Abstract
In healthy monocytes, Toll-like receptor (TLR) engagement induces production of reactive oxygen species (ROS), followed by an antioxidant response involved in IL-1β processing and secretion. Markers of the antioxidant response include intracellular thioredoxin and extracellular release of reduced cysteine. Cryopyrin-associated periodic syndromes (CAPS) are autoinflammatory diseases in which Nod-like receptor family pyrin domain–containing 3 (NLRP3) gene mutations lead to increased IL-1β secretion. We show in a large cohort of patients that IL-1β secretion by CAPS monocytes is much faster than that by healthy monocytes. This accelerated kinetics is caused by alterations in the basal redox state, as well as in the redox response to TLR triggering displayed by CAPS monocytes. Indeed, unstimulated CAPS monocytes are under a mild oxidative stress, with elevated levels of both ROS and antioxidants. The redox response to LPS is quickened, with early generation of the reducing conditions favoring IL-1β processing and secretion, and then rapidly exhausted. Therefore, secretion of IL-1β is accelerated, but reaches a plateau much earlier than in healthy controls. Pharmacologic inhibition of the redox response hinders IL-1β release, confirming the functional link between redox impairment and altered kinetics of secretion. Monocytes from patients with juvenile idiopathic arthritis display normal kinetics of redox response and IL-1β secretion, excluding a role of chronic inflammation in the alterations observed in CAPS. We conclude that preexisting redox alterations distinct from CAPS monocytes anticipate the pathogen-associated molecular pattern molecule–induced generation of the reducing environment favorable to inflammasome activation and IL-1β secretion.
Keywords: antioxidant response, inflammasome, reactive oxygen species, thioredoxin, autoinflammatory diseases
Interleukin-1β is a crucial mediator of host defense produced primarily by activated monocytes. The mechanism of IL-1β secretion can be schematically divided into two steps (1). In the first step, microbial products, such as pathogen-associated molecular pattern molecules (PAMPs), trigger pathogen recognition receptors (PRRs), such as Toll-like receptors (TLRs), inducing gene expression and accumulation of the precursor protein pro–IL-1β. In the second step, stimuli, such as extracellular ATP, crystals, or pathogenic dusts, trigger assembly of the inflammasome, a multiprotein complex required for the activation of caspase-1, ultimately responsible for the cleavage and secretion of the cytokine (2). Although the second signal increases and accelerates IL-1β processing and secretion, in human monocytes, stimulation by PAMPs is sufficient to drive secretion of mature IL-1β, because engagement of PRR causes the release of endogenous ATP (3). Autocrine stimulation of the purinergic receptor P2X7 by the released ATP triggers the cascade of events leading to maturation and secretion of IL-1β (3).
P2X7 triggering by exogenous ATP is followed by redox changes that modulate IL-1β secretion. The actual role of redox is still a matter of debate, however. Reactive oxygen species (ROS) produced by NADPH oxidase after exposure to exogenous ATP have been proposed to activate the inflammasome (4–6). In contrast, Meissner et al. (7) proposed that ROS inhibit caspase-1 activation, and that the inhibition is reverted by the antioxidant enzyme superoxide dismutase-1. Moreover, we have shown that in healthy human monocytes, PRR engagement by PAMPs induces activation of NADPH oxidase, with early production of ROS in turn responsible for the generation of an antioxidant response (8). Both ROS production and the consequent antioxidant response are required for the release of mature, fully processed IL-1β (8). The antioxidant response is characterized by increased activity of the cystine/cysteine redox cycle (9). This cycle involves up-regulation of the functional subunit of the Xc− transporter xCT, which mediates the internalization of cystine, the oxidized form of the amino acid cysteine that prevails extracellularly (10); enhanced uptake of cystine; intracellular conversion to cysteine; and secretion of the reduced amino acid, resulting in a switch of the extracellular redox from the physiological oxidizing to a nonphysiological reduced state. The oxidoreductase thioredoxin (Trx) (11) is also up-regulated and involved in the redox remodeling after monocyte activation (8). Up-regulation of the cystine/cysteine redox cycle occurs and serves previously unrecognized signaling and homeostatic functions in different physiological and pathological responses (12), including antigen presentation (13), B cell differentiation (14), and, in cancer cells, resistance to apoptosis (9) and drug sensitivity (15).
Cryopyrinopathies (CAPS) are a group of rare autoinflammatory diseases characterized by unexplained episodes of fever, severe localized inflammation, and elevated levels of acute-phase proteins (16). CAPS include familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS), and chronic infantile neurologic cutaneous and articular (CINCA) syndrome [also known as neonatal-onset multi-inflammatory disease (NOMID)]. These syndromes are part of a spectrum of symptoms, of which CINCA/NOMID is the most severe and FCAS is the mildest. In most cases, the disease is due to mutations in the NLRP3/CIAS1 gene (17), a key component of the inflammasome (18), which causes excessive production of IL-1β (18–22). Up to 40% of patients with a CAPS phenotype display no NLPR3 mutations, however (19). In all cases, the exquisite dependency on IL-1β has been confirmed by the rapid and sustained resolution of symptoms observed on blocking of IL-1 (20, 21).
To investigate whether redox is implicated in the deregulated IL-1β secretion observed in CAPS, we studied the basal redox and the PAMP-induced redox changes in monocytes from patients with CAPS. Our results show that CAPS monocytes are under oxidative stress, with chronic up-regulation of antioxidant systems. This redox imbalance in unstimulated monocytes causes a deranged response to PAMP stimulation, resulting in accelerated IL-1β secretion.
Results
Activated Monocytes from CAPS Patients Secrete IL-1β More Rapidly Than Healthy Monocytes.
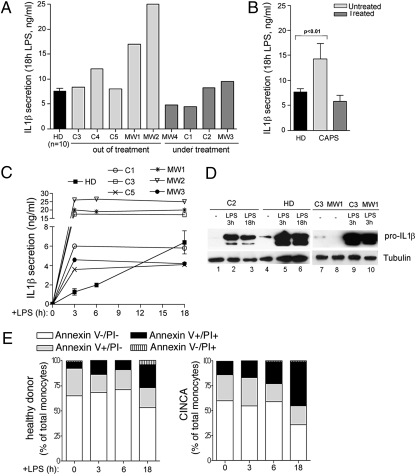
We studied IL-1β secretion in response to TLR triggering in monocytes from five patients with CINCA (C1–C5) and four patients with MWS (MW1–MW4) (Table S1). At the time of the analysis, five patients had completed anti–IL-1 therapy, and four patients were receiving IL-1 blockers. All patients with except C5 carried an NLRP3 mutation. Although monocytes from untreated patients secreted more IL-1β than healthy donors in response to 18 h of LPS stimulation (Fig. 1 A and B), the levels of IL-1β secreted by monocytes from patients under treatment were comparable to those of healthy controls (Fig. 1 A and B), confirming previous observations (18, 20–23).
Fig. 1.
LPS-stimulated CAPS monocytes display accelerated kinetics of IL-1β secretion. (A and B) Monocytes from healthy donors (HD; n = 10) and from the indicated CAPS subjects were cultured for 18 h with 1 μg/mL of LPS. Secreted IL-1β was quantified by ELISA. Data are expressed as ng/mL. (A) Data from single patients. For HDs, mean ± SD is shown. (B) Mean ± SD from untreated patients (n = 5), treated patients (n = 4), and HDs (n = 10). (C) Kinetics of IL-1β secretion by monocytes from 10 HDs and from the indicated CAPS subjects. Monocytes were exposed to LPS for 3, 6, or 18 h, and secreted IL-1β was quantified as in A. (D) Western blots of intracellular pro–IL-1β in monocytes from patients C2 (lanes 1–3), C3 (lanes 7 and 9), and MW1 (lanes 8 and 10) and from one representative HD analyzed in parallel (lanes 4–6), unstimulated (lanes 1, 4, 7, and 8) and stimulated with LPS for 3 h (lanes 2, 5, 9, and 10) or 18 h (lanes 3 and 6). (Lower) The same blots hybridized with anti−β-tubulin. (E) Monocytes from healthy donors and CAPS patients, unstimulated (0) or stimulated for various times with LPS (3, 6, and18 h) were stained with FITC-conjugated anti-annexin V and propidium iodide (PI) and analyzed by flow cytometry (25). Data show the percentage of living cells (annexin V−/PI−), early apoptotic cells (annexin V+/PI−), late apoptotic cells (annexin V+/PI+), and necrotic cells (annexin V−/PI+). Representative data from 2 healthy donors and 2 CINCA patients are shown.
In addition, kinetics studies revealed that IL-1β secretion by LPS-stimulated CAPS monocytes reaches a plateau at 3 h after stimulation, whereas secretion by healthy monocytes is still increasing at 18 h after exposure to LPS (Fig. 1C). This rapid kinetics was observed in monocytes from all CAPS patients, independent of the total amount of IL-1β secreted and of whether the patient was receiving or not receiving treatment. In all cases, the level of IL-1β detected in supernatants of CAPS compared with controls was much higher at 3 h (4- to 26-fold higher) than at 18 h (0- to 4-fold higher).
LPS-induced intracellular pro–IL-1β, although variable in the different individuals (21), was comparable in healthy controls and CAPS patients and was maintained for several hours (Fig. 1D), ruling out that the exhaustion of the cytoplasmic precursor is responsible for the early ending of IL-1β secretion by CAPS monocytes.
The failure of CAPS monocytes to secrete IL-1β after 3 h of LPS stimulation is also unrelated to cell death. As shown in Fig. 1E, the number of cells dying in the first 6 h from exposure to LPS was very similar in healthy donors and CAPS patients. Only at later times after LPS exposure (18 h) was a higher increment of cell death with respect to time 0 observed in CINCA monocytes (40% increase) compared with healthy monocytes (20% increase).
Unstimulated CAPS Monocytes Produce Higher Levels of ROS Than Healthy Monocytes.
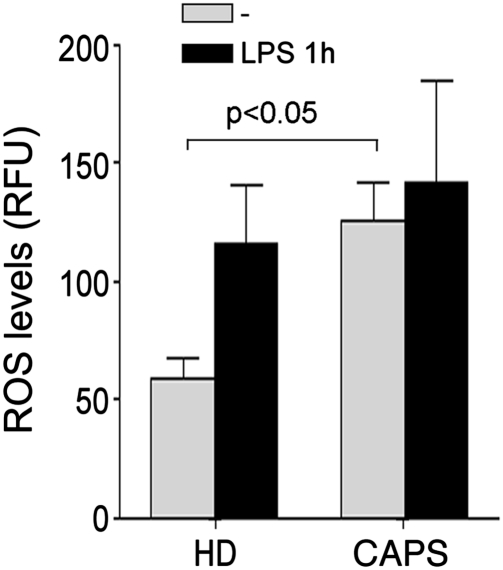
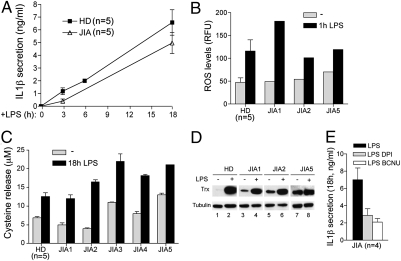
We recently reported that redox changes, with early ROS production followed by a strong antioxidant response, play a crucial role in PAMP-induced IL-1β processing and secretion (8). To explore whether the altered kinetics of IL-1β secretion observed in CAPS patients is related to an abnormal redox state of the monocytes, we investigated basal redox and redox remodeling in unstimulated and LPS-stimulated CAPS monocytes. The results demonstrate that unstimulated monocytes from CAPS patients produce significantly higher levels of ROS than healthy monocytes (Fig. 2). As in healthy monocytes, ROS production was further enhanced by LPS.
Fig. 2.
ROS levels are higher in unstimulated CAPS than in healthy monocytes. Monocytes from four HDs and four CAPS patients (C3, C4, C5, and MW1) were cultured 1 h in the absence (gray columns) or presence (black columns) of LPS, and intracellular ROS levels were quantified by H2DCF-DA fluorometric methods. Data are expressed as mean ± SD relative fluorescence units (RFUs).
CAPS Monocytes Display an Altered Redox Response to PAMP Stimulation.
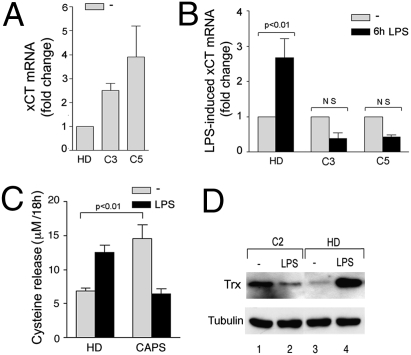
The levels of three related antioxidant markers—the xCT antiporter (10), released cysteine (8, 13) and the oxidoreductase Trx (8, 11)—were compared in CAPS and healthy monocytes both unstimulated and after LPS stimulation. As shown in Fig. 3A, xCT expression was higher in unstimulated CAPS monocytes than in healthy controls. Moreover, although in control monocytes, xCT was significantly up-regulated by 6 h of LPS stimulation, a down-modulation occurred in LPS-stimulated CINCA monocytes (Fig. 3B). Supporting the functional link between xCT expression and externalization of reduced cysteine (8, 9), CAPS monocytes released higher levels of cysteine than healthy monocytes during 18 h of culture in the absence of stimuli (Fig. 3C). But although in healthy monocytes the release of cysteine was strongly induced by LPS, CAPS monocytes secreted less cysteine after LPS stimulation than under unstimulated conditions (Fig. 3C).
Fig. 3.
Impaired antioxidant response in CAPS monocytes. Real-time PCR analysis of xCT mRNA in monocytes from the indicated CAPS patients and from two HDs, untreated (gray columns) or after 6 h of exposure to LPS (black columns). (A) Data are expressed as fold changes (mean ± SD) in untreated CAPS monocytes compared with untreated HD monocytes. (B) Data are expressed as fold changes (mean ± SD) in LPS-stimulated (black columns) compared with unstimulated monocytes (gray columns) of the same subject. NS, not significant. (C) Extracellular cysteine was quantified in 18 h supernatants of monocytes from HDs (n = 6) or CAPS patients (n = 6) cultured without LPS (−; gray columns) or with LPS (18 h LPS; black columns). Data are expressed as mean ± SD. (D) Intracellular Trx in monocytes from a CAPS patient (lanes 1 and 2) and an HD (lanes 3 and 4), unstimulated (lanes 1 and 3) and stimulated for 18 h with LPS (lanes 2 and 4). Results from one representative HD out of the six analyzed are shown. (Lower) The same blot hybridized with anti–β-tubulin.
Like xCT and cysteine release, Trx was abundant in unstimulated CAPS and was decreased after 18 h of LPS treatment, whereas the opposite was observed in control monocytes (Fig. 3D).
Rapid Exhaustion of the Antioxidant Response in LPS-Stimulated CAPS Monocytes.
The opposite trend of antioxidant response in CAPS monocytes compared with healthy donors might be due to the oxidative stress present in unstimulated CAPS monocytes (Fig. 2), as well as to the consequent up-regulation of antioxidant systems aimed at restoring the redox homeostasis. When PAMP stimulation provides a new oxidative challenge, the already expanded antioxidant systems might be unable to sustain a prolonged up-regulation and could experience exhaustion earlier than healthy monocytes.
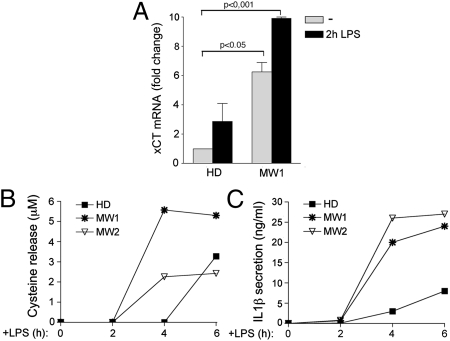
To explore this possibility, we compared the expression of xCT, cysteine release, and IL-1β secretion in healthy and CAPS monocytes at specified short times after LPS exposure. xCT expression was higher in unstimulated MW1 than in control monocytes and was strongly up-regulated after 2 h of LPS stimulation in CAPS monocytes, but only slightly increased in healthy monocytes (Fig. 4A). In keeping with the strong early induction of xCT, the speed of cysteine release was increased, with cysteine detected in supernatants of CAPS monocytes as early as after 4 h after exposure to LPS (Fig. 4B), whereas it did not appear in control monocyte supernatants until more than 6 h after LPS stimulation. But whereas in controls, cysteine release continues to increase throughout the duration of LPS stimulation (see Fig. 3C), in CAPS monocytes, a plateau is reached at 4 h after LPS stimulation. The more rapid kinetics of cysteine release by CAPS monocytes is coincident with the more rapid kinetics of IL-1β secretion (Fig. 4C), both of which reach a plateau early after LPS stimulation.
Fig. 4.
LPS accelerates xCT expression and cysteine release in CAPS monocytes. (A) Real- time PCR analysis of xCT mRNA in monocytes from patient MW1 and from three HDs, untreated (gray columns) or treated with LPS for 2 h (black columns). Data are expressed as fold change (mean ± SD) compared with untreated HD monocytes. (B and C) Kinetics of cysteine release (B) and IL-1β secretion (C) after LPS stimulation by cells from MW1 and MW2 and from one HD analyzed in parallel. Results are representative of three healthy donors analyzed.
Redox-Active Drugs Inhibit IL-1β Secretion by CAPS Monocytes.
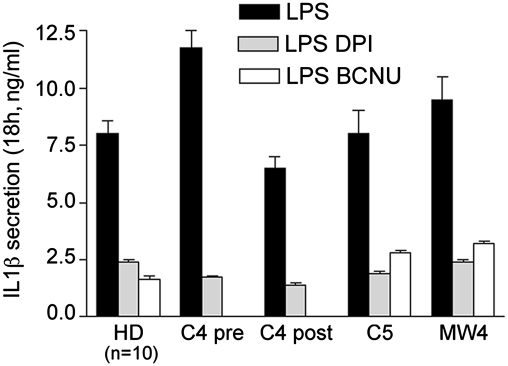
To verify that the redox impairment observed in CAPS monocytes is indeed involved in the process of IL-1β secretion, we investigated the effects of redox-active drugs on monocytes from three CAPS patients, one of which (C4) was analyzed both before and after the start of anti–IL-1 therapy (Fig. 5). Monocytes were stimulated with LPS and treated with diphenylene iodonium (DPI), an inhibitor of NADPH oxidase that prevents ROS production (8), or with 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU), which inhibits Trx activity (8, 11). IL-1β secretion by CAPS monocytes was strongly inhibited by both drugs, independent of treatment or disease state (Fig. 5).
Fig. 5.
Redox active drugs block IL-1β secretion by CAPS monocytes. IL-1β secretion by monocytes from HDs (n = 10) or from the indicated CAPS patients, stimulated for 18 h with LPS in the absence (black columns) or presence of DPI (gray columns; HD, C4, C5, and MW4) or BCNU (white columns; HD, C5, and MW4). Monocytes from patient C4 was analyzed twice, before (C4 pre) and 1 week after (C4 post) the start of anti–IL-1 therapy.
Redox Remodeling and IL-1β Kinetics of Secretion Are Not Altered in Monocytes from SoJIA Patients.
To ascertain whether the disturbed redox remodeling and IL-1β kinetics of secretion are restricted to CAPS or are also present in different but related diseases, we studied five patients affected by systemic-onset juvenile idiopathic arthritis (SoJIA; Table S2), a chronic inflammatory disease with similar pathological manifestations as CAPS but lacking a genetic etiology (16).
At the time of the study, all patients exhibited active disease. As shown in Fig. 6A , the kinetics of IL-1β secretion by SoJIA monocytes was similar to that of healthy controls, as reported previously (24). In addition, ROS production under basal conditions and after exposure to LPS was comparable to that of healthy monocytes (Fig. 6B). Likewise, cysteine release and Trx expression by unstimulated or LPS-stimulated SoJIA monocytes did not differ significantly from that in controls. In a few cases, slightly higher levels of released cysteine and of intracellular Trx were detected in unstimulated monocytes (Fig. 6 C and D), but this did not affect the response to LPS. Indeed, like in healthy monocytes, both cysteine release and Trx expression were still up-regulated at 18 h after LPS exposure. These results indicate that although a mild redox disturbance might be present in resting SoJIA monocytes, the redox response to prolonged LPS stimulation is maintained.
Fig. 6.
Redox remodeling and IL-1β secretion in monocytes from SoJIA patients stimulated with LPS. (A) IL-1β secretion by monocytes from HDs (n = 5) and SoJIA patients exposed to LPS for 3, 6, or 18 h. (B) ROS production by monocytes from HDs (n = 5) and the indicated SoJIA patients cultured for 1 h in the absence (gray columns) or presence (black columns) of LPS. Data are expressed as RFUs. (C) Released cysteine in the 18-h supernatants of unstimulated (gray columns) and stimulated (black columns) monocytes from HDs (n = 5) and the indicated SoJIA patients. (D) Western blot analysis of Trx in monocyte lysates from a representative HD (out of the six performed; lanes 1 and 2), and three SoJIA patients (lanes 3–8), unstimulated (lanes 1, 3, 5, and 7) or stimulated for 18 h with LPS (lanes 2, 4, 6, and 8). (Lower) Same blot hybridized with anti–β-tubulin. (E) IL-1β secreted by monocytes from SoJIA patients (n = 4) stimulated for 18 h with LPS in the absence (black columns) or presence of DPI (gray columns) or BCNU (white columns). Data are expressed as mean ± SD.
Finally, we found that DPI and BCNU dramatically hinder IL-1β secretion by SoJIA monocytes (Fig. 6E), confirming the strong redox dependence of IL-1β secretion in both healthy and pathological conditions.
Discussion
The release of active IL-1β from blood monocytes is tightly controlled, and <20% of the total IL-1β precursor synthesized in response to PRR triggering is actually processed and secreted (1). A number of studies have demonstrated that after LPS stimulation, monocytes from CAPS patients secrete greater amounts of mature IL-1β (18, 20–22), despite cytoplasmic accumulation of the precursor comparable to that seen in healthy individuals (21). This deranged posttranslational control of IL-1β processing and secretion is due to “gain of function” mutations of NLRP3 (16). Just how NLRP3 mutations increase inflammasome activity and pro–IL-1β processing is unclear, however. Dinarello (1) recently pointed out that the increase in IL-1β secretion by CAPS monocytes compared with healthy subjects is modest, raising the question of how a relatively small increase in IL-1β secretion can cause the devastating IL-1–dependent clinical manifestations of CAPS.
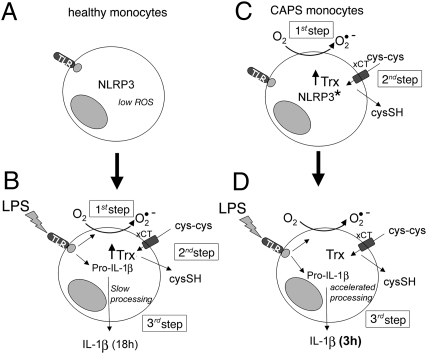
In this study, we have shown that CAPS monocytes not only secrete more IL-1β than healthy subjects, but also have dramatically accelerated IL-1β secretion due to the entanglement of NLRP3 mutations with a deranged redox homeostasis in CAPS monocytes. Our results are summarized in the model shown in Fig. 7. In unstimulated monocytes from healthy subjects (A), a condition of redox homeostasis is maintained, inflammasome components are not assembled, and pro–IL-1β is not expressed (2). On LPS stimulation (B), pro–IL-1β is induced, and redox remodeling occurs, with ROS production followed by a delayed antioxidant response, which generates a reducing environment required for inflammasome assembly and consequently pro–IL-1β processing and secretion (8). In contrast, unstimulated CAPS monocytes display a disturbed redox state with elevated ROS levels and overexpression of antioxidant systems (C). Because of this preexisting overexpression, up-regulation of antioxidant systems in response to LPS-induced ROS generation occurs (and is exhausted) earlier, resulting in faster IL-1β secretion and earlier achievement of plateau level compared with healthy monocytes (D).
Fig. 7.
How do the different redox states and redox responses to LPS in healthy and CAPS monocytes influence the rate of IL-1β secretion? (A) Redox is balanced in resting healthy monocytes (with low basal levels of ROS and of the antioxidant systems Trx and cystine/cysteine cycle). (B) LPS triggering induces pro–IL-1β synthesis and rapid ROS production (first step). To counteract this oxidative hit, an antioxidant response with up-regulation of Trx and increased cysteine release occurs (second step). This redox remodeling, required for pro–IL-1β processing (8), takes several hours. Therefore, IL-1β secretion is slow and peaks at 18 h (third step). (C) In contrast, in resting mutated CAPS monocytes (asterisks), antioxidant systems are up-regulated due to chronic oxidative stress (first and second steps already occurring in resting monocytes). (D) Therefore, on LPS stimulation, IL-1β processing and secretion is anticipated due to the preexisting or rapidly generated antioxidant conditions that promote inflammasome activation and processing (third step).
The inhibitory effect on IL-1β secretion exerted by redox-active drugs demonstrates that redox remodeling is implicated in the process of IL-1β secretion not only in healthy cells (8), but also in cells bearing a gain-of-function mutation. These data also indicate that NLRP3 mutations and redox alterations work together in CAPS monocytes to enhance/anticipate IL-1β secretion.
We have previously shown that unlike healthy monocytes, LPS-stimulated CAPS monocytes do not increase the rate of IL-1β secretion in response to exogenous ATP (21), a powerful trigger of inflammasome activation (2). ATP activates the purinergic receptor P2X7, inducing a strong redox response in normal monocytes, with production of ROS followed by up-regulation of antioxidant systems (4–8). Given our present findings, the failure to increase IL-1β secretion in response to ATP is likely due to exhaustion of the antioxidant systems and the consequent impossibility of mounting a redox response to P2X7 receptor triggering. The breakdown of the antioxidant response also might be the cause of the premature death observed in CAPS monocytes (25). But in our patients, an increase in cell death compared with controls was detected only at later times after LPS exposure, ruling out the possibility that the early attainment of a plateau and cessation of IL-1β secretion is due to cell death. The redox alterations observed in CAPS monocytes might be directly linked to the NLRP3 mutation or might represent a secondary effect of the syndrome, related to, for instance, the chronic inflammatory state. Many genetic diseases display redox impairments. Whereas in some cases the link between mutation and redox is obvious [e.g., in chronic granulomatous disease, in which a defect in NADPH oxidase is directly linked to impaired redox homeostasis (26)], in other cases this relationship is less evident. For instance, mutations of the lysosomal cathepsins inhibitor cystatin B, which cause progressive myoclonus epilepsy, have been unexpectedly found to sensitize neurons to oxidative stress (27). Similarly, a strong oxidative stress in the nervous system is responsible for the severe neurologic disorders that characterize mucopolysaccharidosis IIIB (28), despite the fact that the disease is due to mutations in alpha-N-acetylglucosaminidase, an enzyme required for apparently redox-unrelated functions, such as the degradation of heparan sulfate.
On this basis, we can speculate that redox alterations are a consequence of different genetic defects that perturb the cellular homeostasis. NLRP3 is responsible for a redox-dependent process such as IL-1β maturation and secretion (4–8, 16). Therefore, in the disturbed redox environment of CAPS monocytes, the mutated NLRP3 cooperates with the altered redox response to derange IL-1β secretion, in terms of both amount and kinetics.
Among the patients examined in this study, C5 lacked NLRP3 mutations but displayed clinical manifestations clearly consistent with a CINCA syndrome (29) and the same redox alterations observed in mutated CINCA monocytes (Figs. 2 and 3). This finding might suggest that redox impairment of CAPS can occur independently of the genetic defect. But because C5’s father showed similar, albeit milder, symptoms as those displayed by C5 and the other CINCA patients, it is conceivable that in C5, the syndrome is due to the mutation of a different, as-yet unrecognized or unknown gene.
The observation that the kinetics of IL-1β secretion is much faster in CAPS monocytes than in healthy monocytes has important implications for the development of the inflammatory process. In healthy subjects, weak PRR triggering would induce a poor (if any) inflammatory response, because mechanisms aimed at down-modulating inflammation, including secretion of IL-1 receptor antagonist, will be fully operational when IL-1β secretion reaches the maximum level (1). In contrast, the same weak stimulus will drive dramatic clinical manifestations in CAPS patients, because the amount of IL-1β induced will be not only greater, but also promptly secreted soon after stimulation, before control mechanisms are operative. It is tempting to speculate that the chronic inflammatory state of CAPS patients is the result of recurrent episodes of inflammation due to repeated, short pulses of IL-1β secretion caused by PRR ligands, either exogenous (PAMPs) or endogenous [damage-associated molecular pattern molecules (DAMPs)], in amounts unable to trigger inflammation in healthy individuals, but sufficient to induce a dramatic inflammatory cascade when associated with NLRP3 mutations and redox conditions that strongly accelerate secretion. Interestingly, the accelerated IL-1β release is unrelated to disease severity, level of IL-1β production, or therapeutic regimen, further indicating that it is an intrinsic feature of CAPS monocytes.
The role of chronic inflammation and consequent redox impairment (30) in IL-1β secretion was studied on monocytes from patients affected by SoJIA, who share a number of clinical features (e.g., systemic inflammation, arthritis, rash, persistent elevation of acute phase reactants) with CAPS patients and in almost 45% of cases display the same dramatic response to anti–IL-1 treatment (24, 31), although the etiology remains unclear. We found that SoJIA monocytes display kinetics of IL-1β secretion and redox remodeling similar to those of healthy controls; however, the mild up-regulation of two markers of the antioxidant response, cysteine release and Trx content, in resting monocytes from some SoJIA patients suggests that, at least in some cases, the chronic inflammatory state might slightly perturb the redox homeostasis of circulating monocytes. But the redox derangement is negligible, with no exhaustion of the antioxidant response or modification of the IL-1β secretion rate, indicating that chronic inflammation per se is not the cause of the dramatic alterations in the redox response observed in CAPS.
In conclusion, we have shown that CAPS monocytes display impaired redox homeostasis and response to oxidative stress, responsible for the accelerated secretion of IL-1β. These alterations are absent in patients affected by different chronic inflammatory diseases, such as SoJIA. On this basis, we propose that the redox impairment of CAPS monocytes is not a silent epiphenomenona, but rather is functionally linked to IL-1β processing and secretion and thus is directly connected to the pathophysiology of cryopyrinopathies. Whether and how the redox impairment is directly linked to the NLRP3 mutation remains unclear. Because redox active drugs completely block IL-1β secretion in CAPS patients, it is conceivable that the gain-of-function mutation of NLRP3 is mediated by the redox response.
Materials and Methods
Patients.
Nine CAPS patients (five with CINCA and four with MWS) were enrolled in the study (Table S1). At the time of the study, five of the CAPS patients were not receiving anti–IL-1 treatment and displayed manifestations of active disease (e.g., rash, fever, joint manifestations, headache) associated with elevation of acute-phase reactants. Three patients were receiving an IL-1 blocker (anakinra or canakinumab). Five patients with SoJIA were evaluated as well (Table S2); all five displayed active disease activity (e.g., fever, rash, arthritis) and elevated acute-phase reactants. Twelve age-matched healthy controls were studied in parallel. Blood samples were taken after informed consent was obtained from each patient or a parent. The informed consent was approved by the “G. Gaslini” Ethical Board.
Chemicals.
BCNU, cysteine, DPI, 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB), DTT, and LPS were purchased from Sigma-Aldrich , and 2′7′-dichlorofluorecin diacetate (H2DCF-DA) was purchased from Molecular Probes.
Cell Preparation and Culture.
Monocytes were enriched by adherence from heparinized blood samples and activated with 1 μg/mL of LPS in RPMI 1640 medium supplemented with 10% FCS at 37 °C for prespecified times, as described previously (21, 24).
ELISA Analyses.
The IL-1β content in supernatants was determined by ELISA (R&D Systems) (24).
Intracellular ROS.
Monocytes were stimulated with LPS and 10 μM of H2DCF-DA (32) and added to cultures 30 min before the end of incubation. Fluorescence was measured in cell lysates with a microplate fluorometer (excitation, 480 nm; emission, 530 nm). Data were normalized versus the protein content of cell lysates, as measured by the Lowry method (8).
Determination of Cysteine in Culture Media.
Supernatants (0.1 mL) from monocytes cultured at 4 × 105/0.5 mL in 24-well plates in medium containing 5% FBS were reacted with 10 mM DTNB, and absorption was measured at 412 nm. Cysteine was used as a standard (8).
Western Blot Analysis.
Triton X-100 cell lysates were resolved on 12% SDS/PAGE and electrotransferred. Filters were probed with anti–IL-1β 3ZD (obtained from the National Cancer Institute's Biological Resources Branch), with antiTrx (clone 2B1, a kind gift of Dr. F. Clarke, Brisbane, Australia) or with anti–β-tubulin (Sigma-Aldrich) mAbs, followed by the relevant secondary Ab (DAKO) and developed with ECL-Plus (GE Healthcare) (8).
Real-Time PCR.
Total RNA was isolated from cell using TriPure Isolation Reagent (Roche) and reverse-transcribed with SuperScript III Reverse Transcriptase (Invitrogen). Real-time PCR determination of xCT cDNA was performed using SYBR greenER qPCR Super Mix for iCycler reagent (Invitrogen). The specific primers used were as follows: forward, 5′aaacccaagtggttcagacg 3′; reverse, 5′atctcaatcctgggcagatg 3′ for xCT and forward, 5′atggccttccgtgttcctac 3′; reverse, 5′gcttcaccaccttcttgatgtc 3′ for GAPDH. Relative expression was determined using the ΔCt method (33).
Flow Cytometry.
Monocytes were stained with human annexin V-FITC and propidium iodide (Bender MedSystems). Cells were analyzed with a FACSCalibur flow cytometer and Cell Quest software (BD Biosciences).
Statistical Analysis.
The data were statistically analyzed by one-way ANOVA, followed by a Bonferroni posttest or unpaired t test using GraphPad software. Differences were considered statistically significant at P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank Prof. Frank Clarke and the National Cancer Institute (Biological Resources Branch) for the generous gift of antibodies, and Dr. Serena Matis for the help with the FACS analysis. This work was supported in part by grants from Telethon (GGP09127), Ministero Salute, Compagnia San Paolo, and Istituto Superiore Sanità (ISS).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000779107/-/DCSupplemental.
References
- 1.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 2.Martinon F, Mayor A, Tschopp J. The inflammasomes: Guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 3.Piccini A, et al. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz CM, et al. ATP activates a reactive oxygen species–dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hewinson J, Moore SF, Glover C, Watts AG, MacKenzie AB. A key role for redox signaling in rapid P2X7 receptor–induced IL-1 beta processing in human monocytes. J Immunol. 2008;180:8410–8420. doi: 10.4049/jimmunol.180.12.8410. [DOI] [PubMed] [Google Scholar]
- 7.Meissner F, Molawi K, Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 8.Tassi S, et al. Pathogen-induced interleukin-1beta processing and secretion is regulated by a biphasic redox response. J Immunol. 2009;183:1456–1462. doi: 10.4049/jimmunol.0900578. [DOI] [PubMed] [Google Scholar]
- 9.Banjac A, et al. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 10.Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem. 1999;274:11455–11458. doi: 10.1074/jbc.274.17.11455. [DOI] [PubMed] [Google Scholar]
- 11.Lillig CH, Holmgren A. Thioredoxin and related molecules: From biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 12.Rubartelli A, Sitia R. Chemo-metabolic regulation of immune responses by Tregs. Nat Chem Biol. 2009;5:709–710. doi: 10.1038/nchembio.226. [DOI] [PubMed] [Google Scholar]
- 13.Angelini G, et al. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci USA. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellani P, Angelini G, Delfino L, Matucci A, Rubartelli A. The thiol redox state of lymphoid organs is modified by immunization: Role of different immune cell populations. Eur J Immunol. 2008;38:2419–2425. doi: 10.1002/eji.200838439. [DOI] [PubMed] [Google Scholar]
- 15.Olm E, et al. Extracellular thiol-assisted selenium uptake dependent on the x(c)- cystine transporter explains the cancer-specific cytotoxicity of selenite. Proc Natl Acad Sci USA. 2009;106:11400–11405. doi: 10.1073/pnas.0902204106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: The molecular pathophysiology of autoinflammatory disease (*) Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neven B, et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell–mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood. 2004;103:2809–2815. doi: 10.1182/blood-2003-07-2531. [DOI] [PubMed] [Google Scholar]
- 18.Agostini L, et al. NALP3 forms an IL-1β–processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 19.Aksentijevich I, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): A new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldbach-Mansky R, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattorno M, et al. Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007;56:3138–3148. doi: 10.1002/art.22842. [DOI] [PubMed] [Google Scholar]
- 22.Janssen R, Verhard E, Lankester A, Ten Cate R, van Dissel JT. Enhanced interleukin-1beta and interleukin-18 release in a patient with chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum. 2004;50:3329–3333. doi: 10.1002/art.20494. [DOI] [PubMed] [Google Scholar]
- 23.Lachmann HJ, et al. In vivo regulation of interleukin 1β in patients with cryopyrin-associated periodic syndromes. J Exp Med. 2009;206:1029–1036. doi: 10.1084/jem.20082481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gattorno M, et al. The pattern of response to anti–interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–1515. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 25.Saito M, et al. Disease-associated CIAS1 mutations induce monocyte death, revealing low-level mosaicism in mutation-negative cryopyrin-associated periodic syndrome patients. Blood. 2008;111:2132–2141. doi: 10.1182/blood-2007-06-094201. [DOI] [PubMed] [Google Scholar]
- 26.Jackson SH, Gallin JI, Holland SM. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehtinen MK, et al. Cystatin B deficiency sensitizes neurons to oxidative stress in progressive myoclonus epilepsy, EPM1. J Neurosci. 2009;29:5910–5915. doi: 10.1523/JNEUROSCI.0682-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villani GR, et al. Mucopolysaccharidosis IIIB: Oxidative damage and cytotoxic cell involvement in the neuronal pathogenesis. Brain Res. 2009;1279:99–108. doi: 10.1016/j.brainres.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 29.Caroli F, et al. Clinical and genetic characterization of Italian patients affected by CINCA syndrome. Rheumatology (Oxford) 2007;46:473–478. doi: 10.1093/rheumatology/kel269. [DOI] [PubMed] [Google Scholar]
- 30.Carta S, et al. DAMPs and inflammatory processes: The role of redox in the different outcomes. J Leukoc Biol. 2009;86:549–555. doi: 10.1189/jlb.1008598. [DOI] [PubMed] [Google Scholar]
- 31.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic-onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandra J, et al. Adaphostin-induced oxidative stress overcomes BCR/ABL mutation-dependent and -independent imatinib resistance. Blood. 2006;107:2501–2506. doi: 10.1182/blood-2005-07-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔCT method. Methods. 2001;24:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.