Abstract
Epidemiological theory generally suggests that pathogens will not cause host extinctions because the pathogen should fade out when the host population is driven below some threshold density. An emerging infectious disease, chytridiomycosis, caused by the fungal pathogen Batrachochytrium dendrobatidis (Bd) is directly linked to the recent extinction or serious decline of hundreds of amphibian species. Despite continued spread of this pathogen into uninfected areas, the dynamics of the host–pathogen interaction remain unknown. We use fine-scale spatiotemporal data to describe (i) the invasion and spread of Bd through three lake basins, each containing multiple populations of the mountain yellow-legged frog, and (ii) the accompanying host–pathogen dynamics. Despite intensive sampling, Bd was not detected on frogs in study basins until just before epidemics began. Following Bd arrival in a basin, the disease spread to neighboring populations at ≈700 m/yr in a wave-like pattern until all populations were infected. Within a population, infection prevalence rapidly reached 100% and infection intensity on individual frogs increased in parallel. Frog mass mortality began only when infection intensity reached a critical threshold and repeatedly led to extinction of populations. Our results indicate that the high growth rate and virulence of Bd allow the near-simultaneous infection and buildup of high infection intensities in all host individuals; subsequent host population crashes therefore occur before Bd is limited by density-dependent factors. Preventing infection intensities in host populations from reaching this threshold could provide an effective strategy to avoid the extinction of susceptible amphibian species in the wild.
Keywords: amphibian declines, Batrachochytrium dendrobatidis, chytridiomycosis, emerging infectious disease, Rana muscosa
Earth's biodiversity is increasingly threatened with extinction. The majority of contemporary extinctions are typically attributed to anthropogenic changes such as habitat destruction, overexploitation, and species introductions. Disease is generally not considered a major driving force in extinctions, in part because simple epidemiological theory suggests that a pathogen will fade out when its host population is driven below some threshold density (1, 2). Class Amphibia provides one of the best-documented examples of contemporary biodiversity loss, with ≈43% of the more than 6,600 described species currently threatened with extinction (3). Remarkably, an emerging infectious disease, chytridiomycosis, is directly linked to the recent extinction or serious decline of hundreds of amphibian species (4). The effect of chytridiomycosis on amphibians has been described as the greatest loss of vertebrate biodiversity attributable to disease in recorded history (4), and although doubts about the importance of disease in driving global amphibian declines have been expressed (5), these have largely been overcome by weight of evidence (4, 6–8).
Chytridiomycosis is caused by the fungal pathogen Batrachochytrium dendrobatidis (Bd), whose only known host is larval and adult amphibians. This pathogen was described in the late 1990s (6, 9) and is now known from six continents (4). The infective stage is a free-living flagellated zoospore that encysts in the skin of an amphibian and develops into a zoosporangium. Zoosporangia produce zoospores via asexual reproduction [it remains unclear whether sexual reproduction also occurs (10, 11)], and the zoospores are released into the environment through a discharge tube. Tadpoles are typically little affected by chytridiomycosis, but sublethal and lethal effects are known (12, 13). Effects of chytridiomycosis on frogs are highly variable, with frogs of some species dying from the disease within weeks and others experiencing few negative effects (4). Chytridiomycosis likely causes frog mortality by severely disrupting epidermal functions and causing osmotic imbalance (14, 15). However, it remains unknown how chytridiomycosis is able to cause the extinction of its amphibian hosts, an outcome that would require that Bd not be severely limited by density-dependent factors. The objective of our study was to describe frog–Bd dynamics by measuring both Bd prevalence in populations and infection intensity in individual frogs during chytridiomycosis epizootics (epidemics in nonhuman species) in naive frog metapopulations (we use the term “metapopulation” to mean a collection of populations connected by dispersal) (16, 17). In doing so, we reveal the heretofore unknown importance of infection intensity as a factor allowing Bd to drive amphibian populations to extinction. We also sought to describe the rate of spread by Bd through these metapopulations, which is information critical to understanding the potential vectors of this pathogen.
The rapid decline of California's mountain yellow-legged frog (a species complex consisting of Rana muscosa and Rana sierrae) (18) is emblematic of global amphibian declines (3). Historically, these two species inhabited thousands of lakes and ponds in California's Sierra Nevada (where this study took place) (19). Both of these closely related species are highly aquatic and have a multiyear tadpole stage that allows them to breed successfully in the cold water bodies typical of the high elevation portions of this mountain range. Despite the fact that the majority of their habitat is fully protected, these frogs have disappeared from >93% of their historic range during the past several decades (18). As a consequence of this decline, the mountain yellow-legged frog has gone from being one of the most common vertebrates in the Sierra Nevada to one classified as “critically endangered” (3). One of the earliest recorded cases of Bd infecting amphibians in western North America (1975) was in R. muscosa specimens from the Sierra Nevada (20); these specimens were originally identified as Rana boylii, but subsequent inspection by one of the authors (V.T.V.) indicated that they are actually R. muscosa. Since then, Bd has spread across this mountain range, causing the extinction of hundreds of mountain yellow-legged frog populations (21, 22).
Our study area comprised three lake basins: Milestone, Sixty Lake, and Barrett Lakes in Sequoia–Kings Canyon National Park, CA (Fig. S1). The three basins were separated from each other by 20–50 km. At the beginning of our study, we found no evidence of chytridiomycosis in the frog populations in these lake basins, but all three basins were immediately adjacent to basins in which chytridiomycosis epizootics and subsequent frog population extinctions had recently occurred. At the inception of our study (1996–2000), the three study basins, Milestone, Sixty Lake, and Barrett Lakes, contained 13, 33, and 42 frog populations, respectively, and represented the most intact remaining metapopulations of these species. To quantify trends in population size before and after Bd-caused epizootics, we used repeat surveys of all 88 frog populations over a 9–13-year period. Frog surveys were conducted 1–5 times per year at each population in Milestone Basin (R. muscosa: 2000, 2003–2008), 1–12 times per year in Sixty Lake Basin (R. muscosa: 1996–2008), and once per year in Barrett Lakes Basin (R. sierrae: 1997, 2002–2008), for a total of 1,995 surveys (yearly average = 1.8 surveys × population−1). We measured Bd prevalence and infection intensity (expressed as zoospore equivalents × swab−1) using a real-time quantitative PCR assay (23) conducted on skin swabs (24) collected from frogs in 2004–2008 (n = 4,591). Before the availability of the PCR assay, tadpole mouthpart inspections (25) were used for assessments of Bd prevalence (2002–2005, n = 1,389).
Results
We detected Bd in Milestone Basin in June 2004, in Sixty Lake Basin in August 2004, and in Barrett Lakes Basin in July 2005 (Fig. 1). In the relatively small Milestone Basin, Bd spread to virtually all populations within a single year (Fig. 1 A and B). In the larger Sixty Lake and Barrett Lakes Basins, it took 3–5 years for Bd to spread to all frog populations (Fig. 1 F–O). Our most detailed within-season Bd occurrence data were collected in Sixty Lake Basin, and these data allowed us to quantify the pattern and rate of Bd spread. In Sixty Lake Basin, the distance from the original Bd outbreak site (Fig. 1F) to subsequently infected populations increased linearly with time (linear regression through the origin: R2 = 0.85, P < 0.001), consistent with a wave-like pattern (Fig. 1 F–J). The slope of the regression line indicated an average rate of Bd spread (±1 SE) of 688 ± 64 m·yr−1. The pattern and rate of spread in Barrett Lakes Basin (where we collected skin swabs only once per year; Fig. 1 K–O) were qualitatively similar to those measured in Sixty Lake Basin.
Fig. 1.
Maps of the three study metapopulations showing the spread of Bd and frog population status (adults only) during a 4-year period following the initial detection of Bd. Depicted are Milestone Basin (A–E), Sixty Lake Basin (F–J), and Barrett Lakes Basin (K–O). Lake color (green, yellow, and black) shows the Bd infection and frog population status, and the light gray shaded region surrounds the area in which frog populations were Bd-positive in each year. Lakes shown with a thick black outline are fishless, and a thin gray outline indicates that nonnative fish were present (details on the historic fish distribution are presented in SI Text). The infection status of frog populations depicted in A and K is based on mouthpart surveys of 459 tadpoles. The infection status of frog populations in B–J and L–O is based on 4,591 skin swabs analyzed using a real-time PCR assay.
In 48 of the 88 frog populations, Bd assays (n = 1,341 swabs, 909 mouthpart inspections) were conducted before the beginning of Bd-caused epizootics. We used results from these assays to calculate the probability that Bd was present on frogs at these sites during the early part of our study but not detected (i.e., false-negative result). These calculations were based on the assumption that the true prevalence of Bd was 5%. For 33 (69%) of the 48 populations, the probability of false-negative results was less than 0.05 (median = 0.02; Table S1). For the best-sampled populations (22 populations for which >30 swabs or mouthpart inspections were collected before detection of Bd), the median probability of false-negative results was 1.6 × 10−3 (Table S1). These results strongly suggest that Bd was not present in frog populations in Milestone, Sixty Lake, and Barrett Lakes Basins in the early years of our study.
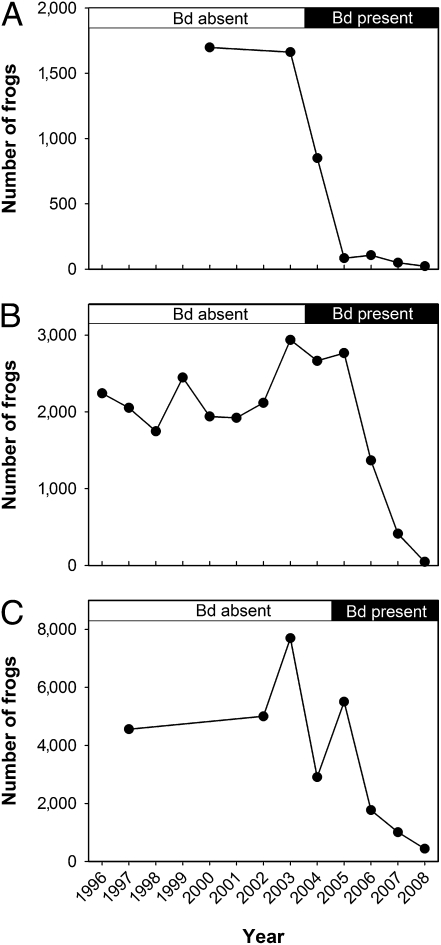
Soon after the detection of Bd, major declines in frog populations were observed in all three study basins (Fig. 2) and were coincident with observations of hundreds of dead and dying frogs (Fig. S2). By 2008, the number of adult frogs in Milestone Basin had declined from 1,680 (frog counts averaged over all surveys conducted before Bd arrival) to 22 (Fig. 2A), from 2,193 to 47 in Sixty Lake Basin (Fig. 2B), and from 5,588 to 436 in Barrett Lakes Basin (Fig. 2C). Similarly, by 2008, adult frogs were extinct from 9 of 13 populations in Milestone Basin, 27 of 33 populations in Sixty Lake Basin, and 33 of 42 populations in Barrett Lakes Basin (Fig. 1 E, J, and O). Based on high rates of population extinctions in nearby basins in the 10 years following Bd arrival, we expect that most, if not all, of the still-extant populations will also go extinct during the next 3 years as the remaining tadpoles metamorphose and succumb to chytridiomycosis (21).
Fig. 2.
Total number of adult and subadult frogs in the three study metapopulations during 1996–2008 before and after the detection of Bd: Milestone Basin (A), Sixty Lake Basin (B), and Barrett Lakes Basin (C).
To quantify the effect of Bd arrival on frog population growth rates, we compared population growth rates in (i) the years before Bd arrival, (ii) the year of Bd arrival, and (iii) the year after Bd arrival. There was a significant decrease in the frog population growth rate in the year of Bd arrival compared with the growth rate in the same populations before Bd arrival [Fig. 3; mean difference in growth rate ([before Bd arrival] − [year of Bd arrival]) = 1.8, paired t test: t = 2.9, df = 42, P < 0.01] and an even larger decrease in the year following Bd arrival [Fig. 3; mean difference in growth rate ([before Bd arrival] − [year after Bd arrival]) = 3.2, paired t test, t = 7.5, df = 36, P << 0.01]. Therefore, the decrease in the frog population growth rate began with the arrival of Bd and was clearly evident within 1 year after the detection of Bd.
Fig. 3.
Box plots showing the effect of Bd arrival on the yearly population growth rate (rt) of three categories of frog populations: (i) populations before detection of Bd (rt for each lake averaged over all years before Bd arrival), (ii) populations during the year in which Bd was detected, and (iii) populations 1 year after Bd was detected. In each case, rt = ln(Nt) − ln(Nt−1), where Nt is the number of adult frogs in the lake in year t. Box plots display the median yearly frog population growth rate (horizontal line), 25th and 75th percentiles (gray boxes), 10th and 90th percentiles (whiskers), and all points that lie outside of the 10th and 90th percentiles (•). Data are from 88 frog populations located in all three study basins (1996–2008).
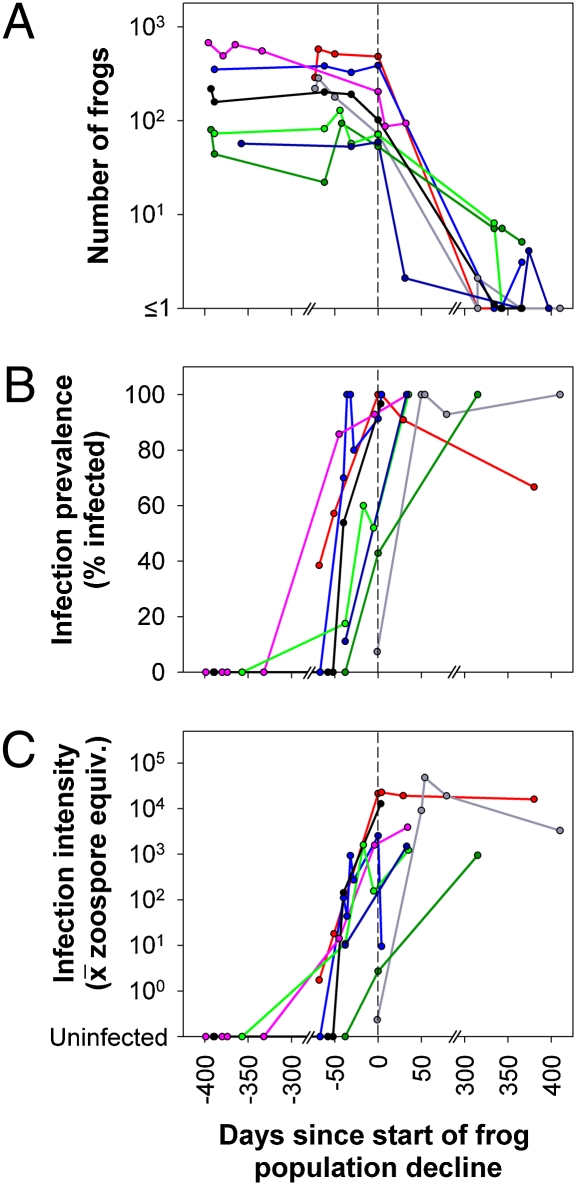
We used detailed within-season data from the eight most intensively sampled populations in Milestone and Sixty Lake Basins to describe the frog–Bd dynamics during epizootics. Following the detection of Bd in these populations, adult frog populations invariably crashed to extinction (n = 7) or near-extinction (n = 1; Fig. 4A). On the date when Bd was detected, both prevalence and infection intensity were relatively low (prevalence: median = 0.42, range = 0.05–1; infection intensity: median = 13.4, range = 0.2–3,843.0). In all populations, Bd prevalence increased rapidly, and in all but one case, it reached 100% (97% in the remaining case), often in less than 50 days (Fig. 4B). Infection intensity increased exponentially; the within-year rate of increase ( ± 1 SE) was 0.15 ± 0.02 × day−1 (Fig. 4C). Declines in frog numbers were generally not evident until an average infection intensity of ≈10,000 zoospore equivalents per swab was reached [maximum infection intensity at time of population crash (
± 1 SE) was 0.15 ± 0.02 × day−1 (Fig. 4C). Declines in frog numbers were generally not evident until an average infection intensity of ≈10,000 zoospore equivalents per swab was reached [maximum infection intensity at time of population crash (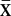 ± 1 SE) = 11,775 ± 5,851 zoospore equivalents × swab−1; Fig. 4 A and C]. Exceeding this threshold consistently resulted in mass mortality and rapid population decline (Fig. 4A). Bd prevalence and infection intensity remained high even in the last surviving frogs following population crashes (Fig. 4 B and C). Frogs swabbed during the second summer after the outbreak (>300 days postoutbreak; Fig. 4 B and C) were all newly metamorphosed subadults (which had survived the winter as tadpoles). The fact that subadults have much higher infection intensities than do adults likely explains the high infection intensities even at the end of the epizootic when very few frogs remained (Fig. 4 A and C).
± 1 SE) = 11,775 ± 5,851 zoospore equivalents × swab−1; Fig. 4 A and C]. Exceeding this threshold consistently resulted in mass mortality and rapid population decline (Fig. 4A). Bd prevalence and infection intensity remained high even in the last surviving frogs following population crashes (Fig. 4 B and C). Frogs swabbed during the second summer after the outbreak (>300 days postoutbreak; Fig. 4 B and C) were all newly metamorphosed subadults (which had survived the winter as tadpoles). The fact that subadults have much higher infection intensities than do adults likely explains the high infection intensities even at the end of the epizootic when very few frogs remained (Fig. 4 A and C).
Fig. 4.
Frog–Bd dynamics in eight intensively sampled populations in Milestone and Sixty Lake Basins before and after detection of Bd: frog counts (adults + subadults) from visual encounter surveys (A); infection prevalence, defined as the fraction of skin swabs collected from each population on each date positive for Bd (B); and infection intensity, defined as the average zoospore equivalents on swabs collected from each population on each date (C). Data are from frog populations that were sampled more than once per year, experienced >80% declines by the end of 2006, and for which the decline in the number of frogs was >10. This last criterion excluded populations that were very small before Bd arrival. Populations were aligned along the x axis such that “0” represents the date on which each frog population began to decline. This was calculated for each population by determining the date at which the number of postmetamorphic frogs dropped below 20% of the average population count before that point.
Discussion
Most of the frog study populations were sampled for Bd for at least 1 year before epizootics began. In all 48 of these frog populations, we found no evidence of Bd until just before the observed frog die-offs. Therefore, we suggest that Bd was absent from the three study metapopulations before 2004. Two studies in Central America (6, 7) also reported the absence of Bd until just before frog die-offs were observed. The apparent absence of Bd before frog die-offs is critically important in resolving the continued debate about whether Bd is a novel pathogen sweeping through naive host populations (7, 8, 26) or a widespread endemic pathogen that has emerged as a result of changing environmental conditions such as those caused by climate warming (Bd thermal optimum hypothesis) (27). Implicit in the Bd thermal optimum hypothesis is the presence of Bd in amphibian populations before chytridiomycosis epizootics (28). Our results indicate that Bd was likely not present on amphibians in our study populations until just before epizootics began. Therefore, our data do not support the Bd thermal optimum hypothesis but are consistent with Bd as a novel pathogen spreading through naive host populations.
Data from the intensively sampled Sixty Lake Basin metapopulation indicated that Bd spread as a distinct wave at a rate of 688 m·yr−1, and rates of spread in Milestone and Barrett Lakes Basin were qualitatively similar. This rate of spread is much lower than rates reported for Bd in Central and South America and Australia (17–282 km·yr−1) (8, 29), but it is unclear if these differences in rate of spread are real or are the result of different spatial scales of sampling used in our study compared with previous studies. In our study system, the observed pattern of Bd spread within a metapopulation is consistent with frog movement patterns, suggesting that frogs may be an important agent of dispersal at this scale (these frogs are known to move only several hundred meters between lakes in a single summer) (17, 30). However, the continuing between-basin spread of Bd and the lack of evidence for interbasin frog movement (17, 18) suggest the involvement of unknown additional vectors. Other possible between-basin dispersal agents include more vagile sympatric organisms, including amphibians (e.g., Pseudacris regilla), insects, or birds.
Before our study, the only data available on frog–Bd dynamics during disease outbreaks showed a temporal correlation between increases in Bd prevalence and amphibian population decline (7), but that study did not include any measurement of infection intensity. As a consequence, the dynamics of this disease were only partially described until now. Our quantification of infection intensity provided a key insight into how Bd causes host extinctions. Temporally intensive sampling at multiple frog populations showed that the very high growth rate and virulence of Bd in mountain yellow-legged frogs allowed the near-simultaneous infection and buildup of high infection intensities in all host individuals. Subsequent host population crashes therefore occurred before Bd could be limited by density dependence, host immune response, or other factors.
Chytridiomycosis is a major driver of an ongoing global mass extinction event (31) in amphibians, but field interventions designed to reduce disease impacts by altering Bd–host dynamics have only just begun. Our results show a primary role for infection intensity in driving the population extinctions that typically follow these epizootics. This suggests that interventions designed to prevent Bd infection intensity on frogs from reaching the critical lethal threshold could reduce the probability of population extinction. Interventions could include capturing frogs immediately in front of the Bd wave and releasing them back into the same habitat after the Bd wave has passed and pathogen pressure has declined following die-offs of resident frog populations or reducing the density of infective Bd zoospores by treating a large proportion of frogs during epizootics with antifungal drugs (32, 33) and releasing them back into the same habitat. In both cases, the goal of interventions would not be to eradicate the pathogen from the targeted habitats, because this would not be feasible, but, instead, to reduce pathogen transmission rates and thus increase host survivorship (34). Given a known rate of Bd spread in our study system and the resulting knowledge of exactly where the Bd front is within remaining frog metapopulations, the results of the current study create a unique opportunity to test these approaches, the results of which will be of critical importance to the global conservation of amphibians.
Methods
Study Area Description.
The three study watersheds are in Sequoia–Kings Canyon National Park (milestone: 36°38′57″ N, 118°27′28″ W; Sixty Lake: 36°49′03″ N, 118°25′24″ W; Barrett Lakes: 37°04′52″ N, 118°31′35″ W; Fig. S1). Milestone and Sixty Lake Basins contain the southern mountain yellow-legged frog (R. muscosa), and Barrett Lakes Basin contains the closely related Sierra Nevada yellow-legged frog (R. sierrae (18). These basins are located in the subalpine and alpine zones and contain 13–42 oligotrophic lakes and ponds (elevation range: 3,030–3,790 m), all of which are naturally fishless. Nonnative trout (primarily Oncorhynchus mykiss and Salvelinus fontinalis) have been introduced into many Sierra Nevada lakes to provide recreational fishing opportunities, and their negative impacts on mountain-yellow legged frogs are well known (35–37). The active season for frogs in the study basins is from early June to mid-October; the basins are typically covered by several meters of snow during the winter.
Frog Surveys.
We used diurnal visual encounter surveys (38) of entire water body perimeters to describe the abundance of adult (≥40 mm snout–vent length) and subadult (<40 mm snout–vent length) mountain yellow-legged frogs at all water bodies in the study basins (36, 39). In these species, counts from surveys are highly correlated with estimates of population size obtained using mark-recapture techniques. These frogs’ high detectability during visual surveys is a consequence of a diurnal habit, spending the majority of the active season at the water–land interface (30, 40), and not in terrestrial habitats (41), and occupying structurally simple habitats (e.g., subalpine lakes, alpine lakes) in which the lack of submerged logs or aquatic vegetation provides few places for frogs to hide.
Disease Prevalence and Infection Intensity.
We used frog skin swabs and a real-time quantitative PCR assay to quantify Bd prevalence and infection intensity (23, 24). Swabs were stroked across a frog's skin in a standardized way: five strokes on each side of the abdominal midline, five strokes on the inner thighs of each hind leg, and five strokes on the foot webbing of each hind leg (total of 30 strokes × frog−1). Swabs were air-dried in the field and stored individually in labeled microcentrifuge tubes before PCR analysis. We used standard Bd DNA extraction and real-time PCR methods (23, 24), except that swab extracts were analyzed singly instead of in triplicate (42). We defined infection intensity as the number of “zoospore equivalents” per swab. Zoospore equivalents were calculated by multiplying the genomic equivalent values generated during the real-time PCR assay by 80; this multiplication accounts for the fact that DNA extracts from swabs were diluted 80-fold during extraction and PCR. For calculations of Bd prevalence, swabs were categorized as Bd-positive when zoospore equivalents were ≥1 and as Bd-negative when zoospore equivalents were <1. Before the availability of the PCR assay, we determined the infection status (infected/uninfected) of frog populations using inspections of tadpole mouthparts (upper jaw sheaths). Tadpole mouthpart anomalies can have numerous causes, but in R. muscosa and R. sierrae, mouthpart anomalies are an accurate indicator of chytridiomycosis (25).
Bd Disinfection Procedures.
To ensure that Bd was not spread between frog populations by field sampling activities, we disinfected all field gear by immersion in 1% sodium hypochlorite or 0.01% quaternary ammonia for 5 min (43). In Milestone and Barrett Lakes Basins, disinfection was performed whenever moving between frog populations. In Sixty Lake Basin, where the distribution of Bd was very well known during each summer, we divided the area into discrete units based on geography and Bd infection status (infected/uninfected) and disinfected gear when moving between units.
Rate of Bd Spread.
Calculations of Bd spread rate in Sixty Lake Basin were based on the date of earliest Bd detection: August 22, 2004. For each newly infected frog population in this basin, we calculated (i) the minimum straight-line distance from the original outbreak sites (Fig. 1F) and (ii) the number of days between August 22, 2004 and the date on which Bd was detected. The slope from a linear regression model of distance as a function of time provided the rate of spread. The regression included only populations that became infected by the autumn of 2006. The intercept of the regression (±1 SE) was not significantly different from zero (189 ± 223 m); thus, the regression line was forced through the origin. Lakes that had not become infected by the autumn of 2006 were situated significantly further from the site of initial Bd detection than lakes that became infected (logistic regression: P < 0.01, df = 28).
Supplementary Material
Acknowledgments
Research permits were provided by Sequoia–Kings Canyon National Park and the University of California, Berkeley; San Francisco State University; and University of California, Santa Barbara Institutional Animal Care and Use Committees. We thank the staff at Sequoia–Kings Canyon National Park for logistical support and many technicians for their help in collecting field data and running PCR assays. This work was funded by National Institutes of Health Grant R01ES12067 and National Science Foundation Grant EF-0723563 as part of the joint National Science Foundation–National Institutes of Health Ecology of Infectious Disease program.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/cgi/content/full/0914111107/DCSupplemental.
References
- 1.Anderson RM, May RM. Population biology of infectious diseases: Part I. Nature. 1979;280:361–367. doi: 10.1038/280361a0. [DOI] [PubMed] [Google Scholar]
- 2.de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol Lett. 2005;8:117–126. [Google Scholar]
- 3.Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 4.Skerratt LF, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- 5.McCallum H. Inconclusiveness of chytridiomycosis as the agent in widespread frog declines. Conserv Biol. 2005;19:1421–1430. [Google Scholar]
- 6.Berger L, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lips KR, et al. Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lips KR, Diffendorfer J, Mendelson JR, Sears MW. Riding the wave: Reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:e72. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. [Google Scholar]
- 10.James TY, et al. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathog. 2009;5:e1000458. doi: 10.1371/journal.ppat.1000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan JAT, et al. Population genetics of the frog-killing fungus Batracho-chytrium dendrobatidis. Proc Natl Acad Sci USA. 2007;104:13845–13850. doi: 10.1073/pnas.0701838104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaustein AR, et al. Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv Biol. 2005;19:1460–1468. [Google Scholar]
- 13.Parris MJ, Cornelius TO. Fungal pathogen causes competitive and developmental stress in larval amphibian communities. Ecology. 2004;85:3385–3395. [Google Scholar]
- 14.Voyles J, et al. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis Aquat Org. 2007;77:113–118. doi: 10.3354/dao01838. [DOI] [PubMed] [Google Scholar]
- 15.Voyles J, et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326:582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- 16.Hanski I, Gilpin ME. Metapopulation Biology: Ecology, Genetics, and Evolution. San Diego: Academic; 1997. [Google Scholar]
- 17.Bingham RE. Differentiation across multiple spatial scales in three Californian amphibians. PhD dissertation. Berkeley: UnivERSITY of California; 2007. [Google Scholar]
- 18.Vredenburg VT, et al. Concordant molecular and phenotypic data delineate new taxonomy and conservation priorities for the endangered mountain yellow-legged frog. J Zool. 2007;271:361–374. [Google Scholar]
- 19.Grinnell J, Storer TI. Animal Life in the Yosemite. Berkeley: Univ of California Press; 1924. [Google Scholar]
- 20.Ouellet M, Mikaelian I, Pauli BD, Rodrigue J, Green DM. Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv Biol. 2005;19:1431–1440. [Google Scholar]
- 21.Rachowicz LJ, et al. Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology. 2006;87:1671–1683. doi: 10.1890/0012-9658(2006)87[1671:eidaap]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Briggs CJ, Vredenburg VT, Knapp RA, Rachowicz LJ. Investigating the population-level effects of chytridiomycosis: an emerging infectious disease of amphibians. Ecology. 2005;86:3149–3159. [Google Scholar]
- 23.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 24.Hyatt AD, et al. Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Dis Aquat Org. 2007;73:175–192. doi: 10.3354/dao073175. [DOI] [PubMed] [Google Scholar]
- 25.Knapp RA, Morgan JAT. Tadpole mouthpart depigmentation as an accurate indicator of chytridiomycosis, an emerging disease of amphibians. Copeia. 2006;2006:188–197. [Google Scholar]
- 26.Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ. Evaluating the links between climate, disease spread, and amphibian declines. Proc Natl Acad Sci USA. 2008;105:17436–17441. doi: 10.1073/pnas.0806368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pounds JA, et al. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature. 2006;439:161–167. doi: 10.1038/nature04246. [DOI] [PubMed] [Google Scholar]
- 28.Rachowicz LJ, et al. The novel and endemic pathogen hypotheses: Competing explanations for the origin of emerging infectious diseases of wildlife. Conserv Biol. 2005;19:1441–1448. [Google Scholar]
- 29.Laurance WF, McDonald KR, Speare R. Epidemic disease and the catastrophic decline of Australian rain forest frogs. Conserv Biol. 1996;10:406–413. [Google Scholar]
- 30.Pope KL, Matthews KR. Movement ecology and seasonal distribution of mountain yellow-legged frogs, Rana muscosa, in a high-elevation Sierra Nevada basin. Copeia. 2001;2001:787–793. [Google Scholar]
- 31.Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc Natl Acad Sci USA. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker JM, Mikaelian I, Hahn N, Diggs HE. Clinical diagnosis and treatment of epidermal chytridiomycosis in African clawed frogs (Xenopus tropicalis) Comp Med. 2002;52:265–268. [PubMed] [Google Scholar]
- 33.Garner TWJ, Garcia G, Carroll B, Fisher MC. Using itraconazole to clear Batrachochytrium dendrobatidis infection, and subsequent depigmentation of Alytes muletensis tadpoles. Dis Aquat Org. 2008;83:257–260. doi: 10.3354/dao02008. [DOI] [PubMed] [Google Scholar]
- 34.Briggs CJ, Knapp RA, Vredenburg VT. (In review) Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians. Proc Natl Acad Sci USA. 107:9695–9700. doi: 10.1073/pnas.0912886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knapp RA, Matthews KR. Non-native fish introductions and the decline of the mountain yellow-legged frog from within protected areas. Conserv Biol. 2000;14:428–438. [Google Scholar]
- 36.Vredenburg VT. Reversing introduced species effects: Experimental removal of introduced fish leads to rapid recovery of a declining frog. Proc Natl Acad Sci USA. 2004;101:7646–7650. doi: 10.1073/pnas.0402321101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knapp RA, Boiano DM, Vredenburg VT. Removal of nonnative fish results in population expansion of a declining amphibian (mountain yellow-legged frog, Rana muscosa) Biol Conserv. 2007;135:11–20. doi: 10.1016/j.biocon.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crump ML, Scott NJ., Jr . In: Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians. Heyer WR, Donnelly MA, McDiarmid RW, Hayek L- AC, Foster MS, editors. Washington, DC: Smithsonian; 1994. pp. 84–91. [Google Scholar]
- 39.Knapp RA, Matthews KR, Preisler HK, Jellison R. Developing probabilistic models to predict amphibian site occupancy in a patchy landscape. Ecol Appl. 2003;13:1069–1082. [Google Scholar]
- 40.Bradford DF. Temperature modulation in a high-elevation amphibian, Rana muscosa. Copeia. 1984;1984:966–976. [Google Scholar]
- 41.Vredenburg VT, Fellers GM, Davidson C. In: Status and Conservation of US Amphibians. Lannoo MJ, editor. Berkeley: Univ of California Press; 2005. pp. 563–566. [Google Scholar]
- 42.Kriger KM, Hero J-M, Ashton KJ. Cost efficiency in the detection of chytridiomycosis using PCR assay. Dis Aquat Org. 2006;71:149–154. doi: 10.3354/dao071149. [DOI] [PubMed] [Google Scholar]
- 43.Johnson ML, Berger L, Philips L, Speare R. Fungicidal effects of chemical disinfectants, UV light, desiccation and heat on the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Org. 2003;57:255–260. doi: 10.3354/dao057255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.