Abstract
Paleontological work carried out over the last 3 decades has established that three major primate groups were present in the Eocene of Africa—anthropoids, adapiforms, and advanced strepsirrhines. Here we describe isolated teeth of a previously undocumented primate from the earliest late Eocene (≈37 Ma) of northern Egypt, Nosmips aenigmaticus, whose phylogenetic placement within Primates is unclear. Nosmips is smaller than the sympatric adapiform Afradapis but is considerably larger than other primate taxa known from the same paleocommunity. The species bears an odd mosaic of dental features, combining enlarged, elongate, and molariform premolars with simple upper molars that lack hypocones. Phylogenetic analysis across a series of different assumption sets variously places Nosmips as a stem anthropoid, a nonadapiform stem strepsirrhine, or even among adapiforms. This phylogenetic instability suggests to us that Nosmips likely represents a highly specialized member of a previously undocumented, and presumably quite ancient, endemic African primate lineage, the subordinal affinities of which have been obscured by its striking dental autapomorphies. Discriminant functions based on measurements of lower molar size and topography reliably classify extant prosimian primates into their correct dietary groups and identify Nosmips and Afradapis as omnivores and folivores, respectively. Although Nosmips currently defies classification, this strange and unexpected fossil primate nevertheless provides additional evidence for high primate diversity in northern Africa ≈37 million years ago and further underscores the fact that our understanding of early primate evolution on that continent remains highly incomplete.
Keywords: Africa, Anthropoidea, Fayum, phylogeny, Strepsirrhini
Despite significant recent discoveries of fossil primates from the Eocene of Algeria (1) and Egypt (2–4), Africa's role in the early evolution and diversification of primates remains mysterious. The oldest candidate crown primate, latest Paleocene Altiatlasius, is from Africa (3, 5–7), but there is limited evidence for primate diversity from that continent's few early and middle Eocene vertebrate fossil localities. Early or middle Eocene Algeripithecus, a primate that was widely considered to be an anthropoid on the basis of limited and fragmentary dental remains (3, 7–10), has recently been reinterpreted as a stem strepsirrhine on the basis of more complete material and placed in the family Azibiidae along with the sympatric genus Azibius; the previously named genera Tabelia and Dralestes are now considered to be invalid (1). In addition to azibiids, other primates from this poorly sampled interval in Africa include the stem strepsirrhine Djebelemur (1, 2, 11–13), one lower molar of an unnamed adapiform (14), and two tooth fragments of an unnamed haplorhine (15).
Recent work at the ≈37 Ma Birket Qarun Locality 2 (BQ-2), in the Fayum Depression of northern Egypt, has nevertheless revealed that by the beginning of the late Eocene, primate communities in northern Africa were characterized by high diversity and morphological disparity. Thus far BQ-2 has yielded remains of djebelemurines, large-bodied caenopithecine adapiforms such as Afradapis (4), the stem galagid Saharagalago, the possible crown strepsirrhine Karanisia (2), and small anthropoids such as Biretia (3). Djebelemurines and crown strepsirrhines are closely related and most parsimoniously interpreted as descendants from an ancient African ancestor (1, 2, 12, 13), whereas caenopithecines are likely derived from an independent colonization from Europe or Asia. The timing of anthropoids’ arrival in Afro-Arabia is unclear, but the presence of multiple anthropoid species at BQ-2 supports a colonization in the late middle Eocene or earlier.
Here we describe a rare and enigmatic primate species from BQ-2 that, unlike other primates from the Eocene of Africa, does not fit unambiguously into either Anthropoidea or Strepsirrhini on the basis of the limited dental evidence available. The absence of clear relatives on northern continents suggests that this genus might be a highly specialized member of an ancient and previously undocumented clade of endemic African primates.
Results
Systematic Paleontology.
Placentalia Owen, 1837; Order Primates Linneaus, 1758; Nosmips, new genus.
Type species.
Nosmips aenigmaticus, new genus and species.
Etymology.
Anagram of Simpson, in honor of the late vertebrate paleontologist and anagram enthusiast George Gaylord Simpson (b. 1902 – d. 1984).
Distribution.
Earliest late Eocene (earliest Priabonian) of northern Egypt.
Diagnosis.
Differs from Eocene-Oligocene anthropoids in exhibiting the following combination of features: (i) relatively large and mesiodistally elongate P3 with a long and curving paracristid, a distinct protocristid, and a large metaconid cusp; (ii) mesiodistally elongate and buccolingually narrow trigonid basin on M1 bearing an internalized metaconid cusp; (iii) well-developed paracristids that surround the trigonid basin on M1–3; (iv) low and crestiform entoconid cusps on M1–3; (v) small and centrally placed hypoconulid cusp on M2; (vi) weak lingual cingulum and no hypocone on M2?; (vii) well-developed buccal cingulum on M2?. Differs from Eocene strepsirrhines, including adapiforms, in exhibiting features i, ii, iv, and v listed above as well as the following: mesiodistally oriented oblique cristids that meet the protoconids on M1–3; relatively broad talonid basins on M1–3; and metaconid cusps that are relatively mesial in placement on M1–3. In strong contrast to Nosmips, African Eocene strepsirrhines such as djebelemurines, Karanisia, Plesiopithecus, and Wadilemur all lack metaconids and have small and poorly developed talonids on P3–4; these taxa also have relatively restricted trigonid basins on M1–2.
Nosmips aenigmaticus, New Species.
Etymology.
Specific epithet is from aenigmaticus, Latin for enigmatic, obscure, or puzzling.
Holotype.
CGM 66002, a left lower fourth premolar (Fig. 1 I and L and Fig. S1).
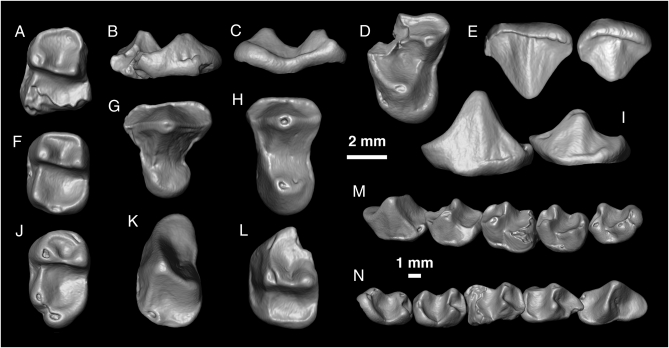
Fig. 1.
Isolated teeth of Nosmips aenigmaticus, gen. et sp. nov., from the earliest late Eocene Locality BQ-2 in northern Egypt. (A) DPC 22442M, left M1 in occlusal view; (B) DPC 21759D, left M2? in mesial view; (C) DPC 21759D, left M2? in distal view; (D) DPC 21759D, left M2? in occlusal view; (E) Left: DPC 21747M, right P3 (reversed); (Right) DPC 21250F, left P4, in buccal view; (F) DPC 21366G, right M2 (reversed), in occlusal view; (G) DPC 21747M, right P3 (reversed) in occlusal view; (H) DPC 21250F, left P4, in occlusal view; (I) (Left) DPC 21359G, left P3 (reversed); (Right) CGM 66002, holotype left P4, in buccal view; (J) DPC 21370K, left M3, in occlusal view; (K) DPC 21359G, left P3 (reversed), in occlusal view; (L) CGM 66002, holotype left P4, in occlusal view; (M) composite lower tooth row (P3–M3) in buccal view; (N) composite lower tooth row (P3–M3) in lingual view.
Type locality.
BQ-2, Fayum, Egypt.
Formation and age.
Umm Rigl Member of Birket Qarun Formation, earliest Priabonian in age (≈37 Ma).
Diagnosis.
As for genus.
Description.
Nosmips aenigmaticus is represented by a small sample of isolated upper and lower molars and premolars including P3–M3, P3–4, and a possible M2 (Fig. 1). After eight field seasons of intensive excavation at BQ-2, the primate fauna from the locality is now well known, and it is clear that the premolar teeth described here are, despite their large size relative to the teeth interpreted as lower molars of Nosmips, nevertheless almost certainly attributable to this genus. The only other mammals known from BQ-2 that are in roughly the same size range are creodonts and anomaluroid rodents, both of which are radically different from Nosmips in dental morphology.
The P3 of N. aenigmaticus (Fig. 1K) is peculiar in being tall and very long relative to maximum width, and in having both a large, lingually placed metaconid cusp that is connected to the protoconid by a tall protocristid and an elongate trigonid that is bounded buccally by a long and gently curving paracristid. The talonid is very short, bordered lingually by only a weak ridge, and bordered buccally by a distinct postprotocristid. The long axis of the distal root's cross-section is oriented obliquely with respect to the long axis of the crown (Fig. S3). As with P3, the P4 (Fig. 1L and Fig. S1) also has an elongate trigonid and a gently curving paracristid, but the P4 metaconid is approximately equal in size to the protoconid. The talonid basin on P4 is broad but mesiodistally short and surrounded distally by a weak hypocristid; there is no distinct entoconid cusp, but a weak ridge, continuous with the hypocristid, encloses the lingual side of the talonid. As with P3, the postprotocristid is distinct. The morphology of the mesial interstitial wear facet on P4 suggests that that the trigonid basin of that tooth slightly overlapped the P3 talonid, reducing the occlusal area available for the P3. Both P3 and P4 have very weak and discontinuous buccal cingulids.
A relatively large molariform tooth with a damaged talonid (DPC 22442M; Fig. 1A and Fig. S4) is here interpreted as an M1. When compared with other living and extinct primates, the tooth is very odd in having an elongate trigonid that is completely surrounded mesially by a distinct and slightly mesiolingually oriented crest that is continuous lingually with the mesial face of the metaconid, the distal end of which could be considered a short premetacristid. Other lower molars in the hypodigm that lack this crest and that lack a projecting hypoconulid lobe are considered to be M2s (Fig. 1F); both M2 and M3 have relatively short and restricted trigonid basins. There is no hint of a paraconid cusp on any of the lower molars. On M1 the metaconid is tall, and its lingual face inclines buccally, giving a somewhat pyramidal shape to that cusp. The cristid obliqua meets the posterior wall of the trigonid low and just distal to the protoconid on all lower molars, and the hypoflexid is accordingly very restricted. The entoconid is crestiform on all of the lower molars, and the lingual halves of the talonids are very low relative to the tall buccal borders, the latter of which are formed by distinct hypoconids and well-developed oblique cristids. The hypoconulid region is not preserved on M1 owing to damage, but on M2 that cusp is very small and located approximately midway between the hypoconid and entoconid. The M3 (Fig. 1J) has a distinct hypoconulid that is centrally or slightly buccally placed and projects distally as a small lobe, leaving a clear notch between the hypoconid and hypoconulid.
The upper dentition is only known from P3, P4, and a possible M2. The buccal aspect of P3 (Fig. 1 E and G) is very long mesiodistally and bears a tall paracone with long and trenchant pre- and postparacone cristae. The protocone is very small, located mesially, and there is no hint of a lingual cingulum. The distal margin of the tooth is distinctly notched between the buccal and lingual moieties, giving the tooth a waisted appearance. When compared with P3, the buccal aspect of P4 (Fig. 1 E and H) is relatively narrow mesiodistally, the paracone is relatively short, and the tooth has a very large and well-developed pro-tocone with a distinct postprotocrista. Each tooth has a minute parastyle and a distinct buccal cingulum. The crown morphology of the possible M2 is very simple; the mesiobuccal aspect of the tooth is missing, but the tooth presumably had only three cusps (paracone, metacone, and protocone) and notably completely lacks a hypocone and has a discontinuous lingual cingulum. There is no hint of a paraconule or metaconule, and the weak postprotocrista courses to the base of the metacone, where it meets a faint crest on that cusp's lingual face [the “lateral posterior transverse crista” of Kay (16)]. The postmetacrista is long, distinct, oriented toward the buccal aspect of the tooth, and terminates distally at a well-developed buccal cingulum.
Phylogenetic Position of Nosmips.
Across 8 of 12 assumption sets (Table 1), parsimony analysis of 361 morphological characters (105 of which could be scored for Nosmips) places Nosmips along the stem lineage of Anthropoidea, in a more basal position than any other Fayum anthropoid. In the remaining four assumption sets, Nosmips is twice placed as an advanced stem strepsirrhine (and as the sister taxon of the latest Eocene Fayum genus Plesiopithecus), and twice within Adapiformes (Fig. 2 and Fig. S2). These results lead us to view Nosmips’ more common placement as a stem anthropoid with considerable caution. Notably, Nosmips is placed as an advanced stem strepsirrhine when the analysis is constrained by what we consider to be particularly well-founded assumptions (i.e., assumption sets 1 and 2, with some multistate characters ordered and scaled, monophyly of Malagasy lemurs and an Arctocebus-Perodicticus clade constrained, and premolar reacquisition not allowed).
Table 1.
Assumption sets (AS), tree statistics, and placement of Nosmips for each of the 12 phylogenetic analyses undertaken
| Assumption set | Some multistate characters ordered and scaled? | Monophyly of Malagasy lemurs and Arctocebus-Perodicticus clade enforced? | Premolar reacquisition allowed? | NMMP 20 skeleton assigned to Pondaungia? | Tree length | Consistency index | Retention index | Placement of Nosmips |
| AS1 | Yes | Yes | No | Yes | 2,175.116 | 0.1979 | 0.5776 | Stem Strepsirrhini |
| AS2 | Yes | Yes | No | No | 2,175.116 | 0.1979 | 0.5776 | Stem Strepsirrhini |
| AS3 | Yes | Yes | Yes | No | 2,164.835 | 0.2007 | 0.5635 | Stem Anthropoidea |
| AS4 | Yes | No | Yes | No | 2,150.420 | 0.2020 | 0.5672 | Stem Anthropoidea |
| AS5 | No | No | Yes | Yes | 3,822 | 0.2486 | 0.5026 | Stem Anthropoidea |
| AS6 | No | Yes | Yes | Yes | 3,833 | 0.2479 | 0.5007 | Stem Anthropoidea |
| AS7 | No | No | Yes | No | 3,822 | 0.2486 | 0.5026 | Stem Anthropoidea |
| AS8 | No | Yes | Yes | No | 3,833 | 0.2479 | 0.5007 | Stem Anthropoidea |
| AS9 | Yes | No | No | Yes | 2,161.291 | 0.1991 | 0.5809 | Adapiformes |
| AS10 | Yes | No | Yes | Yes | 2,164.775 | 0.1988 | 0.5801 | Stem Anthropoidea |
| AS11 | Yes | Yes | Yes | Yes | 2,164.773 | 0.2007 | 0.5635 | Stem Anthropoidea |
| AS12 | Yes | No | No | No | 2,164.775 | 0.1988 | 0.5801 | Adapiformes |
Fig. 2.
Varying phylogenetic placements of Nosmips given different assumptions about morphological character evolution, strepsirrhine phylogeny, and attribution of the NMMP 20 partial skeleton. Strict consensus trees derived from parsimony analysis of (A) assumption sets (AS) 1 and 2 (trees from AS 1 and 2 are congruent); (B) AS 3; (C) AS 9 and 12 (trees from AS 9 and 12 are congruent); and (D) AS 5 and 7 (trees from AS 5 and 7 are congruent). See Fig. S2 for strict consensus trees derived from additional assumption sets and Table 1 for assumptions and tree statistics.
Dietary Reconstruction.
Peculiarities in dental morphology often reflect dietary specializations (e.g., ref. 17). Nosmips is not the only primate at BQ-2 with unusual tooth morphology; Afradapis longicristatus also has a strange dentition, presumably reflecting dietary specializations (4). Sympatric primates in extant communities avoid competition for food resources through differing ecologic specializations. Morphological differences that correspond to differences in ecologic niche and resource use include body size and tooth morphology (17). Given similarities in tooth size and presumably body size, we predict that Nosmips and Afradapis were morphologically specialized for different dietary niches, like sympatric primates of similar size in extant communities [e.g., Alouatta and Ateles (18)].
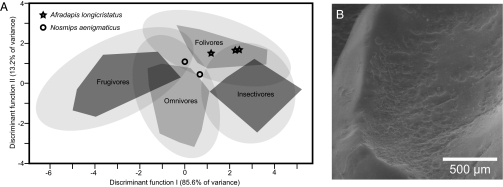
A discriminant function analysis of three variables [orientation patch count (OPC) (19) and relief index (RFI) (20) on M2, and M1 area] measured on 103 extant tarsier and strepsirrhine primates with different diets (i.e., omnivores, primary frugivores, primary folivores, and primary insectivores) classified 92.2% of these individuals into their correct dietary groups (Tables S1, S2, S3, and S4), indicating that this extant comparative data set can be used to infer the dietary proclivities of extinct primates with some confidence. We used the resulting discriminant functions to classify individuals of Nosmips and the sympatric caenopithecine adapiform Afradapis (4) (Fig. 3A). The analysis supports Seiffert et al.’s (4) suggestion, made on the basis of gross dental morphology, that Afradapis was highly folivorous (with Afradapis individuals being assigned to the “folivores” group with probabilities of 0.83, 0.86, and 0.89). The diet of Nosmips is less clear, with both individuals classified as omnivores with probabilities of 0.56 and 0.66 [the second highest probabilities, classifying Nosmips as a folivore, are also low (0.29 and 0.42)]. Nosmips’ nearest neighbors along discriminant functions I and II are individuals of the genera Eulemur and Nycticebus, whereas all of the nearest neighbors of Afradapis are members of the genus Propithecus.
Fig. 3.
Evidence bearing on Nosmips’ dietary preferences. (A) Distribution of individuals assigned to four predefined dietary groups along discriminant functions I and II, derived from discriminant analysis of three variables (first lower molar area, and OPC and RFI measured on second lower molars). Convex polygons demarcate limits of x-, y-points for individuals within each of the predefined dietary groups, whereas translucent ellipses represent the 95% confidence limits for each predefined dietary group. Afradapis and Nosmips individuals are indicated by stars and circles, respectively. (B) Scanning electron micrograph of the talonid of P4 of Nosmips aenigmaticus (DPC 21366G), showing extensive pitting of enamel surface. Similar pitting occurs on lower molars, and is consistently restricted to the talonid basins.
Regardless of DFA classification, the M2s of Nosmips and Afradapis are nonoverlapping in RFI and OPC: the M2s of Nosmips have lower complexity (OPC values of 45–50 as opposed to Afradapis’ values of 51–53) and lower relief (RFI of 0.49–0.53 in Nosmips, 0.55–0.58 in Afradapis). This suggests that Nosmips had a diet that required less molar processing of fibrous or tough foods (i.e., less reliance on adult leaves). Interestingly, Nosmips has a fourth premolar with lower relief but greater complexity (RFI 0.43; OPC 52) than those of Afradapis [n = 4 (DPC 21548D, -F, -H, and -I); RFI 0.54–0.59; OPC 32–37). Two additional sources of information bearing on Nosmips’ diet are (i) the presence of extensive enamel pitting in the talonid basins, particularly on P4–M2 (Fig. 3B), possibly formed by mastication of hard objects such as seeds or pits of fruits, and (ii) its highly specialized premolar dentition, including a tall and elongate P3, which was presumably used to slice foods before mastication by the molar teeth. Again, a diet of fruits or seeds would seem to be consistent with Nosmips’ specialized premolars and expanded talonid basins, although the elongate shearing P3/3 could also conceivably have been used to crop leaves from tough stems.
Discussion and Conclusions
Nosmips may be an odd strepsirrhine bearing some anthropoid-like dental features, a highly specialized early offshoot from the anthropoid stem lineage, or even a bizarre catarrhine, but much more informative material is needed to clarify this strange primate's phylogenetic position. Nosmips shows clear morphological differences from other genera at the base of the anthropoid stem lineage, such as Eosimias (21) and Bahinia (22). Unlike Nosmips, these genera primitively retain large molar paraconids but also have complete lingual cingula on the upper molars. Nosmips is similar to Eosimias and Bahinia, and unlike more derived Eocene anthropoids from the Fayum, in retaining probable primitive features, such as small and centrally placed lower molar hypoconulids, a well developed buccal cingulum and notched distal margin on M2?, the lack of a hypocone on M2?, and a tiny P3 protocone. Layered on top of these primitive features, however, are some clear autapomorphies, such as the elongate and bicuspid P3, the elongate and molariform P4, the enlarged buccal cusps of P3/3 relative to those of P4/4, the peculiar morphology of the first lower molar, the lack of distinct molar paraconids, crestiform molar entoconids, and low lingual walls on M1–3.
Nosmips’ enlarged and elongate P3, molariform P4, absence of lower molar paraconids and upper molar conules, and centrally placed hypoconulids are features that are also seen in early stem catarrhines such as propliopithecids, which appear in the same area later in the Paleogene. However, Nosmips differs significantly from propliopithecids in having an M2? with an incomplete lingual cingulum, broad buccal shelf, and no hypocone; no lingual cingula on the upper premolars; no lingual cingulid on P3; an elongate trigonid basin on M1; small lower molar hypoconulids; and extreme difference in height between the lower molar trigonids and talonids in lingual view. The genus is never placed with early catarrhines in any of our phylogenetic analyses. The upper tooth that shears against Nosmips’ specialized P3 paracristid has never been discovered at BQ-2, but we consider it likely, on the basis of Nosmips’ greatly enlarged P3, that, like early catarrhines and the sympatric adapiform Afradapis, the occluding upper tooth was more likely a canine than an enlarged and caniniform P2. If this is the case, then the honing complex between the upper canine and P3, and loss of P2/2, would have evolved independently three times in the Eocene of Africa (i.e., in catarrhines, Afradapis, and Nosmips).
Nosmips’ consistent proximity to the strange terminal Eocene Fayum primate genus Plesiopithecus, which was originally interpreted as an anthropoid on the basis of limited dental remains (23) but subsequently aligned with strepsirrhines on the basis of more informative cranial remains (24), suggests that Nosmips might turn out to have affinities with plesiopithecids, despite considerable differences in overall dental morphology. Nosmips is also similar to early Eocene asiadapine adapiforms in combining lower molars that have broad talonid basins and reduced or absent paraconids (on M2–3 in asiadapines) with upper molars that lack hypocones, conules, and complete lingual cingula (25). Nosmips is, however, never allied with asiadapines in our phylogenetic analyses.
Information from Nosmips’ anterior dentition will help to further clarify this strange primate's feeding adaptations, but on the basis of available evidence from molar topography and microwear, we consider it likely that Nosmips regularly consumed fruit, presumably supplemented by leaves and insects. Such preferences likely differentiated it from the sympatric folivore Afradapis. The premolar morphology of Afradapis and Nosmips suggests additional differences in ingested food types and/or modes preparation: low relief and high OPC in the P4 of Nosmips indicate a possible fracturing function, whereas higher relief and lower complexity of Afradapis P4s indicate a shearing function. Overall close similarity between the P4 and molars of Nosmips in RFI and OPC could also suggests that these teeth were part of the same functional field, whereas differences between the P4 and the molars of Afradapis suggest these teeth were part of different functional fields. If this is correct, then the two large primates from BQ-2, Afradapis and Nosmips, had diets that were sufficiently different to allow their populations to avoid consistent direct competition for resources.
Materials and Methods
Phylogenetic Analysis.
A reduced version of Seiffert et al.’s (4) morphological character matrix, with highly incomplete taxa (i.e., those missing >75% of the 361 characters) removed (matrix is available at http://www.anat.stonybrook.edu/eseiffert/nosmips.html ), was analyzed under maximum parsimony using the heuristic search option in PAUP* 4.0b10 (26) with random addition sequence and the tree bisection and reconnection branch swapping algorithm across 5,000 replicates. Numerous analyses were undertaken, altering certain assumptions to determine whether the placement of Nosmips changed depending on whether (i) some multistate characters were ordered and scaled; (ii) premolar presence/absence was constrained to be irreversible (i.e., previously lost premolars were not allowed to be reacquired); (iii) a molecular scaffold constrained the monophyly of Malagasy lemurs and an Arctocebus-Perodicticus clade (as supported by abundant DNA sequence and SINE data, e.g., ref. 27); or (iv) the National Museum of Myanmar Primate (NMMP) 20 partial skeleton from the late middle Eocene Pondaung Formation was scored for the Pondaungia cotteri OTU (28). See Fig. S2 for additional results.
Dietary Reconstruction.
OPC (19) and RFI (20) were calculated using three-dimensional surface reconstructions derived from micro-CT scans of tooth casts. Methodology follows that of Boyer et al. (29). For calculation of RFI, each tooth scan was smoothed using 100 iterations in the program Avizo v. 6. For OPC, tooth scans were not smoothed but were downsampled to a standard number of point rows per tooth (50), leaving a final resolution of 80–100 μm for these teeth. OPC values were calculated in a GIS (Geographic Information System) program, SurferManipulator (30), that divides up a 3D tooth surface model into patches that face in any of eight cardinal directions. OPC values represent the total number of patches (consisting of three or more pixels) that are required to describe the surface of the tooth. RFI is a metric describing the 3D surface area (3da) of a tooth model relative to the 2D area of a tooth model in occlusal view (2da), using the equation RFI = ln [(√3da)/(√2da)].
Supplementary Material
Acknowledgments
We thank M. E. Steiper for access to computing facilities; C. T. Rubin and S. Judex for access to micro-CT scanners in the Center for Biotechnology at Stony Brook University; Duke University's Shared Materials Instrumentation Facility for access to their ESEM; and K.C. Beard, G. F. Gunnell, and K. D. Rose for providing helpful comments on the manuscript. This is DLC publication no. 1176. The staff of the Egyptian Mineral Resources Authority, the Egyptian Geological Museum, and the Egyptian Environmental Affairs Agency provided valuable assistance in Egypt. Fieldwork was supported by the US National Science Foundation and The Leakey Foundation. P. Chatrath managed fieldwork in the Fayum area.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1001393107/DCSupplemental.
References
- 1.Tabuce R, et al. Anthropoid versus strepsirhine status of the African Eocene primates Algeripithecus and Azibius: Craniodental evidence. Proc Biol Sci. 2009;276:4087–4094. doi: 10.1098/rspb.2009.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seiffert ER, Simons EL, Attia Y. Fossil evidence for an ancient divergence of lorises and galagos. Nature. 2003;422:421–424. doi: 10.1038/nature01489. [DOI] [PubMed] [Google Scholar]
- 3.Seiffert ER, et al. Basal anthropoids from Egypt and the antiquity of Africa's higher primate radiation. Science. 2005;310:300–304. doi: 10.1126/science.1116569. [DOI] [PubMed] [Google Scholar]
- 4.Seiffert ER, Perry JM, Simons EL, Boyer DM. Convergent evolution of anthropoid-like adaptations in Eocene adapiform primates. Nature. 2009;461:1118–1121. doi: 10.1038/nature08429. [DOI] [PubMed] [Google Scholar]
- 5.Sigé B, Jaeger JJ, Sudre J, Vianey-Liaud M. Altiatlasius koulchii n. gen. n. sp., primate omomyidé du Paléocène du Maroc, et les origines des euprimates. Palaeontographica. Abt A. 1990;214:31–56. [Google Scholar]
- 6.Beard KC. East of Eden: Asia as an important biogeographic center of taxonomic origination in mammalian evolution. Bull Carnegie Mus Nat Hist. 1998;34:5–39. [Google Scholar]
- 7.Godinot M. In: Anthropoid Origins. Fleagle JG, Kay RF, editors. New York: Plenum Press; 1994. pp. 235–296. [Google Scholar]
- 8.Beard KC. In: The Primate Fossil Record. Hartwig WC, editor. Cambridge, UK: Cambridge Unive Press; 2002. pp. 133–149. [Google Scholar]
- 9.Godinot M, Mahboubi M. Earliest known simian primate found in Algeria. Nature. 1992;357:324–326. doi: 10.1038/357324a0. [DOI] [PubMed] [Google Scholar]
- 10.Godinot M, Mahboubi M. Les petits primates simiiformes de Glib Zegdou (Éocène inférieur à moyen d'Algérie) C R Acad Sci Paris. 1994;319:357–364. [Google Scholar]
- 11.Hartenberger JL, Marandat B. A new genus and species of an early Eocene primate from North Africa. Hum Evol. 1992;7:9–16. [Google Scholar]
- 12.Godinot M. Lemuriform origins as viewed from the fossil record. Folia Primatol (Basel) 2006;77:446–464. doi: 10.1159/000095391. [DOI] [PubMed] [Google Scholar]
- 13.Seiffert ER, Simons EL, Ryan TM, Attia Y. Additional remains of Wadilemur elegans, a primitive stem galagid from the late Eocene of Egypt. Proc Natl Acad Sci USA. 2005;102:11396–11401. doi: 10.1073/pnas.0505310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Court N. An enigmatic new mammal from the Eocene of north Africa. J Vert Paleo. 1992;13:267–269. [Google Scholar]
- 15.Tabuce R, et al. Aznag (bassin d'Ouarzazate, Maroc), nouvelle localité à sélaciens et mammifères de l'Eocène moyen (Lutétien) d'Afrique. Bull Soc Geol Fr. 2005;176:381–400. [Google Scholar]
- 16.Kay RF. The evolution of molar occlusion in the Cercopithecidae and early Catarrhines. Am J Phys Anthropol. 1977;46:327–352. doi: 10.1002/ajpa.1330460213. [DOI] [PubMed] [Google Scholar]
- 17.Kay RF. The functional adaptations of primate molar teeth. Am J Phys Anthropol. 1975;43:195–216. doi: 10.1002/ajpa.1330430207. [DOI] [PubMed] [Google Scholar]
- 18.Anthony MRL, Kay RF. Tooth form and diet in ateline and alouattine primates—reflections on the comparative method. Am J Sci. 1993;293A:356–382. [Google Scholar]
- 19.Evans AR, Wilson GP, Fortelius M, Jernvall J. High-level similarity of dentitions in carnivorans and rodents. Nature. 2007;445:78–81. doi: 10.1038/nature05433. [DOI] [PubMed] [Google Scholar]
- 20.Boyer DM. Relief index of second mandibular molars is a correlate of diet among prosimian primates and other euarchontan mammals. J Hum Evol. 2008;55:1118–1137. doi: 10.1016/j.jhevol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Beard KC, Wang J. The eosimiid primates (Anthropoidea) of the Heti Formation, Yuanqu Basin, Shanxi and Henan Provinces, People's Republic of China. J Hum Evol. 2004;46:401–432. doi: 10.1016/j.jhevol.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Jaeger JJ, et al. A new primate from the Middle Eocene of Myanmar and the Asian early origin of anthropoids. Science. 1999;286:528–530. doi: 10.1126/science.286.5439.528. [DOI] [PubMed] [Google Scholar]
- 23.Simons EL. Diversity in the early tertiary anthropoidean radiation in Africa. Proc Natl Acad Sci USA. 1992;89:10743–10747. doi: 10.1073/pnas.89.22.10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simons EL, Rasmussen DT. A remarkable cranium of Plesiopithecus teras (Primates, Prosimii) from the Eocene of Egypt. Proc Natl Acad Sci USA. 1994;91:9946–9950. doi: 10.1073/pnas.91.21.9946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose KD, et al. Early Eocene primates from Gujarat, India. J Hum Evol. 2009;56:366–404. doi: 10.1016/j.jhevol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Swofford DL. Sunderland, MA: Sinauer Associates; 1998. PAUP* Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4. [Google Scholar]
- 27.Roos C, Schmitz J, Zischler H. Primate jumping genes elucidate strepsirrhine phylogeny. Proc Natl Acad Sci USA. 2004;101:10650–10654. doi: 10.1073/pnas.0403852101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciochon RL, Gingerich PD, Gunnell GF, Simons EL. Primate postcrania from the late middle Eocene of Myanmar. Proc Natl Acad Sci USA. 2001;98:7672–7677. doi: 10.1073/pnas.051003298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boyer DM, Evans A, Jernvall J. Evidence of dietary differentiation among late Paleocene-early Eocene plesiadapids (Mammalia, Primates) Am J Phys Anthropol. 2009 doi: 10.1002/ajpa.21211. in press. [DOI] [PubMed] [Google Scholar]
- 30.Evans A. SurferManipulator for Windows. 2009 Available at: http://users.monash.edu.au/~arevans/software.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.