Abstract
Most arthropod-borne and invertebrate viruses are orally ingested and commence infection in cells of the invertebrate intestine. Infection of secondary sites and eventual transmission to other hosts is hindered by basal lamina, a tightly interwoven and virus-impenetrable noncellular layer, lining the intestine and other organ cell layers. The mechanisms for viral escape across basal laminae are unknown. We describe an elegant mechanism mediated by a baculovirus-encoded fibroblast growth factor (vFGF) that signals a previously undescribed stepwise cascade of protease activation wherein matrix metalloproteases activate effector caspases, leading to remodeling of basal lamina lining tracheal cells associated with the intestine and culminating in the establishment of efficient systemic infections. Because FGFs coordinate diverse functions during development, metabolic processes, and tissue repair, it is plausible that the vFGF-mediated pathway described here is widely used during developmental and pathogenic processes that involve basal lamina remodeling.
Keywords: basal lamina, FGF, midgut, virus
The basal lamina (BL) is a tightly woven, dynamic, and interactive protein mesh lining the basal side of cell sheets. It is mainly composed of type IV collagen, laminin, nidogen, and perlecan proteoglycans, and has roles in cell adhesion, cell migration, tissue repair, and morphogenesis. BL prevents adjoining cells from making contact with the epithelial layer, thus defining spatial boundaries among cell types and protecting the epithelium from invasion by macromolecules and pathogens. The intestines of animals are subject to commensal and pathogenic microbial infections. BL lining gut cells serves as a nearly impassable barrier, denying pathogens access to susceptible underlying tissues.
The insect tracheal system functions in gas exchange and bifurcates repeatedly to form a network of fine tube-like structures that service the tissues of the insect. Tracheae are composed of a chitinous tube surrounded by tracheal epithelial cells, which secrete the outermost layer, BL. Tracheal epithelial cells are often subject to infection (1–3), but it is not clear how tracheal cells surrounded by BL are accessed by pathogens.
An important and long-standing question is how insect pathogens and insect-vectored pathogens gain access from midgut epithelial cells, the primary site of infection, to other organs in the main insect cavity or hemocoel, bypassing cellular and noncellular barriers, as well as host defenses. Although the mechanism has not been defined, arthropod-vectored viruses and insect pathogens such as the baculovirus Autographa californica M nucleopolyhedrovirus (AcMNPV) have been observed in tracheal epithelial cells after midgut infections (2–4). Infection of tracheal epithelial cells is thought to be a prerequisite for AcMNPV infection of other tissues (5, 6). It has been hypothesized that tracheoblasts that cross the BL are the secondary sites of infection and serve as a vehicle across the midgut cell BL to allow systemic infection (2).
We previously showed that AcMNPV lacking a viral fibroblast growth factor (vfgf) does not have obvious replication defects in cell culture but results in slower mortality than its wild-type virus in two hosts (7). These findings, functional parallels between cellular and vFGFs and knowledge of AcMNPV pathogenesis, led us to hypothesize that vfgf facilitates the systemic spread of the virus. In this study, we found a defect in the rate of systemic infection following oral infection of insects with the vfgf-knockout virus. We also observed that vFGF expression led to alterations of tracheal BL, and this was dependent on the activation of matrix metalloproteases (MMPs) and effector caspases. Furthermore, we showed that MMPs were able to directly activate effector caspases without apical caspase activation or apoptosis. These findings suggest a unique pathogenesis pathway in which expression of vfgf leads to disassembly of host noncellular barriers to establish efficient systemic infection.
Results
vFGF Accelerates Viral Spread.
AcMNPV vfgf is necessary for the timely death of orally infected insects (7); however, the mechanisms leading to this phenotype are not clear. We hypothesized that vfgf accelerates mortality by facilitating virus spread within the infected host. To determine the rate of infection, we used a virus lacking vfgf, AcBAC–vfgfKO, and its parent virus encoding vfgf, AcBAC, to monitor infection in susceptible tissues. Cell infection was tracked by enhanced green fluorescent protein (eGFP) expression, because both viruses encode egfp. We infected insects either orally, where occlusion-derived virions infect midgut epithelial cells before the establishment of systemic infections, or by injecting budded virus (BV) into the hemocoel, thus bypassing midgut barriers. We found a defect in the timing of systemic infection following oral but not intrahemocoelic infection of insects with AcBAC–vfgfKO (Fig. S1). These findings stress that vFGF has a role at the primary or secondary site of infection but is not required for virus spread after midgut barriers have been breached. We also observed increased phosphorylation of tyrosines in AcBAC-infected tissues compared with mock- or AcBAC–vfgfKO-infected tissues, suggesting FGF receptor (FGFR) activation by vFGF in these tissues (Fig. S2).
vFGF Affects Tracheal Cell BL Dynamics.
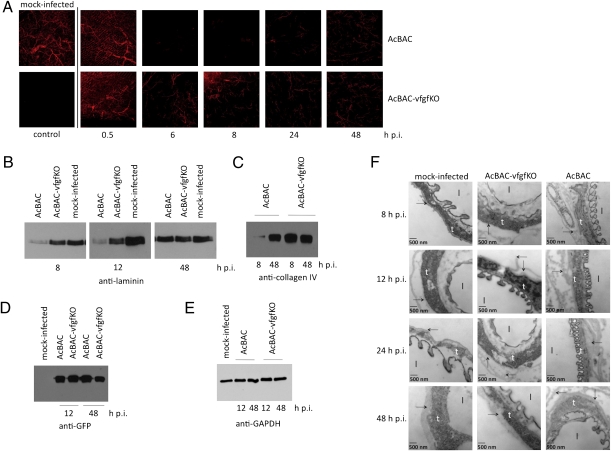
After primary infection of midgut epithelial cells, AcMNPV infects tracheoblasts that cross the midgut BL, serving as conduits to spread infection (2). To explore whether vFGF alters tracheal cell BL, we orally infected larvae with AcBAC or AcBAC–vfgfKO and immunodetected laminin, a major BL component. Mock-infected tracheae showed an elaborate branching pattern (Fig. 1A). As infection progressed, AcBAC-infected trachea no longer cross-reacted with anti-laminin as extensively (Fig. 1A), and at later times, laminin staining recommenced (Fig. 1A and Fig. S3). This pattern suggests tracheal BL remodeling, ending in secretion of BL, a normal function after cell growth or after injury. Complete regeneration of BL was not observed, in part because insects die. In AcBAC–vfgfKO-infected insects, tracheal cell BL was also disrupted, albeit at lower levels than in AcBAC-infected insects, and disruption did not increase with time. We did not observe obvious laminin remodeling in the BL that lines midgut epithelial cells (Fig. S4A). These effects were specific for laminin because antisera against type I collagen, a protein not present in BL but in neighboring cells, showed no differences (Fig. S4B).
Fig. 1.
vFGF affects tracheal BL integrity. Infected larvae were dissected at the indicated hours postinfection. (A) Tracheal laminin (red) was detected using anti-laminin. (B–E) Lysates from infected midguts were immunoblotted using antibodies that recognize laminin (B), type IV collagen (C), eGFP (D), or GAPDH (E). (F) Mock-infected or virus-infected tissues were observed using TEM. BL (arrow), tracheal epithelial cells (t), and trachea lumen (l) are indicated.
BL rearrangements were confirmed by immunoblotting lysates from AcBAC-infected tissues using antisera against laminin (Fig. 1B) or type IV collagen, another major component of BL (Fig. 1C). At early times p.i., the steady-state levels of laminin and type IV collagen were reduced in samples from AcBAC- but not AcBAC–vfgfKO-infected guts. Again, antibody cross-reactivity was renewed at 48 h p.i. The levels of eGFP were similar in all samples, indicating that infection levels were comparable (Fig. 1D). A host protein, GAPDH, did not have the same pattern (Fig. 1E), demonstrating that the transient decrease was specific for BL proteins. These data indicate that after virus infection, BL components were altered, and this effect was more pronounced in the presence of vFGF.
We observed tracheal BL in mock- or virus-infected larvae by transmission electron microscopy. BL of mock- and AcBAC–vfgfKO-infected tracheal epithelial cells bordered cells or exhibited folds, respectively (Fig. 1F). In contrast, AcBAC-infected tracheal cells showed extensive BL rearrangements. By 48 h p.i., BL integrity appeared less disrupted (Fig. 1F Lower), consistent with immunohistochemical and immunoblotting studies at late times p.i. once epithelial cells secreted new BL (Fig. 1 A and B).
Caspase Activation in Response to AcBAC Infection.
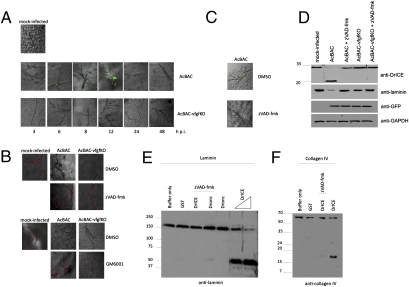
Extracellular matrix proteases can activate various processes, including cell death. Also, heparan sulfate-interacting proteins play a role in apoptosis (8). Given these correlations, we thought that the interactions of vFGF with heparan sulfate and FGFR may mediate signaling events that activate caspases, the executioners of apoptosis. To this end, we orally infected larvae with viruses and tested for caspase activation using antibodies against active DrICE, a Drosophila effector caspase, and eGFP, as evidence of virus infection (Fig. 2A and Fig. S5). AcBAC-infected tracheal cells had a protein that cross-reacted with anti-active DrICE, whereas AcBAC–vfgfKO-infected cells did not, even after 96 h p.i., when AcBAC–vfgfKO infection catches up with that of AcBAC. Protein detection with anti-DrICE was inhibited by the pan-caspase inhibitor zVAD-fmk (Fig. 2C). In addition, lysates from AcBAC- but not AcBAC–vfgfKO-infected midguts show cleavage of an anti-DrICE cross-reactive protein in the absence of zVAD-fmk and reduction of laminin (Fig. 2D).
Fig. 2.
Viruses encoding vFGF induce caspase activation during infection and affect tracheal cell BL. Infected larvae were dissected at the indicated hours postinfection, and tissues were examined. (A and C) Active effector caspases (green) were detected on tracheal cells using an anti-active DrICE. (B) Laminin (red) was detected on tracheal cells from mock- or virus-infected insects cofed with zVAD-fmk, GM6001, or carrier (DMSO) and dissected at 12 h p.i. (D) Lysates from mock- or virus-infected midguts dissected at 12 h p.i. were immunoblotted with the indicated antibodies. Molecular mass standards in kilodaltons are shown to the left. (E and F) Laminin (E) or type IV collagen (F) was incubated with active caspases and samples were immunoblotted using laminin (E) or type IV collagen antisera (F). Molecular mass standards in kilodaltons are shown to the left.
Laminin Disruption in Tracheal Cell BL Following Caspase Activation.
Because vFGF had a role in tracheal cell BL disruption and caspase activation, we investigated whether caspase activation was directly involved in tracheal cell BL alterations. Insects were infected orally in the presence or absence of zVAD-fmk and immunostained with anti-laminin. Laminin was apparent in AcBAC–vfgfKO- but not AcBAC-infected cells (Fig. 2B). Inhibition of caspase activation with zVAD-fmk blocked laminin disruption (Fig. 2B).
The ability of caspases to directly cleave BL proteins was investigated in vitro. Purified Drosophila apical caspase Dronc, effector caspase DrICE, or a nonspecific protein, GST, were incubated with purified laminin or type IV collagen in the presence or absence of zVAD-fmk. DrICE cleaved laminin and type IV collagen, and these activities were inhibited by zVAD-fmk (Fig. 2 E and F). Dronc did not cleave laminin significantly. These data indicate direct cleavage of BL proteins by effector caspases and caspase activation mediated by vFGF (Fig. 2).
MMPs Activate Effector Caspases.
MMPs are zinc endopeptidases present in the extracellular matrix and are involved in BL remodeling. More recently, cleavage of additional substrates by MMPs has been shown to lead to alternative signaling events, including apoptosis (9). We hypothesized that MMPs activated effector caspases, leading to degradation of BL components. To test this, we measured in vitro activity of caspases or MMPs in lysates from mock- or virus-infected midguts.
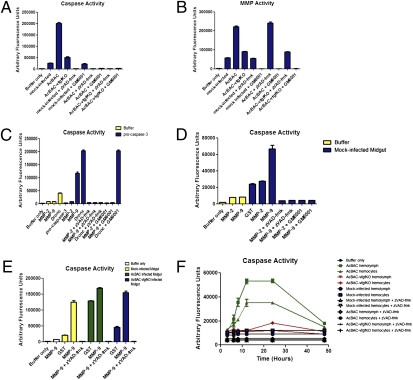
Lysates from AcBAC-infected midguts yielded high levels of effector caspase activity (Fig. 3A), consistent with our immunohistochemistry studies (Fig. 2A), and also exhibited high levels of MMP activity (Fig. 3B). Reactions using AcBAC–vfgfKO-infected material had caspase and MMP activities just slightly higher than mock-infected samples. These activities were specific, because the presence of zVAD-fmk or the MMP inhibitor GM6001 abolished the corresponding enzymatic activities. Moreover, in the presence of GM6001, we did not observe any virus-mediated caspase activity (Fig. 3A; AcBAC + GM6001 lane), whereas zVAD-fmk had no effect on MMP activity (Fig. 3B; AcBAC + zVAD-fmk lane). This finding implied that MMPs acted upstream of caspases, directly or indirectly activating caspases, which cleaved BL components (Fig. 2). In the absence of MMP activity, laminin was immunodetected in AcBAC-infected tissues (Fig. 2B). Inhibitors were specific for their target proteins (Fig. S6).
Fig. 3.
MMPs work upstream of caspase activation and caspases are released into the hemolymph. Larvae were orally infected with AcBAC or AcBAC–vfgfKO, or mock-infected and fed inhibitors zVAD-fmk, GM6001, or DMSO. Insects were dissected at 12 h p.i. or as indicated, and lysates were analyzed for enzymatic activity. (A, B, and E) Midgut lysates were analyzed for caspase (A and E) or MMP (B) activity using DEVD-afc caspase or MMP fluorescent substrates, inhibitors, and proteins. (C) Active MMP or initiator caspase Dronc was incubated with procaspase-3 in the presence or absence of zVAD-fmk or GM6001, and caspase activity measured. (D) Lysates from midguts or buffer only were used to measure caspase activity in the presence of active MMPs. (F) Hemolymph and hemocytes were collected from mock- or virus-infected insects cofed with inhibitors and tested for caspase activity.
To determine whether MMPs were able to directly cleave and activate effector caspases, we incubated purified MMP-9 or -2, type IV collagen-specific MMPs, and purified procaspase-3. The caspase substrate DEVD-afc was not cleaved by procaspase-3 alone, but addition of MMP-9 activated procaspase-3 in the absence of GM6001 (Fig. 3C). Dronc, an initiator caspase that activates procaspase-3, cleaved procaspase-3 but was not inhibited by GM6001. In contrast to MMP-9, MMP-2 was unable to activate procaspase-3. Similarly, procaspases present in mock- or AcBAC–vfgfKO-infected midgut lysates were activated by addition of MMP-9 (Fig. 3 D and E), indicating a procaspase-3-like enzyme in midguts. In contrast, initiator or effector caspases were unable to activate pro-MMP-9 (Fig. S7). Hence, exogenous active MMP-9 or AcBAC-activated MMPs activated effector caspases.
Are Caspases Available Extracellularly?
Caspases are normally found intracellularly, but there is evidence of extracellular caspase activity during apoptosis (10) or after secretion of unconventional inflammation-responsive caspases (11). In our system, for effector caspases to be active in the extracellular matrix and remodel BL, they would need to be released from the cell.
We tested for effector caspase activity in infected insects in the hemolymph and in hemocytes. Effector caspase activity increased from 6 to 12 h p.i. in hemocytes and hemolymph from AcBAC-infected insects, whereas levels in AcBAC–vfgfKO-infected hemocytes or hemolymph were similar to those in mock-infected insects (Fig. 3F). We hypothesized that procaspases may be either shuttled to the extracellular matrix of cells or released from apoptotic cells, and once they reach the extracellular matrix of cells, they become activated by MMPs and degrade BL components. Thus, we explored the possibility of apoptosis occurring during virus infection.
Interrelationships Among vFGF, Caspase, and Apoptosis.
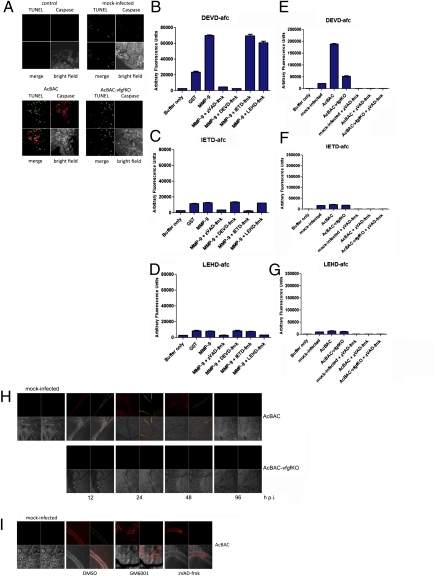
Because effector caspases were activated following infection with a virus expressing vfgf but not with one lacking vfgf, we questioned whether infected cells with active caspases underwent apoptosis. Apoptotic cells in infected insects were identified by TUNEL, and active caspases were examined using an antibody against active effector caspases (12). AcBAC-infected tissues had approximately the same number of TUNEL-positive foci but more cells with active caspases compared with AcBAC–vfgfKO-infected tissues (Fig. 4A). It is possible that because AcBAC–vfgfKO has slower infection kinetics, caspase activation was not as high at this point, but later time points did not show a relative increase in TUNEL-positive cells compared to cells with active caspases. DNA extracted from virus-infected midguts did not show DNA laddering, characteristic of apoptosis (Fig. S8).
Fig. 4.
Increased caspase activity is observed during infection with AcBAC. Infected larvae (A) were dissected at 12 h p.i., and TUNEL-positive cells (green) and active caspase (red) were detected. Lysates from uninfected (B–D), virus-, or mock-infected (E–G) midguts were incubated with the effector (DEVD-afc) or initiator (IETD-afc or LEHD-afc) caspase fluorescent substrates and the corresponding caspase inhibitors, DEVD-fmk, IETD-fmk, and LEHD-fmk, and purified proteins. Larvae were infected in the presence or absence of inhibitors or carrier and dissected at the times shown (H) or 12 h p.i. (I), and receptor activation and effector caspase activity were detected. (Upper Left) Red, anti-phosphotyrosine staining; (Upper Right) green, anti-active DrICE staining; (Lower Left) bright field; (Lower Right) merge.
We next asked if effector caspases were activated by initiator caspases, consistent with effector caspase activation during apoptosis, or whether this activation pathway was bypassed and effector caspases were directly activated by MMPs. We used effector caspase or initiator caspase substrates to test which caspase was being activated. MMP-9 induced effector but not initiator caspase activity in mock-infected midgut lysates, and this activity was inhibited by effector caspase inhibitors only (Fig. 4 B–D). AcBAC-infected samples had effector but not initiator caspase activity, and activity was higher than in AcBAC–vfgfKO-infected preparations (Fig. 4 E–G).
Because caspase activation correlated with vfgf expression, we investigated whether these activities overlapped in AcBAC-infected tracheal cells. AcBAC-infected tracheal epithelial cells positive for caspase activation also displayed tyrosine phosphorylation, suggestive of FGFR activation by vFGF (Fig. 4H). Neither caspase-positive cells nor tyrosine phosphorylation was apparent with AcBAC–vfgfKO-infected cells. In addition, tyrosine phosphorylation was independent of caspase and MMP activity (Fig. 4I), implying that FGFR activation occurred upstream of these proteases. In vitro, addition of vFGF did not activate procaspase-3 (Fig. S9A), indicating that an MMP or additional factors were necessary. Midguts from AcBAC–vfgfKO-infected insects could be complemented by addition of vFGF, resulting in MMP and effector caspase activity (Fig. S9 B and C). Our findings are consistent with FGFR, MMP, and effector caspase activation in response to vFGF in tracheal epithelial cells and/or in vitro.
MMP and Effector Caspase Activity Is Required for Establishment of Systemic Infection.
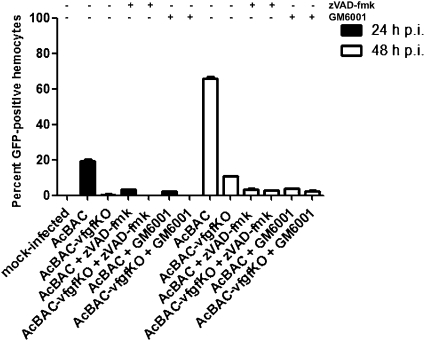
To evaluate systemic infection dependency on MMP and effector caspase acitivities, we enumerated eGFP-positive hemocytes in zVAD-fmk-, GM6001-, or carrier-fed insects. Hemocytes are present in the hemocoel and are readily infected once the virus escapes from the midgut (13). Significantly higher numbers of infected hemocytes were present in AcBAC- than in AcBAC–vfgfKO-infected larvae, and infection of hemocytes was dependent on both effector caspase and MMP activities (Fig. 5).
Fig. 5.
Inhibition of MMPs or caspases prevents spread of infection to hemocytes. Larvae were orally infected and fed inhibitors. Insects were dissected at the indicated hours postinfection, and infected hemocytes were detected with GFP antibody and quantified.
Discussion
AcMNPV relies on the expression of vfgf to induce a cascade of events involving the choreographed activation of MMPs and effector caspases, bypassing initiator caspases and the induction of apoptosis, to degrade abundant BL components and gain access to secondary cell targets. These studies identify the involvement of a virus-encoded gene, vfgf, in mediating BL remodeling to establish systemic infection but also define a pathway that may be used by other pathogens that do not encode fgfs, including mosquito-vectored pathogens, because AcBAC–vfgfKO activated the same proteases but at lower levels. In addition, to our knowledge, our work ties together the sequential steps between FGF signaling, MMP and effector caspase activation, terminating in BL remodeling.
vFGF Accelerates the Rate of Virus Infection.
Hosts infected with AcBAC–vfgfKO take longer to die, especially following oral infection (7, 14). Slower time of death implies that vfgf accelerates the rate of infection and insect death. Evidence for this is shown here and with another baculovirus (14).
vFGF Activates MMPs.
Our findings suggest that vFGF initiates a cascade of signaling events that leads to successful virus propagation. FGFRs are present at the tips of tracheal cells (15, 16) and share membranous environments with MMPs, which could be activated upon FGFR engagement. There are reports of FGFs up-regulating MMP expression or activity via the MAP kinase or NFkB pathway. Myogenic stem-cell migration through the BL is arbitrated by basic FGF up-regulating MMP-9 via tumor necrosis factor-α (17). Also, the walls of varicose veins overexpress acidic FGF via the MAP kinase pathway, and this is thought to play a role in extracellular matrix turnover (18). Thus, a link between FGFs and MMPs has been hypothesized in developmental and pathological processes in vitro. Our work establishes in vivo and in vitro relationships between these factors during invertebrate pathogenesis, suggesting that the pathways are conserved between vertebrate and invertebrate animals.
MMPs Activate Effector Caspases and Mediate BL Degradation.
After vFGF-mediated activation of MMPs, effector caspases are activated. We hypothesize that effector caspases are released to the extracellular matrix where active MMPs are present, but other scenarios are also possible. A relationship between MMPs and caspase activation or caspase secretion has been documented (10, 11, 19–21). These examples include extracellular caspase-3 activity in apoptotic cells and their involvement in extracellular matrix degradation (10), active caspase-1/FGF-2 association and cosecretion (11), and activation of procaspase-3 by metalloproteases (19, 21). During palatogenesis, BL degradation was blocked by caspase inhibitors, leading to the conclusion that cell death was required for BL degradation (22); in contrast, we do not detect apoptosis. In addition to identifying the factors necessary for BL degradation, we determined the linear sequence of activation events. Lack of apoptosis here could be due to the expression of the viral caspase inhibitor P35 (23). In addition, T. ni are relatively resistant to apoptosis, and apoptosis may be more prevalent during infection of other insect species. If effector caspases are activated extracellularly, they would not be expected to lead to cell death.
We show in vivo and in vitro evidence of a correlation between a triad of enzymatic events: MMP activation, effector caspase activation, and the end product, tracheal cell BL protein degradation. Our work delineates a BL remodeling pathway and provides insight into how viruses use this pathway to escape BL barriers by possibly inducing tracheal cell motility and then propagating throughout the host.
Model for AcMNPV Midgut Escape.
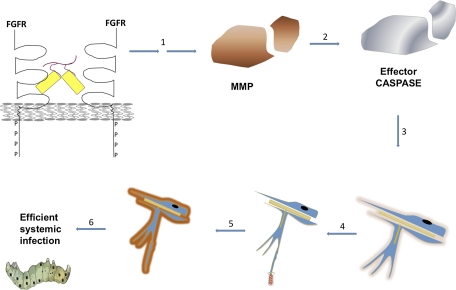
Upon vFGF signaling, tracheoblasts, expressing FGFR (15) and servicing midgut epithelial cells, migrate toward vFGF (infected midgut epithelial cells), and tracheal cell BL is degraded during vFGF-stimulated cell motility. vFGF expression thus sets off a cascade of protease activation whereby MMPs functionally homologous to mammalian MMP-9 activate effector caspases independent of initiator caspase activation. Effector caspases then act extracellularly to degrade BL components. Once tracheal cell BL is degraded, cells become more susceptible to virus infection for a period before regeneration of BL (Fig. 6). The tracheal system then serves as a conduit for virus escape across the midgut, carrying virus to hemoceolic tissues, and effectively establishing systemic infection.
Fig. 6.
Model for AcMPNV–T. ni midgut escape. vFGF-heparan sulfate complexes (yellow/red) activate the FGFR. MMPs are then activated where intermediary factors are unknown (1). Active MMPs activate effector caspases (2) and remodel tracheal BL (3). BL remodeling is concomitant with tracheal cell motility toward vFGF and delaminated tracheal cells are infected (4). Later, tracheal cells secrete new BL (5). Rearrangements in BL lead to trachea-mediated virus escape from the midgut and establishment of efficient systemic infection (6).
Baculoviruses usurped a host gene to benefit their replication capabilities and mimicked its cellular function, stimulating transient cell motility of tracheal cells and culminating with efficient virus spread. Because induction of cell motility is part of branching organ morphogenesis, tissue repair, and metastasis, it is likely that these processes also use MMPs and effector caspases to dispose of an impending BL to accommodate organ morphogenesis or tissue regeneration. Thus, our work provides insight into normal developmental processes, disease mechanisms, and disease etiology. Moreover, mosquito-vectored pathogens also face the dilemma of escaping the midgut barrier. Other insect-borne pathogens may activate the pathway described here to facilitate midgut escape. Understanding the escape tactics employed by insect pathogens and insect-vectored pathogens may lead to the development of strategies that block their transmission by preventing escape beyond the primary infection site.
Materials and Methods
Hemolymph Extraction and Hemocyte Examination.
At various times p.i., cohorts of 20–25 T. ni were chilled for 30 min at 4 °C. Hemolymph was collected on ice-cold anticoagulant buffer (4 mM NaCl, 40 mM KCl, 1.7 mM Pipes, 146 mM sucrose, 0.1% polyvinyl pyrrolidine, 8 mM EDTA, 9.5 mM citric acid, and 27 mM sodium citrate) after cutting an anal proleg. Hemocytes were fixed overnight in 4% paraformaldehyde/PBS at 4 °C, immmunostained as described in supporting information. A minimum of 200 cells were scored. For caspase activity assays, hemolymph was centrifuged at 960 × g for 5 min at 4 °C to separate cells from hemolymph. Cells were resuspended in caspase buffer (24) and hemolymph was diluted 1:1 with caspase buffer. Protein concentration was determined using the BCA Protein Assay Kit (Thermo Scientific), and equal amounts of protein were used.
Caspase and MMP Activity Assays.
Caspase or MMP activity was determined as an increase in fluorescence by the enzymatic cleavage of the substrate DEVD-AFC (MP Biomedicals) or Dnp-Pro-Leu-Gly-Leu-Trp-Ala-D-Arg-NH2 (Calbiochem), respectively, and according to instructions of the manufacturer.
TUNEL Staining.
Dissected midguts were placed in 4% paraformaldehyde/PBS (pH 7.4) and incubated overnight at 4 °C. TUNEL was performed using the In Situ Cell Death Detection Kit, TMR red (Roche) as described by the manufacturer and anti-CM-1 polyclonal to label active caspases.
Supplementary Material
Acknowledgments
We thank Bruce Hay for active DrICE antibody, Rollie Clem for caspases, and Casey Devore for assistance. We also thank Rollie Clem, Brian Spooner, and Anna Zolkiewska for discussions on virus-induced apoptosis, tracheal morphogenesis, and metalloproteases. This work was supported in part by the National Institutes of Health Grant R21AI063089 and US Department of Agriculture Award 2008-35302-18849. This article is contribution no. 10-052J from the Kansas Agricultural Experiment Station.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0913582107/-/DCSupplemental.
References
- 1.Bowers DF, Abell BA, Brown DT. Replication and tissue tropism of the alphavirus Sindbis in the mosquito Aedes albopictus. Virology. 1995;212:1–12. doi: 10.1006/viro.1995.1447. [DOI] [PubMed] [Google Scholar]
- 2.Engelhard EK, Kam-Morgan LNW, Washburn JO, Volkman LE. The insect tracheal system: A conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc Natl Acad Sci USA. 1994;91:3224–3227. doi: 10.1073/pnas.91.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romoser WS, et al. Pathogenesis of Rift Valley fever virus in mosquitoes—tracheal conduits and the basal lamina as an extra-cellular barrier. Arch Virol Suppl. 2005;19:89–100. doi: 10.1007/3-211-29981-5_8. [DOI] [PubMed] [Google Scholar]
- 4.Chandler LJ, Blair CD, Beaty BJ. La Crosse virus infection of Aedes triseriatus (Diptera: Culicidae) ovaries before dissemination of virus from the midgut. J Med Entomol. 1998;35:567–572. doi: 10.1093/jmedent/35.4.567. [DOI] [PubMed] [Google Scholar]
- 5.Engelhard EK, Volkman L. Developmental resistance in fourth instar Trichoplusia ni orally inoculated with Autographa californica M nuclear polyhedrosis virus. Virology. 1994;209:384–389. doi: 10.1006/viro.1995.1270. [DOI] [PubMed] [Google Scholar]
- 6.Washburn JO, Kirkpatrick BA, Volkman LE, Volkman LE. Comparative pathogenesis of Autographa californica M nuclear polyhedrosis virus in larvae of Trichoplusia ni and Heliothis virescens. Virology. 1995;209:561–568. doi: 10.1006/viro.1995.1288. [DOI] [PubMed] [Google Scholar]
- 7.Detvisitsakun C, Cain EL, Passarelli AL. The Autographa californica M nucleopolyhedrovirus fibroblast growth factor accelerates host mortality. Virology. 2007;365:70–78. doi: 10.1016/j.virol.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 8.Liu J-J, et al. Heparin/heparan sulfate interacting protein plays a role in apoptosis induced by anticancer drugs. Carcinogenesis. 2004;25:873–879. doi: 10.1093/carcin/bgh081. [DOI] [PubMed] [Google Scholar]
- 9.Mannello F, Luchetti F, Falcieri EP, Papa S. Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis. 2005;10:19–24. doi: 10.1007/s10495-005-6058-7. [DOI] [PubMed] [Google Scholar]
- 10.Hentze H, et al. In vivo and in vitro evidence for extracellular caspase activity released from apoptotic cells. Biochem Biophys Res Commun. 2001;283:1111–1117. doi: 10.1006/bbrc.2001.4918. [DOI] [PubMed] [Google Scholar]
- 11.Keller M, Rüegg A, Werner S, Beer H-D. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan A, et al. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 1998;5:1004–1016. doi: 10.1038/sj.cdd.4400449. [DOI] [PubMed] [Google Scholar]
- 13.Clarke TE, Clem RJ. Lack of involvement of haemocytes in the establishment and spread of infection in Spodoptera frugiperda larvae infected with the baculovirus Autographa californica M nucleopolyhedrovirus by intrahaemocoelic injection. J Gen Virol. 2002;83:1565–1572. doi: 10.1099/0022-1317-83-7-1565. [DOI] [PubMed] [Google Scholar]
- 14.Katsuma S, Horie S, Daimon T, Iwanaga M, Shimada T. In vivo and in vitro analyses of a Bombyx mori nucleopolyhedrovirus mutant lacking functional vfgf. Virology. 2006;355:62–70. doi: 10.1016/j.virol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Katsuma S, Daimon T, Mita K, Shimada T. Lepidopteran ortholog of Drosophila breathless is a receptor for the baculovirus fibroblast growth factor. J Virol. 2006;80:5474–5481. doi: 10.1128/JVI.00248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell. 1996;87:1091–1101. doi: 10.1016/s0092-8674(00)81803-6. [DOI] [PubMed] [Google Scholar]
- 17.Allen DL, Teitelbaum DH, Kurachi K. Growth factor stimulation of matrix metalloproteinase expression and myoblast migration and invasion in vitro. Am J Physiol Cell Physiol. 2003;284:C805–C815. doi: 10.1152/ajpcell.00215.2002. [DOI] [PubMed] [Google Scholar]
- 18.Kowalewski R, Malkowski A, Sobolewski K, Gacko M. Evaluation of aFGF/bFGF and FGF signaling pathway in the wall of varicose veins. J Surg Res. 2009;155:165–172. doi: 10.1016/j.jss.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 19.Kim HY, et al. Procaspase-3 activation by a metalloprotease secreted from Vibrio vulnificus. Int J Mol Med. 2007;20:591–595. [PubMed] [Google Scholar]
- 20.Lee S-R, Lo EH. Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2004;24:720–727. doi: 10.1097/01.WCB.0000122747.72175.47. [DOI] [PubMed] [Google Scholar]
- 21.You W-K, Seo H-J, Chung K-H, Kim D-S. A novel metalloprotease from Gloydius halys venom induces endothelial cell apoptosis through its protease and disintegrin-like domains. J Biochem. 2003;134:739–749. doi: 10.1093/jb/mvg202. [DOI] [PubMed] [Google Scholar]
- 22.Cuervo R, Covarrubias L. Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogenesis. Development. 2004;131:15–24. doi: 10.1242/dev.00907. [DOI] [PubMed] [Google Scholar]
- 23.Clem RJ, Fechheimer M, Miller LK. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991;254:1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- 24.Means JC, Muro I, Clem RJ. Lack of involvement of mitochondrial factors in caspase activation in a Drosophila cell-free system. Cell Death Differ. 2006;13:1222–1234. doi: 10.1038/sj.cdd.4401821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.