Abstract
Probing gene–environment interactions that affect neural processing is crucial for understanding individual differences in behavior and disease vulnerability. Here, we tested whether the current environmental context, which affects the acute brain state, modulates genotype effects on brain function in humans. We manipulated the context by inducing acute psychological stress, which increases noradrenergic activity, and probed its effect on tonic activity and phasic responses in the amygdala using two MRI techniques: conventional blood oxygen level–dependent functional MRI and arterial spin labeling. We showed that only carriers of a common functional deletion in ADRA2B, the gene coding for the α2b-adrenoreceptor, displayed increased phasic amygdala responses under stress. Tonic activity, reflecting the perfusion of the amygdala, increased independently of genotype after stress induction. Thus, when tonic activity was heightened by stress, only deletion carriers showed increased amygdala responses. Our results demonstrate that genetic effects on brain operations can be state dependent, such that they only become apparent under specific, often environmentally controlled, conditions.
Keywords: ADRA2B, gene–environment interactions, fMRI, imaging genetics
Interindividual differences in both normal and pathological brain function are caused by genetic and environmental factors, as well as the interactions between the two. Although the understanding of the effects of genetic variance on the brain has grown, and multiple polymorphisms have been found to affect brain structure and function (1, 2), insight as to the interactions between genetic and environmental factors remains limited. Investigating these interactions is crucial for obtaining a complete understanding of mechanisms leading to individual variation (3), and both genetic and environmental effects might not be uncovered without taking these interactions into account. It has been shown that the influence of genetic variation on brain activity (4) and risk for depression (5) is modulated by the accumulation of stressful life events. This supports the diathesis–stress theory, which predicts that genetic vulnerability interacts with stressful life events in determining psychological functioning (6). These effects are presumably mediated by epigenetic mechanisms that lead to long-lasting alterations in gene expression (7). However, variable reactions to present environmental challenges are also dependent on certain stable characteristics (8), suggesting that genetic influences on brain function may be modulated by the current environmental context. To investigate this, we designed an experiment in which we manipulated the present environment and measured its ability to modulate genetic effects on brain function in humans.
Animal models suggest that a state of acute stress modulates genetic effects on the brain (9, 10), underlining the importance of the stressful state. Indeed, stressful situations are evolutionarily relevant and lead to a fundamental shift in the state of the brain. Acute stress is associated with a surge in vigilance, which enables the organism to respond quickly and adaptively to any threat to its homeostasis (11). This surge is mediated by the release of stress hormones from the hypothalamus (12) and widespread noradrenergic projections from the locus coeruleus (13, 14), which affect neuronal signaling via multiple receptors (15). A key target of noradrenergic projections is the amygdala, the central structure for threat detection, vigilance regulation, and facilitation of memory for arousing experiences (16, 17), as has been shown in both animals (18) and humans (19, 20). The effects of different neurotransmitters on emotional memory even converge in regulating noradrenaline release in the amygdala (21). Vulnerability to stress has been shown to depend on amygdala predisposition, thereby indicating that the effects of stress on the amygdala are likely mediated by genetic factors (22).
A common functional deletion in the gene coding for the presynaptic α2b-adrenoreceptor (ADRA2B) leads to the loss of three glutamic acid residues (residues 301–303) in the third intracellular loop of the receptor, changing its negative feedback function. In vitro data suggest that the deletion exerts both agonistic and antagonistic effects (23). This deletion has been found to be associated with increased reexperiencing of traumatic memories in humans (24), which suggests that the deletion acts as a reduced-function variant. Furthermore, this finding indicates that carriers of this deletion are more strongly affected by stressful events. It has been shown that carriers of the ADRA2B deletion display increased amygdala activity while encoding arousing negative pictures, but not when encoding less-arousing positive pictures (25). This suggests that the neural effects of this genotype are dependent on arousal. However, the level of arousal was inherently confounded with the stimulus content in that study, precluding this conclusion. To investigate this possibility, we tested whether experimentally induced acute stress modulates the influence of ADRA2B variation on amygdala processing for salient but non-arousing stimuli. Additionally, we disentangled state-related changes from stimulus-related changes in brain activity by using different functional MRI (fMRI) techniques.
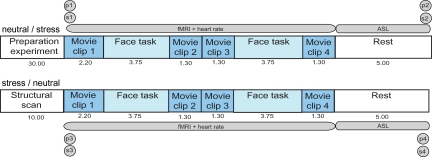
To this end, 41 healthy, young, Caucasian men participated in an fMRI experiment. Each participant took part in two sessions, consisting of a stress condition and a control condition, of which the order was counterbalanced across participants (Fig. 1). Acute stress was induced by showing short movie clips with highly aversive content and a self-reference instruction, which directly preceded and followed the task. This method of stress induction has been shown previously to elicit an acute stress response (26–28). To assess the effectiveness of this method in our study, subjective negative affect, salivary cortisol and α-amylase, and heart rate were measured. The latter two can be seen as correlates of adrenergic activity (29). In the control condition, movie clips with emotionally neutral content were implemented. We investigated the effect of acute stress on amygdala reactivity using a task that robustly engages the amygdala (26), in which participants passively viewed photographed faces with expressions morphing dynamically into either happy or fearful. During this task, we measured conventional fMRI based on blood oxygen level–dependent (BOLD) contrast to assess phasic responses of neural activity induced by changing experimental conditions. Thus, we measured the response of the amygdala to faces with emotional expressions during the stress condition and during the neutral condition. Additionally, stress may lead to more slowly modulated state changes, and therefore we measured regional perfusion by obtaining continuous arterial spin labeling (CASL) data after the fourth movie clip in both experimental conditions (30). These CASL data were used as a correlate of tonic activity, complementing the data on phasic responses, which were obtained with conventional fMRI. ADRA2B genotype was determined for each participant, and 56% were carriers of the deletion variant. All participants were college students, and the genotype groups (carriers vs. noncarriers) did not differ significantly in age (P > 0.1), trait anxiety (P > 0.6), nor in baseline levels of cortisol (P > 0.8) and α-amylase (P > 0.2) as measured the day before the experiment at home. We predicted that the state of acute stress would lead to a larger increase in noradrenaline for deletion carriers and expected that this increase would be reflected by increased phasic activity, increased tonic activity, or increases in both phasic and tonic activity in the amygdala for deletion carriers as compared with nondeletion carriers.
Fig. 1.
Overview and timeline of the experimental design. All participants went through both conditions. For half of the participants the first part of the experiment (Upper) consisted of the stress condition, whereas for the other half the first part consisted of the neutral condition. Preparation took place outside the scanner. Between the second and third movie n-back data were acquired, which are not reported here. The numbers below the movies and tasks indicate the time in minutes. P, PANAS; S, saliva sampling. Acquisition of each PANAS and each saliva sample took 5 min, thereby causing a 20-min gap between conditions.
Results
Physiological and Behavioral Measures Reveal a State of Acute Stress.
To establish whether the stress induction was successful, heart rate was recorded throughout the stress and control conditions, and measures of salivary cortisol, salivary α-amylase, and subjective negative affect were taken before and after each experimental condition. A stress × time repeated-measures ANOVA showed an interaction effect for heart rate [F(2,37) = 34.79, P < 0.001], with heart rate being higher before (mean beats per minute: 64.9 vs. 60.2), during (62.3 vs. 61.2), and after (66.1 vs. 60.0) the amygdala activation task in the stress condition than in the control condition. Similar stress × time interactions were found for salivary cortisol [F(1,36) = 4.46, P = 0.042], salivary α-amylase [F(1,36) = 7.97, P = 0.008], and negative affect [F(1,39) = 20.43, P < 0.001]. Increases were observed during the stress condition [mean cortisol (nmol/l): from 7.68 to 8.32; mean α-amylase (U/L): from 47.77 to 55.30; mean negative affect: from 12.85 to 16.29], whereas decreases were observed during the neutral condition (cortisol: from 7.97 to 6.39; α-amylase: from 58.06 to 49.36; negative affect: from 13.23 to 12.55). Heart rate, salivary cortisol, salivary α-amylase, and negative affect did not vary as a function of ADRA2B genotype (heart rate: P > 0.6; cortisol: P > 0.4; α-amylase: P > 0.1; negative affect: P > 0.07). Together, these findings indicate that a similar state of acute stress was induced during the stress condition in deletion as well as nondeletion carriers.
ADRA2B Affects Phasic Amygdala Responses Only During a State of Acute Stress.
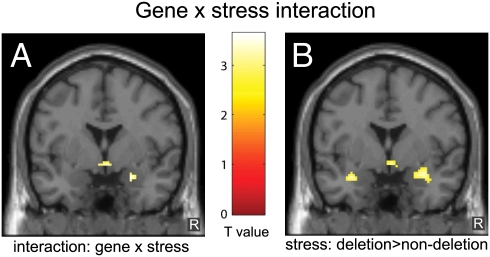
Results obtained with conventional fMRI based on BOLD contrast showed that our task elicited bilateral phasic responses in a well-defined set of brain regions, including the ventral visual stream ranging from the primary visual cortex to the fusiform gyrus, and medial temporal lobe regions including the amygdala and hippocampus [P < 0.05, familywise error (FWE)-corrected]. To evaluate whether the results were moderated by specific emotional expressions, the interactions with emotion type were tested first. However, neither the three-way interaction of ADRA2B genotype × stress × emotion nor the stress × emotion and genotype × emotion interactions were significant (FWE-corrected, P > 0.05). This suggests that the results were not influenced by emotion type. Therefore, we evaluated the influence of ADRA2B genotype independent of emotional expression. Importantly, the results revealed an ADRA2B genotype × stress interaction (Fig. 2A) in the right amygdala [MNI coordinates: (x = 24, y = 0, z = −20), Z = 3.35, P = 0.013 small volume corrected (SVC)]. Independent t tests for simple effects showed larger amygdala responses in deletion carriers as compared with nondeletion carriers (Fig. 2B) during the stress condition [(28, −2, −18), Z = 3.26, P = 0.018 (SVC)], whereas no significant difference between the groups was observed in the control condition [P > 0.5 (SVC)]. Thus, these tests for simple effects demonstrated that there was only an effect of genotype in the stress condition. This pattern of results suggests that the influence of ADRA2B variation on amygdala reactivity is dependent on the stress-related brain state as induced by an environmental context of acute stress.
Fig. 2.
The influence of the common deletion in ADRA2B on amygdala responses is modulated by acute stress. (A) Significant interaction between ADRA2B genotype and acute stress in the right amygdala. (B) Larger conventional fMRI responses in deletion carriers than nondeletion carriers in the acute stress condition. The figures show the statistical comparisons (P < 0.005 uncorrected for visualization purposes) superimposed on a single-subject T1-weighted image (y = 0).
Strikingly, subsequent paired-sample t tests showed that the stress condition reduced amygdala responses in nondeletion carriers [(x = 24, y = 0, z = −20), Z = 4.52, P < 0.001 (SVC)], whereas no significant difference between conditions was observed in deletion carriers [P > 0.5 (SVC)]. Because conventional BOLD fMRI measures the differential response between the face processing task and fixation baseline, the reduced phasic amygdala response could also reflect enhanced tonic baseline activity throughout the stress condition, leading to reduced differential responses. This would suggest that ADRA2B nondeletion carriers do not show phasic amygdala responses at higher baseline activity, whereas amygdala responses increase even beyond the stress-enhanced baseline in ADRA2B deletion carriers. To investigate this possibility, we looked at tonic baseline activity as measured with CASL.
Stress Increases Tonic Amygdala Activity Independently of Genotype.
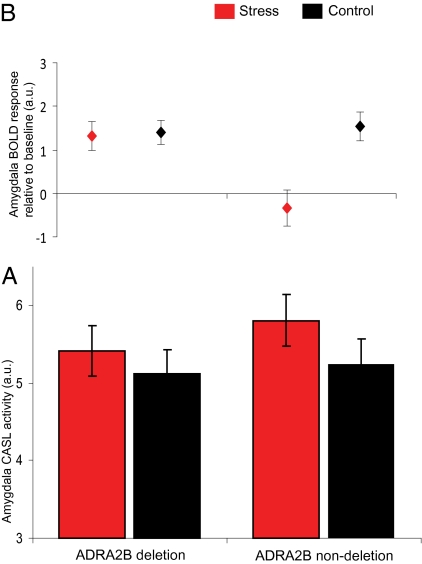
Acute stress leads to more slowly modulated state changes and has been shown to affect tonic activity in the amygdala (31). Therefore, we measured regional brain perfusion using CASL to investigate the stress-induced changes in tonic amygdala activity (32). Knowledge of changes in tonic activity forms an indication of stress-induced state changes and will lead to a better interpretation of the pattern of phasic responses. We extracted perfusion data, calculated by pairwise subtraction of the label/control image pairs, from the cluster of amygdala activation found with conventional fMRI by creating regions of interest (ROIs) for each individual participant. The results showed an increase in tonic amygdala perfusion during the stress condition as compared with the neutral condition [F(1,34) = 6.17, P = 0.018] but no significant ADRA2B genotype × stress interaction [F(1,34) = 0.57, P = 0.46] or effect of genotype [F(1,34) = 0.363, P = 0.55]. Thus, tonic amygdala activity was affected by stressful environmental conditions but not by ADRA2B genotype. These results indicate that although acute stress leads to increased tonic amygdala activity, this increase seems not directly responsible for the genotype-dependent differences in phasic responses as observed with conventional fMRI. In addition, this result supports the conventional fMRI data by showing that stress increased tonic baseline activity in both genotypes. Therefore, the combination of the conventional fMRI and CASL results suggest that both genotypes show augmented amygdala perfusion under stress, with deletion carriers showing an increase in phasic responses on top of that (Fig. 3).
Fig. 3.
The influence of acute stress and the common deletion in ADRA2B on amygdala perfusion and amygdala responses. Shown is the combination of tonic amygdala activity (CASL fMRI) and phasic amygdala responses (BOLD fMRI). (A) Tonic activity: amygdala perfusion was higher in the stress than control condition, and this effect was not significantly different between ADRA2B genotypes. (B) Phasic responses: deletion carriers were able to increase amygdala responses in both the stress and neutral condition despite the differences in perfusion, whereas nondeletion carriers only increased amygdala activity during the neutral condition. a.u., arbitrary units.
Discussion
In the present study we examined whether and how genotype effects can be modulated by the current environmental context in humans. The results indicate that the influence of ADRA2B genotype on brain processes is dependent on a state of acute stress. Whereas under non-stressful conditions no effect of ADRA2B on amygdala processing was revealed, deletion carriers showed increased phasic amygdala responses compared with nondeletion carriers in a state of acute stress. These results suggest that the increased amygdala activity in deletion carriers that was found previously (25) might be due to the arousal caused by the stimuli used in that study. These arousing stimuli might have led to a state of stress, thereby allowing the genotype effects to become apparent. Our ADRA2B genotype by acute stress design demonstrated that acute stress can lead to state changes by using both physiological stress measures and measures of tonic brain activity. Thereby, we revealed that the effects of genes on the brain can be state dependent, such that they only become apparent under specific, often environmentally controlled, conditions.
The finding that none of the physiological measures, serving as a reflection of the stress response, showed ADRA2B dependency might be explained by a lesser sensitivity of physiological measures as compared with measures of brain activity. However, future studies with larger subject numbers could use path analyses (33) to demonstrate significant effects of genetic variation on behavior through effects on brain function. Because CASL was always performed at the end of the scanning session, it is theoretically possible that the lack of stress × genotype interaction is due to a position effect. However, we think the CASL data as measured at the end of the experiment are representative for the brain state that was present throughout the experiment. The CASL data are a reflection of slowly modulated changes in amygdala perfusion in a time window that has the same temporal relation to the stressor as the BOLD fMRI data measured during the task of interest. Therefore, we can assume a similar brain state of increased tonic activity during the BOLD fMRI and CASL measurements.
The increased amygdala response we observed in deletion carriers as compared with nondeletion carriers may result from changes in noradrenaline availability, because the deletion leads to a perturbation of the relationship between incoming noradrenaline signals and receptor responsiveness (23). The α2b-adrenoreceptor is a presynaptic receptor and has a negative feedback function. In vitro data suggest that the deletion exerts both agonistic and antagonistic effects, making it impossible to predict whether the deletion leads to increased or decreased noradrenergic signaling (23). However, behavioral data indicate that the deletion acts as a reduced-function or even loss-of-function variant, thereby leading to increased noradrenaline availability (24). Our finding of a relative increase in amygdala activity for deletion carriers provides additional evidence for the idea that the ADRA2B deletion potentiates noradrenergic activity. We found that during the neutral condition both deletion and nondeletion carriers are still able to increase phasic responses. However, when there is an increase in tonic activity during the stress condition and therefore a change in baseline activity, nondeletion carriers no longer display increased phasic activity in the amygdala. This is in line with studies indicating that increases in tonic activity lead to decreased phasic responses (14). Nevertheless, the deletion group, with putatively increased noradrenaline availability, seems able to increase noradrenaline further and thus show phasic responses to emotional stimuli. Increased noradrenaline in the amyg-dala leads to enhanced emotional memory (21), and it has been proposed that overactivation of this system may contribute to the development of posttraumatic stress disorder (34, 35). One may therefore speculate whether the interaction revealed here could act as a vulnerability factor for stress-related mental disorders.
The context-dependent influence of genes on brain function is in line with recent theories about human personality and development. Classical personality theories have emphasized either the influence of stable characteristics on behavior or the impact of the situation on behavior. However, our results support the more recent theory that reconciles both viewpoints and posits that personality determines the response to certain situations (36). These conditional influences are thought to develop through the interaction between genes and the environment (8), which may strengthen the initial characteristics (37). Our results suggest that these context-dependent genetic characteristics are mediated by those brain regions important for the particular behavior.
In sum, in this study we disentangled the effects of ADRA2B genotype on tonic and phasic amygdala processing by inducing acute stress. Most importantly, the results demonstrate that the influence of genes on brain function can be dependent on the current context. This finding underlines the importance of studying gene–environment interactions for the understanding of the mechanisms leading to interindividual differences in behavior, disease vulnerability, and underlying brain function. Future studies may reveal whether previously observed effects of other polymorphisms show the same state dependency.
Materials and Methods
Participants.
Forty-one Caucasian men (aged 18–35 years) with normal or corrected-to-normal vision participated in this study. Only men were included because hormonal fluctuations across the menstrual cycle and hormonal contraceptives influence the stress response (38). Participants reported no history of psychiatric, neurological, or endocrine diseases and no current use of psychoactive drugs or corticosteroids. All had participated in previous MRI experiments to ensure that no stress response would be evoked by unfamiliarity with the environment and procedures. After the experiment, ADRA2B genotype was determined for each participant, and 56% were carriers of the deletion variant leading to the loss of three glutamic acid residues (residues 301–303) in the third intracellular loop of the receptor protein, which has an observed allele frequency of ≈31% in a Caucasian population (23). Only three participants were homozygous for the deletion. Written informed consent was obtained before the experiment, and the study was carried out in accordance with the guidelines of the local ethics review board (Commissie Mensgebonden Onderzoek Region Arnhem-Nijmegen, The Netherlands) and in accordance with the declaration of Helsinki. Data of one participant were excluded owing to technical failure, and MRI data of one participant were excluded owing to excessive head movement.
General Procedure.
In the week preceding the experiment, participants were contacted by telephone and answered a set of questions that formed the exclusion criteria for this experiment. After the phone call, participants were sent a salivette collection device (Sarstedt) and were asked to take a baseline saliva sample in the late afternoon on the day before the experiment. The experiment took place in the afternoon or early evening to ensure low and relatively stable levels of endogenous cortisol. Upon arrival, participants completed the trait version of the State-Trait Anxiety Inventory (39) and had the experiment explained to them. All individuals participated in both the stress and the control condition, and this was done in counterbalanced order. Each condition consisted of the amygdala activation task, which was interleaved with stressful or neutral movies. Immediately after the last movie, perfusion was measured with CASL. Before and after the stress and control condition, saliva samples and subjective affect ratings were collected, and heart rate was measured throughout the experiment. The two conditions were separated by ≈20 min, during which a structural MRI scan was made for anatomical normalization purposes.
Stress Induction.
Moderate psychological stress was induced by showing short movie clips within the MRI scanner, containing scenes with strongly aversive content (extreme violence) selected from a commercially available movie (40). During the control condition participants watched equally long movie clips from another movie (41), which were equal in luminance and similar in language but contained only non-arousing scenes. After short introductory texts, participants were asked to watch the movies attentively and take an eye-witness perspective as to involve them maximally in the action taking place in the movie clips. The present stress induction method closely corresponds to the determinants of the human stress response as described by Mason (42) (i.e., unpredictability, novelty, and uncontrollability). Moreover, previous studies have shown that this method elicits a measurable physiological and psychological stress response (26–28).
Amygdala Activation Task.
Between the movie clips, participants passively viewed blocks of faces morphing dynamically into happy or fearful expressions. The perceptual processing of emotional faces has been shown to reliably and robustly engage the amygdala (43) and even more so with dynamic rather than static presentation of facial expressions (44). Stimuli consisted of short 133-ms animation clips for each of 10 different faces [taken from a standardized set and equalized in luminance and contrast (45)] showing a morphing sequence consisting of four frames (55%, 70%, 85%, and 100% emotional expression) repeated at 2 Hz. The amygdala activation task lasted 7.45 min and consisted of six blocks of 25 s of each emotion and six blocks of 25 s of fixation cross. The whole task was divided into two parts so that it was fully embedded into a stressful context. The first part of the task was carried out between the first and second movie and the second part between the third and fourth movie. Because the two parts of the face task did not lead to different activation patterns (FWE-corrected, P > 0.05), we collapsed the data for further analyses. Blocks were presented in a mirrored design avoiding covariation with linear drift, and adjacent blocks of the same emotion or fixation cross were avoided. The order of blocks was counterbalanced across participants. Participants were requested to press a button with their right index finger when they saw a fixation cross, to ensure that they were paying attention.
Subjective and Physiological Measurements.
Subjective mood was assessed by obtaining scores on the Positive and Negative Affect Scale (PANAS) (46) before and after the stress and the control condition. Ten items for positive and 10 for negative affect had to be rated on a 5-point scale ranging from 1 (not at all) to 5 (extremely). A mean score was calculated for subjective negative affect.
To assess the autonomic response and the hypothalamic–pituitary–adrenal axis response to the context manipulation, saliva was sampled with salivette collection devices to determine the levels of α-amylase and cortisol. Samples were taken on the day before the experiment and before and after both conditions (five in total) and were stored at −20 °C until analysis. The analysis was carried out at the Biopsychology Department in Dresden, where samples were prepared for biochemical analysis by centrifuging at 1,500 × g for 5 min, which resulted in a clear supernatant of low viscosity. Salivary-free cortisol concentrations were determined with a chemiluminescence assay with high sensitivity of 0.16 ng/mL (IBL). Concentration of α-amylase in saliva was measured by an enzyme kinetic method: saliva was processed on a Genesis RSP8/150 liquid handling system (Tecan). First, saliva was diluted 1:625 with double-distilled water by the liquid handling system. Twenty microliters of diluted saliva and standard were then transferred into standard transparent 96-well microplates (Roth). Standard was prepared from “Calibrator f.a.s.” solution (Roche Diagnostics) with concentrations of 326, 163, 81.5, 40.75, 20.38, 10.19, and 5.01 U/L α-amylase, respectively, and double-distilled water as zero standard. After that, 80 mL of substrate reagent (α-amylase EPS Sys; Roche Diagnostics) were pipetted into each well using a multichannel pipette. The microplate containing sample and substrate was then warmed to 37 °C by incubation in a water bath for 90 s. Immediately afterward, a first interference measurement was obtained at a wavelength of 405 nm with a standard ELISA reader (Anthos Labtech Instruments HT2). The plate was then incubated for another 5 min at 37 °C in the water bath, before a second measurement at 405 nm was taken. Increases in absorbance were calculated for unknowns and standards. Increases of absorbance of diluted samples were transformed to α-amylase concentrations using a linear regression calculated for each microplate (Graphpad Prism 4.0c for MacOSX; Graphpad Software). For one subject no data were acquired and for one subject the analysis did not succeed, whereas data of a third subject were not taken into account because he consumed caffeine shortly before the experiment.
To assess autonomic activity throughout the experiment, we continuously recorded heart rate with an infrared pulse oximeter (accompanying the MRI scanner; Siemens) placed on a finger of the left hand. Offline artifact correction and analysis of the heart rate frequency were done with in-house software. The heart rate frequency was averaged for the duration of each movie clip and the task. For one subject, data were not available.
fMRI Data Acquisition.
During both conditions, whole brain T2*-weighted BOLD fMRI data were acquired using echoplanar imaging (EPI) with a Siemens TIM Trio 3.0 Tesla MR scanner using an ascending slice acquisition sequence [37 axial slices, volume repetition time (TR) = 2.18 s, echo time (TE) = 25 ms, flip angle = 90°, slice matrix size = 64 × 64, slice thickness = 3.0 mm, slice gap = 0.3 mm, field of view (FOV) = 212 mm]. Two hundred five images were acquired during the amygdala activation task.
At the end of both conditions, resting-state CASL data were recorded with an ascending slice acquisition sequence (labeling time = 2 s, post-label delay time = 1 s, label offset = 8.0 cm, TR = 3.69 s, TE = 11 ms, flip angle = 90°, matrix size = 64 × 64, slice thickness = 5 mm, slice gap = 1.5 mm, FOV = 224 mm, bandwidth = 2,694 Hz per pixel). Eighty images were acquired for each participant in each condition. For three participants, no CASL data were acquired owing to technical failure.
High-resolution structural images were acquired using a T1-weighted 3D magnetization-prepared rapid gradient echo sequence (TR = 2.3 s, TE = 3.03 s, flip angle = 8°, 192 contiguous sagittal slices, slice matrix size = 256 × 256, FOV = 256 mm).
fMRI Data Analysis.
Image preprocessing and statistical analysis of the BOLD fMRI data were performed using SPM5 (Wellcome Department of Imaging Neuroscience, London). The first five EPI volumes were discarded to allow for T1 equilibration. Remaining functional images were realigned with rigid body transformation and coregistered to the anatomical T1-weighted MR image. Subsequently, images were transformed into a common stereotactic space (MNI152 T1-template) and resampled into 2 × 2 × 2-mm3 isotropic voxels. Spatial smoothing was performed with an isotropic 3D Gaussian kernel of 8 mm full-width at half-maximum.
Statistical analysis was performed within the framework of the general linear model. The presentation of emotional faces was modeled as boxcar regressor and convolved with the canonical hemodynamic response function of SPM5. Additionally, realignment parameters were included to model potential movement artifacts. Contrast parameter images generated at the single subject level (emotion vs. fixation) were submitted to second-level group analysis. This group analysis was a 2 (genotype) × 2 (stress) mixed-model ANOVA. Statistical tests were corrected for multiple comparisons across the entire brain or for the search volume for the amygdala using a small-volume correction (47), which was anatomically defined using the Wake Forest University Pickatlas (48).
Preprocessing of the CASL data was carried out with the SPM-based ASL data processing toolbox using standard settings (49). Images were realigned, and spatial smoothing was applied with a 3D isotropic kernel with 9-mm full-width at half-maximum, followed by image coregistration between the raw EPI and structural images. Perfusion difference images were calculated by pairwise subtraction of the label/control image pairs. The amygdala cluster observed in the conventional fMRI analysis at a threshold of P < 0.05 uncorrected was defined and transformed back into the anatomical space for each individual subject, thereby creating ROIs for all individual participants. Mean perfusion data were extracted for these ROIs and subjected to a 2 (genotype) × 2 (stress condition) ANOVA to determine differences in perfusion.
Genetic Analysis.
For the analysis of the insertion/deletion polymorphism in ADRA2B, we used fragment length analysis on a genetic analyzer. In short, amplification of a fragment containing the variant was performed in a total volume of 10 μL containing 50 ng of DNA and 1× PCR buffer II (Applied Biosystems), 2.5 mM MgCl2 (Applied Biosystems), 0.25 mM dNTPs (GE Healthcare), 0.5 μL (10 pmol/μL) of each primer (Applied Biosystems) [i.e., a NED-labeled forward primer (NED-AGAAGGAGGGTGTTTGTGGGG) and a reverse primer carrying a “PIG tail” (ACCTATAGCACCCACGCCCCT-GTTTCTT)], 0.5 M betaine (Sigma), and 0.04 U AmpliTaq Gold (Applied Biosystems). The amplification protocol consisted of an initial 12 min at 95 °C, followed by 32 cycles of 1 min at 94 °C, 1 min at 66 °C, and 1 min at 72 °C, and finishing with a step of 7 min at 72 °C. For the fragment length analysis on an ABI Prism 3730 Genetic Analyzer, 1 μL of (diluted) PCR product was added to 8.7 μL formamide (Applied Biosystems) and 0.3 μL Liz600 standard (Applied Biosystems). The results were analyzed using GeneMapper Software, version 4.0 (Applied Biosystems). Testing for Hardy-Weinberg equilibrium did not show deviations from the expected distribution [χ2(1) = 0.65, P = 0.42]. In further analyses, a group of deletion carriers (heterozygous and homozygous) was compared with nondeletion carriers.
Acknowledgments
We thank E. R. de Kloet for helpful discussions; E. van Dongen for assistance with data collection; and M. Naber, J. Eernstman, A. Heister, M. Laverman, and S. van der Waal for assistance with data analysis. This project was supported by Grant 918.66.613 from the Dutch Organization for Scientific Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Green AE, et al. Using genetic data in cognitive neuroscience: From growing pains to genuine insights. Nat Rev Neurosci. 2008;9:710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- 2.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 3.Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: Joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 4.Canli T, et al. Neural correlates of epigenesis. Proc Natl Acad Sci USA. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspi A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 6.Abramson LY, Metalsky FI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychol Rev. 1989;96:358–372. [Google Scholar]
- 7.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular biology of resilience. Nat Rev Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mischel W, Shoda Y. Reconciling processing dynamics and personality dispositions. Annu Rev Psychol. 1998;49:229–258. doi: 10.1146/annurev.psych.49.1.229. [DOI] [PubMed] [Google Scholar]
- 9.Kalin NH, et al. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008;13:1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DL, et al. Genetic perspectives on the serotonin transporter. Brain Res Bull. 2001;56:487–494. doi: 10.1016/s0361-9230(01)00622-0. [DOI] [PubMed] [Google Scholar]
- 11.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 12.Wolf OT. The influence of stress hormones on emotional memory: Relevance for psychopathology. Acta Psychol (Amst) 2008;127:513–531. doi: 10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 14.Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- 15.Ramos BP, Arnsten AFT. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Strange BA, Dolan RJ. β-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci USA. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGaugh JL. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annu Rev Neurosci. 1989;12:255–287. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- 19.Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 20.Cahill L, Prins B, Weber M, McGaugh JL. β-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 21.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 22.Admon R, et al. Human vulnerability to stress depends on amygdala's predisposition and hippocampal plasticity. Proc Natl Acad Sci USA. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Small KM, Brown KM, Forbes SL, Liggett SB. Polymorphic deletion of three intracellular acidic residues of the α2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J Biol Chem. 2001;276:4917–4922. doi: 10.1074/jbc.M008118200. [DOI] [PubMed] [Google Scholar]
- 24.De Quervain DJF, et al. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- 25.Rasch B, et al. A genetic variation of the noradrenergic system is related to differential amygdala activation during encoding of emotional memories. Proc Natl Acad Sci USA. 2009;106:19191–19196. doi: 10.1073/pnas.0907425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Marle HJF, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: How acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Henckens MJ, Hermans EJ, Pu Z, Joëls M, Fernández G. Stressed memories: How acute stress affects memory formation in humans. J Neurosci. 2009;29:10111–10119. doi: 10.1523/JNEUROSCI.1184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin S, Hermans EJ, van Marle HJF, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Chatterton RT, Jr, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary α-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, et al. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tillfors M, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: A PET study. Am J Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, et al. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: Feasibility study. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- 33.Fakra E, et al. Effects of HTR1A C(-1019)G on amygdala reactivity and trait anxiety. Arch Gen Psychiatry. 2009;66:33–40. doi: 10.1001/archpsyc.66.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntyre CK, Power AE, Roozendaal B, McGaugh JL. Role of the basolateral amygdala in memory consolidation. Ann N Y Acad Sci. 2003;985:273–293. doi: 10.1111/j.1749-6632.2003.tb07088.x. [DOI] [PubMed] [Google Scholar]
- 35.Southwick SM, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 36.Mischel W, Shoda Y. A cognitive-affective system theory of personality: Reconceptualizing situations, dispositions, dynamics, and invariance in personality structure. Psychol Rev. 1995;102:246–268. doi: 10.1037/0033-295x.102.2.246. [DOI] [PubMed] [Google Scholar]
- 37.Caspi A, Roberts BW, Shiner RL. Personality development: Stability and change. Annu Rev Psychol. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- 38.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Publishing; 1970. [Google Scholar]
- 40.Noé G. Irréversible [motion picture], director Noé G (120 Films) 2002 [Google Scholar]
- 41.Fieschi J, Fontaine A. Comment j'ai tué mon père [motion picture], director Fontaine A (Ciné B) 2001 [Google Scholar]
- 42.Mason JW. A review of psychoendocrine research on the sympathetic-adrenal medullary system. Psychosom Med. 1968;30:576–607. doi: 10.1097/00006842-196809000-00022. [DOI] [PubMed] [Google Scholar]
- 43.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Sato W, Kochiyama T, Yoshikawa S, Naito E, Matsumura M. Enhanced neural activity in response to dynamic facial expressions of emotion: An fMRI study. Brain Res Cogn Brain Res. 2004;20:81–91. doi: 10.1016/j.cogbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Ekman P, Friesen V. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Publishing; 1976. [Google Scholar]
- 46.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 47.Worsley KJ, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 48.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, et al. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26:261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]