Abstract
Increasing UV-B radiation (UV-B; 290–320 nm) due to stratospheric ozone depletion has been a leading explanation for the decline in amphibians for nearly 2 decades. Yet, the likelihood that UV-B can influence amphibians at the large spatial scales relevant to population declines has not yet been evaluated. A key limitation has been in relating results from individual sites to the effect of UV-B for populations distributed across heterogeneous landscapes. We measured critical embryonic exposures to UV-B for two species of montane amphibians with contrasting physiological sensitivities, long-toed salamander (Ambystoma macrodactylum) and Cascades frog (Rana cascadae), at field sites spanning a gradient of UV-B attenuation in water. We then used these experimental results to estimate the proportion of embryos exposed to harmful UV-B across a large number of breeding sites. By combining surveys of the incubation timing, incident UV-B, optical transparency of water, and oviposition depth and light exposure of embryos at each site, we present a comprehensive assessment of the risk posed by UV-B for montane amphibians of the Pacific Northwest. We found that only 1.1% of A. macrodactylum and no R. cascadae embryos across a landscape of breeding sites are exposed to UV-B exceeding lethal levels. These results emphasize that accurately estimating the risk posed by environmental stressors requires placing experimental results in a broader ecological context that accounts for the heterogeneity experienced by populations distributed across natural landscapes.
Keywords: amphibian declines, ultraviolet radiation, risk analysis, dissolved organic matter, oviposition behavior
Species declines and extirpations in seemingly well-protected habitats represent an alarming component of global biodiversity loss. Declining amphibian populations in particular have been heralded as sentinels of subtle environmental degradation in otherwise intact systems, and have been a focal point of intense study for more than 20 years (1–6). Although diverse causes have been proposed, a leading hypothesis for amphibian declines in western North America has been that increasing UV-B radiation (290–320 nm wavelengths) due to anthropogenic ozone depletion (7–9) has increased mortality rates (10, 11). Assessments of the impact of ambient UV-B exposure have been conducted for more than 50 species of amphibians and have focused primarily on embryonic and larval survival under a variety of laboratory and field conditions (reviewed in refs. 11–14). Despite this level of investigation, few if any conclusions regarding the effect of ambient UV-B can be drawn for specific populations, due to the difficulty in translating the results of studies conducted at individual experimental sites to the broad spatial and temporal scales relevant to amphibian population dynamics (5, 15–17). A critical limitation preventing such analyses is the lack of quantitative data regarding actual UV-B exposures across the range of conditions experienced by individuals within a population, coupled to experimentally identified thresholds of lethal UV-B exposure. Although measuring cumulative exposure to UV-B over time at multiple sites in the field is currently limited by instrumentation and expense, site-level UV-B exposures have been estimated from the primary features of the terrestrial and aquatic environment that affect UV-B transmission, including geographic location, elevation, cloud cover, topographic and vegetative shading, and the attenuation of UV-B in water (16, 18). However, an additional limitation of even these sophisticated models is the need to incorporate not only variation among sites, but also the variation in UV-B exposure among individuals within a site that may result from variation in individual behaviors (19, 20).
Exposure of embryonic and larval amphibians to UV-B in aquatic environments is strongly influenced by the concentration of colored dissolved organic matter (DOM), which explains 85–92% of the variation in the rate at which UV-B is attenuated (Kd) with water depth (21, 22). Colored DOM represents a suite of compounds derived primarily from the decomposition of terrestrial vegetation (22, 23) and is transported to surface waters in montane areas by runoff associated with precipitation and snowmelt, resulting in seasonal variation in UV-B transparency (24, 25). Variation in the extent and type of watershed vegetation and soil generates tremendous site-to-site heterogeneity in the UV-B environment experienced by freshwater taxa (26–28); thus, DOM serves to link the dynamics of terrestrial vegetation and soils to the mosaic of exposures experienced by amphibians in aquatic ecosystems.
Landscape-scale variation in water transparency among individual lakes and ponds is compounded with variation in exposure among individuals within each site. Differences in UV-B exposure of embryos within sites is generated by maternal oviposition behaviors that result in deposition of embryos at different water depths and in microhabitats with differing exposure to light (e.g., deposited in vegetation, under rocks or debris, or on exposed surfaces). For some montane amphibians, maternal oviposition behavior appears to track site-level DOM concentration, resulting in reduced embryonic UV-B exposures in highly transparent sites by laying eggs deeper or in shaded microhabitats (19). As such, simple extrapolations from laboratory-based physiological assays or assessments of field exposures will overestimate UV-B risk if such behaviors are not accounted for.
We evaluated the context dependency of UV-B effects for the embryonic survival of two montane amphibians of the Pacific Northwest, Cascades frogs (Rana cascadae) and long-toed salamanders (Ambystoma macrodactylum), by experimentally estimating lethal UV-B exposures and accounting for landscape-scale variation in DOM concentrations and patterns of maternal oviposition behavior. Importantly, these two species exhibit contrasting physiological sensitivity to UV-B (10, 19), with Cascades frogs among the least sensitive of Pacific Northwest species and long-toed salamanders among the most sensitive. We used field experiments in breeding sites spanning a natural gradient of water transparency to UV-B (i.e., DOM concentration) to identify the cumulative exposure to UV-B associated with significant embryonic mortality for each species. We then used the experimental results to evaluate the risk posed by UV-B to embryos of each species across a large number of breeding sites. Our assessment of risk is based on estimates of the cumulative UV-B exposure experienced by individual embryos in each of 22 breeding sites (R. cascadae, n = 107,352; A. macrodactylum, n = 4,159). These exposures were estimated by combining site-specific estimates of incident UV-B, seasonal variation in the UV-B transmission properties of water (Kd), measurement of naturally occurring embryos and their microhabitats (depth and light exposure), and duration of incubation. By comparing the level of UV-B–induced mortality from the field experiments with natural field exposures, we are better able to estimate the frequency with which lethal conditions are experienced by individuals distributed across this heterogeneous montane landscape.
Results
Field Experiments.
To evaluate the context dependency of UV-B effects for R. cascadae and A. macrodactylum, we estimated the survival of embryos incubated in near-ambient and reduced–UV-B environments at seven different breeding sites in Olympic National Park, Washington, spanning a gradient of optical transparency to UV-B (Kd) (four sites for R. cascadae, five sites for A. macrodactylum, and two sites in common). Embryos were incubated at 10 cm below the water surface. The embryos in near-ambient UV-B exhibited significantly higher mortality than those shielded from UV-B (6.7% for R. cascadae and 41.7% for A. macrodactylum) in the most UV-B–transparent sites (Table S1). More specifically, the difference in survival between UV-B–shielded and –exposed treatments was directly related to the cumulative UV-B experienced by embryos in the exposed treatment over the course of the incubation period. UV-B–exposed embryos suffered significantly higher mortality at UV-B >10,534 KJ·m−2 for A. macrodactylum, and at UV-B >22,918 KJ·m−2 for R. cascadae (Fig. 1). The higher UV-B exposures tolerated by R. cascadae embryos compared with A. macrodactylum embryos confirm differences in physiological sensitivity documented under controlled laboratory exposures (10, 19).
Fig. 1.
Summary of cumulative UV-B exposure estimates for R. cascadae (Upper) and A. macrodactylum (Lower) incubation experiments. Data are presented as effect sizes, calculated as the average difference in percent survival between embryos incubated in reduced and near-ambient UV-B environments at a water depth of 10 cm at each site (± SE). Asterisks denote effect sizes significantly different from 0 (α = 0.05).
UV-B Risk Assessment.
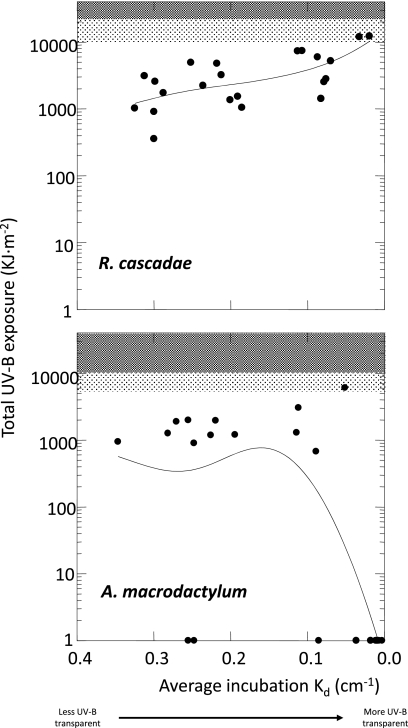
To estimate the risk posed by UV-B exposure for R. cascadae and A. macrodactylum embryos at broader spatial scales than could be evaluated experimentally, we surveyed the depth distribution of embryos and their exposure to light, seasonal timing and duration of incubation, and water transparency at 22 breeding sites for each species spanning a gradient of UV-B transparency. To accomplish this, we combined the cumulative UV-B exposure distributions estimated for each survey site with the results of the egg incubation experiments for each species (Fig. S1). We estimated that none of R. cascadae and 1.1% of A. macrodactylum embryos encounter cumulative UV-B exposures that exceed the levels associated with significant mortality in the experiments (22,918 KJ·m−2 for R. cascadae and 10,534 KJ·m−2 for A. macrodactylum) (Fig. 2). To account for the uncertainty in defining exact thresholds of exposure associated with significant mortality, we also estimated risk across the survey sites using a more conservative threshold based on the highest UV-B exposure from the experimental incubations that resulted in no difference in survival between UV-B treatments (10,534 KJ·m−2 for R. cascadae vs. 5,321 KJ·m−2 for A. macrodactylum). Using these lower thresholds, we estimate that 5.7% of R. cascadae embryos and 11.2% of A. macrodactylum embryos experience cumulative UV-B that equals or exceeds those levels (Fig. 2). We also evaluated how the median UV-B exposure received by embryos changes as a function of the UV-B attenuation coefficient (Kd) at each site, and found a pattern of increasing exposure with increasing water clarity for R. cascadae (Fig. 3 Top) and a pattern of exposure that appears to be decoupled from water transparency for A. macrodactylum, because the lowest median exposures occur in sites with the greatest water transparency to UV-B (Fig. 3 Bottom). These patterns suggest that site-level optical transparency (Kd) might represent a reasonable proxy for estimating UV-B exposures of R. cascadae, but not of A. macrodactlyum.
Fig. 2.
Total cumulative UV-B exposure estimates for all R. cascadae (Upper; 107,352 embryos) and A. macrodactylum (Lower; 4,519 embryos) embryos surveyed across 22 breeding sites for each species. Thresholds for both panels correspond to cumulative exposures received in field experiments that had no effect on embryonic survival (Lower) and that resulted in a significant reduction in survival (Upper). For R. cascadae, 5.7% of all embryos received UV-B exceeding the lower threshold, and none received UV-B exceeding the upper threshold; for A. macrodactylum, these values were 11.2% and 1.1%, respectively. Ten embryos of A. macrodactylum had an estimated UV-B exposure exceeding the range of the x-axis; these are denoted by (10) just beyond the x-axis.
Fig. 3.
Log median cumulative UV-B exposure of R. cascadae (Top) and A. macrodactylum (Bottom) embryos as a function of the average UV-B transparency (Kd) for each site. Shaded areas represent UV-B exposures from egg incubation experiments corresponding to the highest incubation exposure associated with no survival effect (light shading, R. cascadae = 10,534 KJ·m−2; A. macrodactylum = 5,321 KJ·m−2) and the lowest exposure associated with significantly reduced survival (dark shading, R. cascadae = 22,918 KJ·m−2; A. macrodactylum = 10,534 KJ·m−2). Bold line represents a smoothed trend in median cumulative UV-B exposures across transparency fit by distance-weighted least squares. UV-B transparency decreases with larger Kd values.
Discussion
Our results demonstrate that very few R. cascadae and A. macrodactylum embryos are exposed to lethal levels of UV-B across a montane landscape of the Pacific Northwest. Whereas critical UV-B levels are expected to be encountered by some embryos, we estimate that the vast majority are exposed to only very low levels of UV-B (<2,500 KJ·m−2) over the incubation period (R. cascadae, 53%; A. macrodactylum, 80%). This pattern of low exposure is driven by a combination of factors that ameliorate the potentially high levels of UV-B otherwise present in montane environments, including the optical properties of water at each site (Kd) and variation in the depth and light exposure of embryos (maternal oviposition behavior). As UV-B transparency increases across sites, R. cascadae embryos are exposed to higher median cumulative UV-B, whereas A. macrodactylum median exposures are highest at intermediate levels of UV-B transparency (Kd) (Fig. 3). This pattern confirms an earlier observation that oviposition by adult female R. cascadae is relatively static across sites, with egg masses generally deposited in full sunlight at a water depth of ~10 cm (19). In contrast, oviposition site choice by adult female A. macrodactylum is known to correlate with site level water transparency to UV-B, with nearly 100% of embryos laid in shaded areas of sites with the highest UV-B penetration (small Kd) (19). Given the high physiological sensitivity of A. macrodactylum embryos, this pattern suggests the possibility of behavioral avoidance in sites that might otherwise be characterized as having lethal UV-B exposures. Our results support the conclusion that variation in water transparency combined with patterns of egg-laying serve to reduce A. macrodactylum UV-B exposures to below lethal levels.
Our approach to estimating in situ cumulative UV-B exposures allows us to evaluate the specific contribution of oviposition behavior and site-level water transparency (Kd) to each species’ exposure estimates. For example, if we assumed that oviposition was constant across sites, with all embryos exposed to direct sunlight near the water surface, then our estimates of UV-B risk would be 3- to 17-fold higher for A. macrodactylum (Table 1). Similarly, if we assumed that water transparency to UV-B was consistently high, with all embryos incubated in water equal to the most UV-B transparent breeding site for each species, then the percentage of embryos exposed to harmful levels of UV-B would increase 5- to 51-fold for A. macrodactylum and 0- to 1.3-fold for R. cascadae (Table 1). Despite the much greater physiological sensitivity of A. macrodactylum embryos compared with R. cascadae embryos (10, 19), the risk posed by UV-B measured in this study suggests that embryos of the two species are equally unlikely to be affected by UV-B at spatial scales that capture the landscape variation in site-level transparency to UV-B and oviposition behavior.
Table 1.
Effect of behavior and DOM on estimates of UV-B risk
| Species | Scenario | > High threshold | > Low threshold |
| A. macrodactylum | |||
| Actual conditions | 1.1% | 11.2% | |
| No behavior, actual DOM | 18.9% | 37.9% | |
| Actual behavior, constant DOM* | 56.2% | 56.3% | |
| No behavior, constant DOM* | 99.4% | 100% | |
| R. cascadae | |||
| Actual conditions | 0% | 5.7% | |
| Constant DOM† | 0% | 7.7% | |
The percentage of embryos exposed to UV-B (KJ·m−2) exceeding high and low thresholds determined experimentally for each species: for A. macrodactylum, high =10,534 KJ·m−2, low = 5,321 KJ·m−2; for R. cascadae, high = 22,918 KJ·m−2, low = 10,534 KJ·m−2. Scenarios represent calculations of risk based on actual observed conditions, including behavior and landscape variation in DOM, or assumptions of constant DOM across all sites (most transparent site for each species:
*Kd = 0.00037;
†Kd= 0.00897) or of no behavioral response to UV-B exposure (all embryos classified as “exposed”). R. cascadae exhibited no variation in oviposition behavior relevant to UV-B; therefore, only the constant DOM scenario is shown.
Our results suggest that it is exceedingly unlikely that UV-B–induced embryonic mortality could affect the overall population status for either of these two species. However, we are not yet able to explicitly link patterns of embryonic mortality with adult population dynamics for most amphibian species, due to the limited data regarding population status and stage-specific demographic rates. Despite this limitation, many amphibian species undergo strong density-dependent growth and survival during the aquatic larval stage (29, 30), which has been shown to temper the effect of additional mortality in embryonic and larval stages. These results suggest that even large reductions in embryonic survival might not translate into changes in population size or population growth rates (31, 32).
Because embryonic survival at natural breeding sites is governed by many factors other than UV-B, including predation, hydroperiod, disease, and temperature, we specifically isolated the contribution of UV-B by comparing the mean difference in survival between embryos shaded from most UV-B and those incubated under near-ambient conditions in each experiment. As a result, this analysis accounts for the direct mortality associated with UV-B exposure, but does not necessarily predict differences in the overall survival of embryos between sites, or nonlethal but potentially detrimental effects of UV-B that might not be expressed until later life-history stages or that might interact with other stressors (15, 33–35). For example, embryonic mortality in UV-B–shielded treatments varied across experimental sites from 1% to 65% for A. macrodactylum and from 2% to 57% for R. cascadae, suggesting that the importance of direct mortality by UV-B is small relative to other sources of mortality, even in sites with statistically significant UV-B effects (UV-B mortality, 42% in A. macrodactylum and 7% in R. cascadae). Although estimating the importance of mortality due to factors other than UV-B was not the focus of this experiment, these values emphasize the variability in amphibian reproduction and embryonic survival, and represent the broader ecological context within which potential UV-B effects are embedded.
It is important to evaluate the assumptions made in this analysis for our conclusions regarding the level of risk posed by current UV-B. In all cases in which a simplifying assumption was required in our calculations, we intentionally chose the assumption that would favor overestimating UV-B exposure, and as a result we have confidence that these estimates represent a near-maximum effect of UV-B on embryos of these two species. This approach increases the confidence in our conclusion that current UV-B levels are not causing widespread mortality across this montane landscape.
We found significant reductions in the survival of embryos experimentally exposed to UV-B in breeding sites with high optical transparency, corresponding to survival decreases of 7% for R. cascadae embryos and 42% for A. macrodactylum embryos. However, given the tremendous variation in UV-B transparency among amphibian breeding sites, experiments conducted at any one site will not accurately characterize the importance of UV-B for amphibian populations distributed across heterogeneous landscapes encompassing hundreds to thousands of individual sites. When variation in UV-B transparency of breeding sites and oviposition behavior are not included in our estimates of risk, we predict that 9- to 90-fold more embryos would be exposed to potentially harmful UV-B levels (Table 1). This is analogous to conducting an exposure experiment at the most UV-B–transparent breeding site, anchoring embryos at a water depth of 10 cm in full sunlight, and extrapolating those results to the full complement of sites used by each species. These results highlight the fundamental importance of DOM in predicting UV-B exposures in ponds across the landscape, and underscore the fact that individual behaviors may further buffer the effect of otherwise harmful environmental stressors (36). To critically evaluate threats to specific populations, experiments must go beyond testing local conditions in isolation of the broader ecological context within which populations occur (37), and explicitly consider the suite of physical, chemical, and biological features that might ameliorate or exacerbate impacts to individuals. By combining data from multiple spatial scales and levels of biological organization, we are able to improve predictions of the extent of harmful UV-B effects at the spatial scales relevant to amphibian population dynamics.
Materials and Methods
Field Experiments.
To evaluate the context dependency of UV-B effects on the survival of A. macrodactylum and R. cascadae embryos, we monitored the survival of embryos incubated in near-ambient and reduced UV-B environments at seven different breeding sites spanning a gradient of optical transparency to UV-B during July–September 2002. All sites are at subalpine elevations (1278–1500 m) within the headwaters of the Sol Duc drainage of Olympic National Park, Washington (N 47.917°, W 123.784°), and have little direct anthropogenic disturbance. Sites were selected to span the range of UV-B water transparency previously documented for the area (17), and selection was based specifically on seasonal water transparency and amphibian breeding distribution data collected in 2001. At each site, we monitored the timing of breeding for R. cascadae and A. macrodactylum, and collected ~400–1,000 eggs for each experiment within 48 h of egg deposition (Table 1). Previous work has shown that R. cascadae in the Pacific Northwest breed in sites with moderate to high levels of DOM (17, 38), and as a result R. cascadae breeding sites with high optical transparency to UV-B are relatively rare. Results from a preliminary incubation experiment conducted in the clearest site with R. cascadae breeding in 2001 suggested no difference in embryonic survival between UV-B–exposed and UV-B–shaded treatments. Therefore, to identify a threshold cumulative exposure of UV-B associated with significant embryonic mortality for R. cascadae, in 2002 embryos were moved to one site with much higher UV-B penetration than the clearest R. cascadae breeding site within the upper Sol Duc drainage (site Y-015; Table 1). Embryos were collected for this experiment from the nearest natural breeding site (~0.2 km away) and otherwise handled identically to those in parallel experiments. Immediately after collection at each site, egg masses were gently separated into small groups (~1–10 embryos) and randomly assigned to treatment, with a total of 25–70 eggs per replicate.
At each experimental site, we compared embryonic survival in near-ambient and reduced–UV-B environments by incubating embryos at a water depth of 10 cm in floating clear plastic enclosures with 1-mm mesh screened bottoms and light selective films attached to the top (13 × 13 × 9 cm). Near-ambient conditions were maintained with 0.13-mm cellulose acetate film, which allows an average of 84% ± 4.3% transmission of UV-B wavelengths (10, 39, 40), and a reduced UV-B environment was created with 0.13-mm mylar-D film (Dupont), which is known to selectively reduce the intensity of most UV-B wavelengths, with ~50% transmission at 315 nm. The optical properties of mylar-D and acetate film across the UV-B, UV-A, and visible spectrum wavelengths (290–900 nm) were confirmed by spectrophotometry (Shimadzu UV-Vis 2100) before and after the experiments and were similar to those published elsewhere (10, 39, 40). Each of the two experimental treatments (acetate and mylar-D) was replicated six times at each site for each species. Embryos were visually monitored every 2–6 days to track development, because development time varies among sites due primarily to water temperature, and survival to hatching was determined when all embryos had either hatched or died (i.e., arrested development) (Table S1).
Data Analysis.
Whereas embryonic incubations conducted at each site for each species can be considered separate factorial experiments assessing the impact of UV-B exposure on embryonic survival, the motivation for conducting experiments at multiple sites (four sites for R. cascadae and five sites for A. macrodactylum) was to describe how the effect of ambient UV-B changes as a function of the transparency of water to UV-B. Therefore, to compare the importance of UV-B across sites for each species, we calculated the effect size of UV-B exposure by bootstrapping the difference in survival between pairwise combinations of treatments at each site 6,000 times (41). The statistical significance of UV-B exposure on embryonic survival was evaluated based on whether the 95% confidence interval (CI) of the effect size, calculated from the bootstrapped sample [CI = sample mean ± (t1,n-1 · SE)], overlapped with 0.
Embryonic Survey.
To characterize the exposure of R. cascadae and A. macrodactylum embryos to UV-B at broader spatial scales than could be evaluated experimentally, we surveyed 22 breeding sites for each species spanning a gradient of UV-B transparency over the course of the embryonic incubation period in 2002 (Kd, 0.00286–0.34653 cm−1). All sites were within the same drainage system in which the incubation experiments were conducted (elevation, 1,077–1,472 m), and surveys were conducted simultaneously with the field experiments. The site selection was based on previous surveys of water transparency and species occupancy (i.e., breeding), and represent >90% of R. cascadae breeding sites and >50% of A. macrodactylum breeding sites within this headwater basin.
The timing of oviposition by each species was monitored every 1–4 days at each site, and the distribution of egg depths (in cm) beneath the surface of the water was measured ~7 days after initiation of breeding. A. macrodactylum embryos are often laid singly or in loose clumps and can be attached to debris or vegetation. Individual females can produce clutches of 50 to several hundred eggs. As such, we have low confidence that all A. macrodactylum embryos were sampled within each site, but have confidence that the distribution of water depths and light exposures were accurately characterized for each site by carefully searching all oviposition habitats (under layers of loose rock, undercut pond edges, emergent vegetation, woody debris, and open substrate). In contrast, R. cascadae lay conspicuous, often conglomerate, egg masses ranging in size from 50 to 5,000 eggs per mass. We have high confidence that all egg masses of R. cascadae were detected at each survey site and that for each egg mass, the minimum and maximum depths (in cm) were recorded, and dimensional volume (in mL) was estimated. Based on the relationship between egg mass volume and number of embryos per mass for embryos of approximately the same stage (Neggs = 1.091·volumeeggmass), we estimated the total number of embryos within each mass. To associate a water depth with each embryo within each R. cascadae egg mass, we allocated embryos to depths (in cm) according to the volume–depth relationship for a sphere occurring between the minimum and maximum depth of each mass. Importantly, R. cascadae embryos within each egg mass were assumed to receive no shading from overlying embryos, because weighting the relative exposures of embryos at different positions within a mass would require understanding of the optical properties of the jelly matrix and embryonic tissue, which is currently unknown. Coincident with the measurement of egg depths, we described the light exposure of each individual egg (for A. macrodactylum) or egg mass (for R. cascadae) as either fully shaded (with no direct light reaching the eggs) or exposed to light. The eggs in partial shade were categorized as exposed; we intentionally applied this conservative definition to ensure that the light exposure of embryos was not underestimated. Sites were continually monitored over the course of embryonic incubation to estimate the total time that embryos were exposed to UV-B radiation. In cases where either oviposition or hatching was not directly observed, the incubation window was assumed to be the same as that at the next-nearest site, given that temporal asynchrony generally increases with distance between sites within the study area, and imparts at most a ±1- to 2-day level of uncertainty to our estimates.
Site UV-B Transparency.
To estimate the UV-B transparency of water at each experimental and survey site, we collected between two and eight water samples over the course of embryonic incubation to estimate the UV-B attenuation coefficient (Kd). The amount of UV-B reaching any depth (z, in cm) in the water column can be estimated as the exponential decay of surface UV-B (UV-B0) according to the formula UV-Bz = UV-B0·e-Kd·z (21). Water samples (125 mL) were filtered sequentially through 1.2- and 0.2-μm syringe filters (1.2 μm: Whatman GF-C; 0.2 μm: Millipore PTFE; 47 mm diameter), and stored in the dark at ~5–10 °C until being transported to the laboratory. Absorbance (A) of 440-nm light passed through a 10-cm quartz cuvette (path length, z) containing each filtered water sample was determined using a Shimadzu UV-2100 double-beam spectrophotometer, and was related to absorption (a) at 440 nm according to Lambert's and Beer's laws, where a440 = ((2.303 × A440)/z). Based on a previously documented relationship between absorption at 440 nm in a spectrophotometer and in situ UV-B attenuation profiles for lakes in this drainage [Kd (cm−1) = 0.0795 · a440 (cm−1); R2 = 0.98; P < 0.00001] (17) and elsewhere (16, 18), we calculated the integrated extinction coefficient (Kd) for UV-B wavelengths. To account for the temporal variation in UV-B transparency of individual sites in our UV-B exposure estimates, we linearly interpolated daily estimates of Kd between water samples at each site.
Incident UV-B Measurements.
We estimated incident daily UV-B exposure for each experimental and survey site for each day of the incubation period for each species. High spectral resolution (0.5 nm) incident UV flux was recorded at a nearby site (~20 km) in Port Angeles, Washington as part of the Environmental Protection Agency's network of UV spectrophotometers (Mark IV spectrophotometer; Brewer #147; N 48.097°, W 123.426°; elevation, 9.3 m above sea level). Cumulative daily integrated UV-B estimates (287–320 nm) were provided by the National UV Monitoring Center (ftp://ftp.epa.gov/nerlpb/uvnet/olympic/) according to a cosine-corrected trapezoidal integration method. To account for the increase in UV-B irradiance with altitude (elevation, in m), daily cumulative exposures were scaled to the elevation of each breeding site according to the formula UV-Belev = UV-B0 · ([elevation/1,000] · 1.18) (42). No adjustments were made for site-level differences in landscape position, topography, or aspect; thus, reported daily UV-B estimates represent overestimates for some sites, but can be interpreted to be the theoretical maximum daily exposure received at each site.
UV-B Exposure Estimates.
To estimate the distribution of UV-B exposures for embryos of R. cascadae and A. macrodactylum at each survey site we combined (i) cumulative daily UV-B estimates scaled to the elevation of each site, (ii) interpolated daily estimates of Kd at each site, and (iii) the distribution of eggs with water depth and their exposure to light (no exposure = 0 KJ·m−2 UV-B) (Fig. S1). Cumulative daily UV-B estimates were made on a per embryo basis for each day of the observed incubation period for each species at each site, and then these were summed across all days to arrive at a total cumulative UV-B exposure received by each measured embryo. To date, no wavelength-specific damage rates, also referred to as action spectra (43), have been established for any amphibian species. As a result, we chose to use unweighted cumulative exposure (KJ·m−2·day) integrated across the UV-B range as the basis for comparing differences in exposure between sites. Previous risk assessments for amphibian breeding sites in midwestern US wetlands and US national parks also have used this maximum theoretical exposure (16, 18) in the absence of a more mechanistic understanding of wavelength-specific damage rates.
UV-B Risk Assessment.
To assess the risk posed by current UV-B levels for embryos occurring across breeding sites with different depth distributions, light exposures, and water transparency to UV-B, we combined the cumulative UV-B exposure distributions estimated for each survey site with the results of the egg incubation experiments for each species (Fig. S1). Because all embryos in the field experiments were incubated at a common depth of 10 cm, and the transmission properties of the near-ambient treatment are well described (0.13 mm cellulose acetate = 84% ± 4% ambient UV-B transmission) (10, 39, 40), we can calculate cumulative UV-B exposures over the course of incubation for each species at each experimental site in the same way as was done for each survey site (see above). Based on these estimates and the level of statistical significance for the effect of UV-B exposure in each experiment, we are able to bound the threshold of direct lethal UV-B effects for each species as occurring between (i) the maximum cumulative UV-B exposure that produced no significant difference in embryonic survival and (ii) the minimum cumulative UV-B exposure that significantly increased mortality. By evaluating the percentage of embryos of each species exposed to both the “highest known safe” (low) and the “lowest known lethal” (high) cumulative UV-B exposures, we acknowledge the considerable uncertainty that exists in defining an exact dose–response threshold.
Supplementary Material
Acknowledgments
We thank the US National Park Service for access to field sites and permission to conduct the experiments; Michael Kimlin, Jack Shreffler, and Roger Hoffman for UV-B irradiance measurements from Olympic National Park; Mike Adams for logistical support; Mike Brett for use of his spectrophotometer; Brice Semmens, Mary Power, Sarah Kupferberg, K. B. Suttle, and three anonymous reviewers for constructive feedback on earlier drafts; and Jennifer Jones, Adam Goodwin, Kristel Dillon, Jon Moore, Monika Winder, Justin Fox, Tessa Francis, Jackie Carter, Kemp Jones, Anne Salomon, Laura Payne, and Eric Wagner for field assistance. This work was supported by the US Geological Survey, US National Park Service Inventory and Monitoring Program, Canon National Park Science Scholars Program, and the Department of Biology, University of Washington.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.E.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912970107/-/DCSupplemental.
References
- 1.Blaustein AR, Wake DB. Declining amphibian populations: A global phenomenon. Trends Ecol Evol. 1990;5:203–204. [Google Scholar]
- 2.Wake DB. Declining amphibian populations. Science. 1991;253:860. doi: 10.1126/science.253.5022.860. [DOI] [PubMed] [Google Scholar]
- 3.Drost CA, Fellers GM. Collapse of a regional frog fauna in the Yosemite area of the California Sierra Nevada, USA. Conserv Biol. 1996;10:414–425. [Google Scholar]
- 4.Lips KR. Decline of a tropical montane amphibian fauna. Conserv Biol. 1998;12:106–117. [Google Scholar]
- 5.Alford RA, Richards SJ. Global amphibian declines: A problem in applied ecology. Annu Rev Ecol Syst. 1999;30:133–165. [Google Scholar]
- 6.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 7.Kerr JB, McElroy CT. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science. 1993;262:1032–1034. doi: 10.1126/science.262.5136.1032. [DOI] [PubMed] [Google Scholar]
- 8.Madronich S. Increases in biologically damaging UV-B radiation due to stratospheric ozone reductions: A brief review. Archiv für Hydrobiol Beiheft Ergebnisse Limnol. 1994;43:17–30. [Google Scholar]
- 9.World Meterological Organization and United Nations Environment Program (WMO/UNEP) Scientific Assessment of Ozone Depletion. Geneva: World Meteorological Organization; 2006. Global Ozone Research and Monitoring Project Report No. 50. [Google Scholar]
- 10.Blaustein AR, et al. UV repair and resistance to solar UV-B in amphibian eggs: A link to population declines? Proc Natl Acad Sci USA. 1994;91:1791–1795. doi: 10.1073/pnas.91.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaustein AR, Romansic JM, Kiesecker JM, Hatch AC. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers Distrib. 2003;9:123–140. [Google Scholar]
- 12.Corn PS. Amphibian declines: Review of some current hypotheses. In: Sparling DW, Linder G, Bishop CA, editors. Ecotoxicology of Amphibians and Reptiles. Pensacola, FL: Society of Environmental Toxicology and Chemistry; 2000. pp. 663–696. [Google Scholar]
- 13.Licht LE. Shedding light on ultraviolet radiation and amphibian embryos. Bioscience. 2003;53:551–561. [Google Scholar]
- 14.Bancroft BA, Baker NJ, Blaustein AR. Effects of UVB radiation on marine and freshwater organisms: A synthesis through meta-analysis. Ecol Lett. 2007;10:332–345. doi: 10.1111/j.1461-0248.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 15.Tietge JE, et al. Ambient solar UV radiation causes mortality in larvae of three species of Rana under controlled exposure conditions. Photochem Photobiol. 2001;74:261–268. doi: 10.1562/0031-8655(2001)074<0261:asurcm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Diamond SA, Peterson GS, Tietge JE, Ankley GT. Assessment of the risk of solar ultraviolet radiation to amphibians, III: Prediction of impacts in selected northern midwestern wetlands. Environ Sci Technol. 2002;36:2866–2874. doi: 10.1021/es011197d. [DOI] [PubMed] [Google Scholar]
- 17.Palen WJ, et al. Optical characteristics of natural waters protect amphibians from UV-B in the US Pacific Northwest. Ecology. 2002;83:2951–2957. [Google Scholar]
- 18.Diamond SA, et al. Estimated ultraviolet radiation doses in wetlands in six national parks. Ecosystems. 2005;8:462–477. [Google Scholar]
- 19.Palen WJ, Williamson CE, Clauser AA, Schindler DE. Impact of UV-B exposure on amphibian embryos: Linking species physiology and oviposition behaviour. Proc R Soc B Biol Sci. 2005;272:1227–1234. doi: 10.1098/rspb.2005.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belden LK, Wildy EL, Blaustein AR. Growth, survival and behaviour of larval long-toed salamanders (Ambystoma macrodactylum) exposed to ambient levels of UV-B radiation. J Zool. 2000;251:473–479. [Google Scholar]
- 21.Morris DP, et al. The attentuation of solar UV radiation in lakes and the role of dissolved organic carbon. Limnol Oceanogr. 1995;40:1381–1391. [Google Scholar]
- 22.Scully NM, Lean DRS. The attenuation of ultraviolet radiation in temperate lakes. Arch Hydrobiol. 1994;43:135–144. [Google Scholar]
- 23.McKnight DM, et al. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol Oceanogr. 2001;46:38–48. [Google Scholar]
- 24.Brooks PD, et al. Spatial and temporal variability in the amount and source of dissolved organic carbon: Implications for ultraviolet exposure in amphibian habitats. Ecosystems. 2005;8:478–487. [Google Scholar]
- 25.Boyer EW, Hornberger G, Bencala KE, McKnight DM. Response characteristics of DOC flushing in an alpine catchment. Hydrol Process. 1997;11:1635–1647. [Google Scholar]
- 26.Williamson CE, et al. Ultraviolet radiation and zooplankton community structure following deglaciation in Glacier Bay, Alaska. Ecology. 2001;82:1748–1760. [Google Scholar]
- 27.Hope D, Billett MF, Milne R, Brown TAW. Exports of organic carbon in British rivers. Hydrol Process. 1997;11:325–344. [Google Scholar]
- 28.Gergel SE, Turner MG, Kratz TK. Dissolved organic carbon as an indicator of the scale of watershed influence on lakes and rivers. Ecol Appl. 1999;9:1377–1390. [Google Scholar]
- 29.Wilbur HM. Complex life-cycles. Annu Rev Ecol Syst. 1980;11:67–93. [Google Scholar]
- 30.Alford RA. In: Tadpoles: The Biology of Anuran Larvae. McDiarmid RW, Altig R, editors. Chicago: Univ Chicago Press; 1999. pp. 240–278. [Google Scholar]
- 31.Vonesh JR, De la Cruz O. Complex life cycles and density dependence: Assessing the contribution of egg mortality to amphibian declines. Oecologia. 2002;133:325–333. doi: 10.1007/s00442-002-1039-9. [DOI] [PubMed] [Google Scholar]
- 32.Biek R, Funk WC, Maxell BA, Mills LS. What is missing in amphibian decline research: Insights from ecological sensitivity analysis. Conserv Biol. 2002;16:728–734. [Google Scholar]
- 33.Pahkala M, Laurila A, Merila J. Carry-over effects of ultraviolet-B radiation on larval fitness in Rana temporaria. Proc R Soc Lond B Biol Sci. 2001;268:1699–1706. doi: 10.1098/rspb.2001.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belden LK, Blaustein AR. Exposure of red-legged frog embryos to ambient UV-B radiation in the field negatively affects larval growth and development. Oecologia. 2002;130:551–554. doi: 10.1007/s00442-001-0843-y. [DOI] [PubMed] [Google Scholar]
- 35.Smith MA, Kapron CM, Berrill M. Induction of photolyase activity in wood frog (Rana sylvatica) embryos. Photochem Photobiol. 2000;72:575–578. doi: 10.1562/0031-8655(2000)072<0575:iopaiw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Huey RB, Hertz PE, Sinervo B. Behavioral drive versus behavioral inertia in evolution: A null model approach. Am Nat. 2003;161:357–366. doi: 10.1086/346135. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez MJ, Frost TM. Comparisons of laboratory bioassays and a whole-lake experiment: Rotifer responses to experimental acidification. Ecol Appl. 1994;4:69–80. [Google Scholar]
- 38.Adams MJ, Schindler DE, Bury RB. Association of amphibians with attenuation of ultraviolet-B radiation in montane ponds. Oecologia. 2001;128:519–525. doi: 10.1007/s004420100688. [DOI] [PubMed] [Google Scholar]
- 39.Nozais C, Desrosiers G, Gosselin M, Belzile C, Demers S. Effects of ambient UVB radiation in a meiobenthic community of a tidal mudflat. Mar Ecol Prog Ser. 1999;89:149–158. [Google Scholar]
- 40.Blaustein AR, Kiesecker JM, Chivers DP, Anthony RG. Ambient UV-B radiation causes deformities in amphibian embryos. Proc Natl Acad Sci USA. 1997;94:13735–13737. doi: 10.1073/pnas.94.25.13735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. London: Chapman & Hall; 1994. [Google Scholar]
- 42.Blumthaler M, Ambach W, Ellinger R. Increase in solar UV radiation with altitude. J Photochem Photobiol B. 1997;39:130–134. [Google Scholar]
- 43.McKinlay AF, Diffey BL. In: Human Exposure to Ultraviolet Radiation: Risks and Regulations. Passchler WR, Bosnajokovic BFM, editors. Amsterdam: Elsevier; 1987. pp. 83–87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.