev is the wild-type revertent of the pink1B9 mutant in the same genetic background. To address the specificity of DJ-1 rescue of pink1, we determined that DJ-1 up-regulation failed to rescue polyglutamine toxicity and Hid-induced apoptosis in the fly eye (Fig. S4). These results confirmed that DJ-1 interacts with the Pink1 pathway and does not generally mitigate deleterious effects in flies. Thus, up-regulation of DJ-1 can rescue pink1 mutants in a manner comparable to parkin, placing DJ-1 either downstream of pink1 or in a parallel pathway that converges with the pink1/parkin pathway (Fig. S5).
Fig. 4.
Up-regulation of DJ-1 rescues Pink1 muscle defects. (A) Cryosections of the thorax stained with phalloidin to highlight muscle structure of young (<3 days) male flies. Visible vacuoles (arrows) are observed in the pink1 deleted mutant, pink1B9. Both DJ-1a and parkin up-regulation ameliorate the defects; compare with the pink1Rev control, a normal revertant of the original pink1B9 mutation. (B) Widespread apoptosis revealed by TUNEL staining (Upper) in thorax sections of pink1B9 flies is rescued by DJ-1a up-regulation. (C) Relative mtDNA levels from male thoraces was assayed by real-time PCR and adjusted to the levels in the control pink1Rev (mean ± SEM, four to six independent experiments). The mtDNA level was rescued by DJ-1a and parkin up-regulation (two-tailed t test, P = 0.004 between pink1 with driver and pink1 with parkin up-regulated samples, P = 0.006 between pink1 with driver and pink1 with DJ-1a up-regulated samples. A p value of <0.0167 was considered significant after correction for multiple tests). (D) Real-time PCR assays for levels of parkin (Upper), drp1 (white, Lower), and dMfn2 (gray, Lower) mRNA in young (<5 days) male flies (mean ± SEM, three to four independent experiments). The RNA levels were adjusted to pink1Rev levels. Flies overexpressing parkin had dramatically higher (≈650×) parkin mRNA levels, whereas up-regulation of DJ-1a did not change parkin mRNA levels. DJ-1a up-regulation did not change the mRNA levels of drp1 or dMfn2. (E) Up-regulation of DJ-1 does not rescue parkin mutation. Cryosections of thoraces stained with phalloidin (red) for muscle structure (white arrows, vacuoles highlighted deteriorated structure). TUNEL for apopotosis (green) and DAPI (blue). Vacuoles and TUNEL signal of parkin deleted mutant, parkind25, was unchanged with up-regulation of DJ-1a.
Genetic Pathway of DJ-1.
To further address the mechanism of rescue, we tested whether DJ-1 up-regulation induced a change in the expression level of parkin, which rescues pink1 mutants when up-regulated (17, 18). We measured parkin mRNA levels in pink1 mutants, and in pink1 mutants with DJ-1a up-regulation; these studies showed that parkin levels did not increase in DJ-1 rescued animals (Fig. 4D). Changes in expression of genes that modulate mitochondrial fusion/fission (drp1 and dMfn2/marf) have also been shown to rescue pink1 (24, 25). The transcripts level of these genes did not change upon up-regulation of DJ-1a in the pink1 mutant background (Fig. 4D). In addition, DKO flies and in DJ-1a up-regulated flies both showed normal mitochondrial morphology, suggesting that DJ-1 is not important for mitochondrial fission or fusion (Fig. S6). These results indicate that the mechanism of DJ-1 rescue of pink1 is not through changes in the mRNA levels of parkin, drp1, or dMfn2. We were unable to assess the protein levels owing to a lack of antibodies against these proteins in flies.
These data suggest that DJ-1 is downstream of pink1 and does not affect drp1, dMfn2, or parkin gene levels. To further define the placement of DJ-1 activity relative to that of pink1, we tested whether DJ-1 could also rescue parkin mutants. DJ-1a up-regulation failed to rescue the wing posture defect, thoracic muscle morphology, and TUNEL-positive signal in parkin mutant flies (Fig. 4E). Thus, although DJ-1 can rescue pink1 loss of function, DJ-1 cannot rescue parkin mutation. We then determined whether parkin up-regulation could rescue DJ-1 loss of function. Strikingly, we found that up-regulation of either parkin or pink1 in DJ-1 DKO background was lethal (Table 2). Taken together, these data place DJ-1 in a pathway parallel to that of pink1/parkin and show that both of these pathways, and their carefully balanced activity, are critical for mitochondrial function (Fig. S5).
Table 2.
Up-regulation of parkin and pink1 in the DKO mutant background
| Genotype | Adult viability* |
| UAS-parkin;;da-GAL4†/+ | Viable |
| UAS-pink1;;da-GAL4/+ | Viable |
| UAS-parkin;d72‡;da-GAL4 d93§/d93 | Lethal |
| UAS-pink1;d72;da-GAL4 d93/d93 | Lethal |
*Flies were scored for viability 14d after the cross at 25 °C.
†da-GAL4 drives expression ubiquitously.
‡d72 denotes DJ-1a deletion allele.
§d93 denotes DJ-1b deletion allele.
Discussion
Mutations in DJ-1/PARK7, Parkin/PARK2, and PINK1/PARK6 lead to early-onset, autosomal recessive parkinsonism. Studies in Drosophila indicate that pink1 and parkin are genetically in the same pathway, which impacts mitochondrial function (17, 18). The link between DJ-1 and mitochondria, however, has been circumstantial and limited to cell culture studies (20, 21). Here we demonstrate that DJ-1 loss of function leads to mitochondrial dysfunction in an age-dependent manner in both fly and mouse. We further provide genetic evidence that DJ-1 interacts with the pink1/parkin pathway, because DJ-1 up-regulation can compensate for reduction of pink1 activity. Consistent with important interactions between DJ-1 and pink1/parkin, up-regulation of either pink1 or parkin is deleterious in a DJ-1 mutant background. These findings indicate that DJ-1 and pink1/parkin fall into two parallel pathways whose function critically impacts mitochondrial activity (Fig. S5).
Age-Dependent Mitochondrial Dysfunction in DJ-1 DKO Flies.
DKO mutant flies showed several classic defects that reflect mitochondrial dysfunction. First, DKO flies have an overall reduced fitness (shortened lifespan), consistent with mitochondrial involvement in reduced lifespan and accelerated aging in worms, flies, and mice (26). Second, DKO flies have defects in spermatogenesis reflective of disrupted mitochondrial function. In the fly, mitochondrial mutants are often infertile because germ cell maturation requires both energy and mitochondrial morphological changes (27). Similarly, DJ-1 protein levels correlate with infertility in pharmacologically treated male rats that leads to reduced glycolytic enzyme activities in the sperm (28–31). Third, DKO flies showed age-dependent declines in both climbing activity and ATP level. The age-dependent onset of these deficiencies is consistent with the notion that nonlethal mitochondrial mutants can compensate for mitochondrial inefficiency until older ages, when more pressure is exerted on the system. Our data show that DJ-1 DKO mitochondria have a lower RCR, suggesting that the mitochondria can function, albeit at lower capacity and with lower reserve capacity. Over time, DKO cells may no longer effectively compensate, and defects manifest. These data indicate that DJ-1 activity is important for proper mitochondrial function over time.
Mitochondrial Dysfunction in DJ-1 Null Mice.
As in fly DJ-1 mutants, we observed mitochondrial dysfunction in DJ-1 knockout mice skeletal muscle. Although we did not see dysfunction in mitochondria isolated from mouse brain, muscle is one of the high energy demanding tissues. These data agree with previous findings that DJ-1 knockout mice do not have a significant change in lifespan or reduction in dopamine levels with age (32). The age-dependent reduction in rotorod endurance in DJ-1 knockout mice is consistent with findings showing specific age-dependent impairments in endurance in DJ-1 knockout mice (33). Interestingly, Chandran et al. (33) did not report a change in neuromuscular junction or muscle by histology in DJ-1 knockout mice. The changes in mitochondrial function and efficiency we observed can explain the loss of endurance. Alteration in muscle-related activities was not observed in another study of DJ-1 null mice, suggesting that genetic background may affect the presentation of this impairment (34). It is also intriguing that murine muscle seems to be more affected than brain, in that ATP reductions were detected in mouse muscle but not brain (Fig. 3). It is possible that, unlike humans, the energy demand of murine muscle, like that of Drosophila, is higher than that of brain, thus the consequence of mitochondrial dysfunction in the absence of DJ-1 manifests first in the muscle. Taken together, we suggest that loss of DJ-1 activity may lead to age-dependent mitochondrial dysfunction in tissues with high energy demands.
Common Pathway for pink1, parkin, and DJ-1.
We present evidence that DJ-1 loss of function causes similar mitochondrial defects in aged Drosophila. Moreover, we also show that DJ-1 can rescue pink1, although not parkin, loss (Fig. 4). DJ-1a does not ameliorate all pink1 defects: DJ-1a could not rescue the pink1 mutant infertility (0 of 40 male pink1 with DJ-1a up-regulated were fertile). The selective rescue of pink1 mutants by DJ-1 places DJ-1 downstream of pink1. The findings that DJ-1 cannot rescue parkin mutants, and that parkin cannot rescue DKO mutants, suggest that DJ-1 may not be directly downstream of pink1 (neither in between pink1 and parkin nor downstream of parkin). We propose that DJ-1 defines a pathway parallel to that of pink1/parkin. It is likely that there is partial convergence/overlap downstream between the pathways, given the common effects. We cannot, however, rule out the possibility that DJ-1 selectively rescues a target downstream of pink1 pathway that is responsible for the effects on thoracic mitochondria. It is interesting that Omi/HtrA2 has also been suggested to partially rescue pink1 mutants in Drosophila in a pathway that is similarly independent of parkin (35).
Our data indicate a critical balance of activities between the DJ-1 and pink1/parkin pathways, because up-regulation of either pink1 or parkin leads to lethality in DKO flies. In accord to the finding that up-regulation of pink1 leads to a deleterious Drosophila eye effect in a wild-type background (24), our data further suggest that in DKO, a background sensitized for mitochondrial dysfunction, an increase in pink1 levels simply causes lethality. The ability of DJ-1 to respond to oxidative stress through C104 modification or as an atypical peroxidase (8, 32) fits nicely with the hypothesis that DJ-1 is important for proper mitochondrial function. However, it should be noted that C104 might sense oxidative stress independent of the proposed peroxidase function. As such, when DJ-1 activity is compromised, cells accumulate more oxidative stress and mitochondrial dysfunction with age. pink1 deletion may also lead to increased oxidative stress that can be partially reduced through up-regulation of DJ-1. In line with this, when we mitigated the ability of DJ-1b to respond to oxidative stress by mutation of C104, but not C45, rescue of pink1B9 wing posture was abrogated. Interestingly, DJ-1b with either C104A or C45A also failed to rescue pink1B9 fertility phenotype (0 of 15 fertile for DJ-1b C104A and 0 of 10 for DJ-1b C45A). Taken together, these data argue that DJ-1 rescue of pink1B9 mutants requires its ability to respond and protect against oxidative stress in specific tissues.
Although previous studies suggest that DJ-1 cannot rescue pink1 mutants (36), several important differences exist between those data and our findings: first, the method of pink1 reduction (RNAi vs. gene deletion in the present study); and second, the method by which DJ-1 was up-regulated (muscle-specific vs. ubiquitous driver in the present study). Moreover, when assessing rescue, we used a quantitative measurement because the wing effect was not 100% penetrant in pink1B9 mutants. This approach allowed assessment of genes that partially rescue pink1 mutants. Additionally, we found that the expression level of DJ-1 transgenes seems to be critical for rescue, with higher levels of expression being less likely to rescue. These findings indicate that DJ-1 rescues the pink1 mutant in an expression level–dependent manner. Understanding the detailed mechanism by which DJ-1 rescue of pink1 is so critically dose dependent may provide key insight into the intersection of the pathways.
The critical role of mitochondria in neurodegenerative diseases is recognized (37–39). Our findings strongly indicate that mitochondrial dysfunction plays an important role in inherited forms of early-onset parkinsonism, as well as in sporadic disease, which may involve similar genetic players and pathways. These findings highlight the value of defining when mitochondrial function decline occurs relative to disease onset, and the central role of mitochondria as a target in PD therapeutic research.
Methods
Drosophila Assays.
DJ-1a and DJ-1b mutations were generated previously (12). pink1 and parkin mutant flies are described elsewhere (16, 18); the control pink1Rev is a precise excision of the P-element insertion that was used to generate the pink1B9 allele, and is wild type. For lifespan, flies <3 days old were collected and kept together for 3 days before separation according to sex and transferred every 2 to 3 days, with the number of dead flies recorded. Negative geotaxis was done as previously described (40). To test fertility, the number of pupae casings was counted 10 days after single 3-day male and virgin flies were crossed. For investment cone staining, testes were dissected in PBS and fixed in 4% paraformaldehyde, washed in PBS, and permeabilized with 0.3% Triton/PBS followed by PBS washes. Testes were then stained with phalloidin Alexa633 (Invitrogen) and washed in PBS. For wing posture, 3-day males were place with virgin flies and scored 6 days later.
Statistics.
Mean ± SEM are shown on all graphs. Log–rank test was performed on lifespan analysis. Male fertility was performed on a two-tailed Fisher's exact test. For other analysis, Student's t test, two tailed, was performed with Bonferroni-corrected α level. SI Methods provides complete information on materials and methods.
Supplementary Material
Acknowledgments
We thank L. Pallanck, B. Lu, and J. Chung for reagents; Drs. S. DiNardo, M. Selak, R. DeBerardinis, and J. Cross for technical assistance; the University of Pennsylvania Imaging Core for electron microscopy images; and Drs. R. Wilson and M. Selak and the Bonini laboratory for discussion and critical reading of the manuscript. This work was supported by a Developmental Biology Training Grant (to L.-Y.H.) and National Institutes of Health Grant P01-AG09215 (to B.I.G. and N.M.B.). N.M.B. is a Howard Hughes Medical Institute Investigator.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0911175107/-/DCSupplemental.
References
- 1.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Forman MS, Lee VM, Trojanowski JQ. Nosology of Parkinson's disease: Looking for the way out of a quagmire. Neuron. 2005;47:479–482. doi: 10.1016/j.neuron.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Pakkenberg B, Møller A, Gundersen HJ, Mouritzen Dam A, Pakkenberg H. The absolute number of nerve cells in substantia nigra in normal subjects and in patients with Parkinson's disease estimated with an unbiased stereological method. J Neurol Neurosurg Psychiatry. 1991;54:30–33. doi: 10.1136/jnnp.54.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 5.Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S16–S23. doi: 10.1002/ana.10487. discussion S23–S15. [DOI] [PubMed] [Google Scholar]
- 6.Bonifati V, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 7.Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meulener MC, et al. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci USA. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg MS, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Kim RH, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J, et al. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Meulener M, et al. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr Biol. 2005;15:1572–1577. doi: 10.1016/j.cub.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 13.Shimura H, et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 14.Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5:706–708. doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- 15.Valente EM, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 16.Greene JC, et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark IE, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 18.Park J, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 19.Moore DJ, West AB, Dawson VL, Dawson TM. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci. 2005;28:57–87. doi: 10.1146/annurev.neuro.28.061604.135718. [DOI] [PubMed] [Google Scholar]
- 20.Canet-Avilés RM, et al. The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, et al. Mitochondrial localization of the Parkinson's disease related protein DJ-1: Implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 22.Pagliarini DJ, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menzies FM, Yenisetti SC, Min KT. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Poole AC, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci USA. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- 28.Wagenfeld A, Yeung CH, Strupat K, Cooper TG. Shedding of a rat epididymal sperm protein associated with infertility induced by ornidazole and alpha-chlorohydrin. Biol Reprod. 1998;58:1257–1265. doi: 10.1095/biolreprod58.5.1257. [DOI] [PubMed] [Google Scholar]
- 29.Welch JE, Barbee RR, Roberts NL, Suarez JD, Klinefelter GR. SP22: A novel fertility protein from a highly conserved gene family. J Androl. 1998;19:385–393. [PubMed] [Google Scholar]
- 30.Whyard TC, Cheung W, Sheynkin Y, Waltzer WC, Hod Y. Identification of RS as a flagellar and head sperm protein. Mol Reprod Dev. 2000;55:189–196. doi: 10.1002/(SICI)1098-2795(200002)55:2<189::AID-MRD8>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 31.Oberländer G, Yeung CH, Cooper TG. Influence of oral administration of ornidazole on capacitation and the activity of some glycolytic enzymes of rat spermatozoa. J Reprod Fertil. 1996;106:231–239. doi: 10.1530/jrf.0.1060231. [DOI] [PubMed] [Google Scholar]
- 32.Andres-Mateos E, et al. DJ-1 gene deletion reveals that DJ-1 is an atypical peroxiredoxin-like peroxidase. Proc Natl Acad Sci USA. 2007;104:14807–14812. doi: 10.1073/pnas.0703219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandran JS, et al. Progressive behavioral deficits in DJ-1-deficient mice are associated with normal nigrostriatal function. Neurobiol Dis. 2008;29:505–514. doi: 10.1016/j.nbd.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno-Loshuertos R, et al. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat Genet. 2006;38:1261–1268. doi: 10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- 35.Tain LS, et al. Drosophila HtrA2 is dispensable for apoptosis but acts downstream of PINK1 independently from Parkin. Cell Death Differ. 2009;16:1118–1125. doi: 10.1038/cdd.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, et al. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci USA. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Outeiro TF, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 39.Schapira AH, et al. Mitochondrial complex I deficiency in Parkinson's disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 40.Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.