Abstract
A bibliometric analysis of the Bacillus anthracis and Ebola virus archival literature was conducted to determine whether negative consequences of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism” (USA PATRIOT) Act and the 2002 Bioterrorism Preparedness Act on US select agent research could be discerned. Indicators of the health of the field, such as number of papers published per year, number of researchers authoring papers, and influx rate of new authors, indicated an overall stimulus to the field after 2002. As measured by interorganizational coauthorships, both B. anthracis and Ebola virus research networks expanded after 2002 in terms of the number of organizations and the degree of collaboration. Coauthorship between US and non US scientists also grew for Ebola virus but contracted for the subset of B. anthracis research that did not involve possession of viable, virulent bacteria. Some non-US institutions were dropped, and collaborations with others intensified. Contrary to expectations, research did not become centralized around a few gatekeeper institutions. Two negative effects were detected. There was an increased turnover rate of authors in the select agent community that was not observed in the control organism (Klebsiella pneumoniae) research community. However, the most striking effect observed was not associated with individual authors or institutions; it was a loss of efficiency, with an approximate 2- to 5-fold increase in the cost of doing select agent research as measured by the number of research papers published per millions of US research dollars awarded.
Keywords: biosecurity policy, research productivity, research networks, Bacillus anthracis, Ebola virus
In October 2001, President Bush signed the “Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism” Act, otherwise known as the USA PATRIOT Act (1). It was followed in June 2002 by the Public Health Security and Bioterrorism Preparedness and Response Act, otherwise known as the 2002 Bioterrorism Preparedness Act. Sections of these laws deal with select agent (pathogens and toxins listed by the US government that pose a severe threat to public health and safety) research in the US, and include procedures for registration, inventory, and transfer of these organisms and toxins and the physical security required for facilities where research is performed. Regulations implementing these laws require US laboratories that possess, use, or transport select agents to register with the Department of Health and Human Services (2).* Federal Bureau of Investigation (FBI) background checks were required of all personnel with access to select agents. Research facilities had to meet stringent security standards. Mandatory protocols for select agent transfer and inventory; safety and security training and inspections; notification after theft, loss, or release of a listed agent; and record maintenance were instituted. Certain ex-criminals, drug abusers, illegal aliens, mentally ill people, citizens from the Attorney General's list of terrorist nations, and suspected national or international terrorists were prohibited from working with select agents.† Violations of the regulations result in penalties as severe as incarceration (3).
A 2002 Congressional Research Service report warned of potential negative impacts of these laws, including additional financial costs associated with high security and tracking, inhibited scientific information exchange and scientific inquiry, and the loss of skilled foreign technical workers (4). Over 20% of select agent researchers surveyed in 2004 and 2005 noted that the regulation was affecting their ability to collaborate domestically and internationally, and about 40% claimed that they had to use research funding to make security upgrades (5). A 2006 Stimson Center survey found the main complaints of select agent researchers to be monetary and time costs of security upgrades and procedures, bureaucratic time sinks, the tedium of inventorying samples, and barriers to international collaboration (6). Researchers have turned down Department of Homeland Security funding because of the bureaucratic overhead of the compliance review (7). A group of members of the National Science Advisory Board for Biosecurity recently lamented the unmeasurable cost of select agent research that was not done, suggesting that unnecessary inhibition of this science amounts to a national security and public health threat (8).
A National Research Council panel has been tasked with evaluating the safety measures at biosecurity laboratories and the impact of biosecurity policies and regulations on the ability of the scientific community to conduct select agent research (9). A recent report of the US Commission on the Prevention of Weapons of Mass Destruction Proliferation and Terrorism called for tightening government oversight of high-containment laboratories (10). Governments across the globe are grappling with the problem of how best to secure dangerous pathogens, and there is an acute need for quantitative measurements of the impacts of the existing oversight before decisions are made to strengthen or relax biosecurity rules.
If the counter bioterrorism laws have had detrimental effects on select agent research, the impacts should be detectable in the published literature, the output by which scientific production is judged. Previous studies addressing the impacts of the biosecurity laws and regulations have relied entirely on expert opinion and surveys. This paper analyzes the archival experimental research record for evidence of impacts on select agent research.
Results
The archival laboratory research literature concerning two select agents, the bacterium Bacillus anthracis and the Ebola virus,‡ and one control pathogen, Klebsiella pneumoniae, was examined in this study. After removing papers that would not be subject to the biosecurity laws (e.g., review articles), the remaining papers were sorted by whether they entailed the possession of viable, virulent microbes. This was done to determine whether the choice of research methods responded to the passage of the laws.
Was the Volume of Select Agent Research Reduced After the Laws Were Passed?
Although the number of annual publications increased, there was a steep decline in the number of papers per million dollars of US funding for the select agents. This was not observed with the control organism (Fig. 1). Before 2002, the average number of B. anthracis research papers per million dollars was 17. After 2002, the average number was only 3. For Ebola virus, before 2002 the average number of papers was 14, which subsequently fell to 6 per million dollars. In contrast, the average number of papers per million dollars for the control organism declined from 26 to 17. Admittedly, the funding data cannot be directly matched to specific research papers, and errors may have been introduced by the data cleaning process. Nevertheless, there should be no particular difference in errors introduced before and after 2002. Therefore, although we recognize that these figures are soft, they clearly indicate that the efficiency of select agent research fell sharply after the passage of the laws, perhaps by factors in the vicinity of 2- to 5-fold.
Fig. 1.
Annual peer-reviewed research publications and US funding time series for (A) B. anthracis, (B) Ebola virus, and (C) K. pneumoniae. (D) Annual number of papers per million dollars of US funding in the previous year. The vertical bar indicates an approximate boundary between pre- and post-biosecurity law eras.
Did the Laws Accelerate a Switch from Research Involving Live Select Agents to Methods Involving Avirulent or Subcellular Fractions of the Organisms?
One way for researchers to continue working on select agents without having to comply with the most stringent new regulations would be to switch to nonpathogenic strains or to research on subcellular components of the select agents. Using a binary logistic regression to assess whether scientists turned away from research in the more regulated “live-pathogen” category after the laws were passed, we find mixed results (Table 1).
Table 1.
Ratio of the odds of “live-pathogen” research before and after 2002
| Organism | Odds ratio |
| B. anthracis | 0.54* |
| Ebola virus | 2.25 |
| K. pneumoniae | 1.57 |
*P ≤ 0.10.
After 2002, the propensity to publish research involving viable, virulent B. anthracis decreased compared to research involving subcellular fractions or nonpathogenic strains. The same was not true for Ebola virus or K. pneumoniae research. The mixed results for the two select agents suggest that the choice of methods was not influenced by the biosecurity laws.
Was There a Detectable Exodus of Expertise or Were Fewer Researchers Attracted to Working with Restricted Organisms?
In the wake of the biosecurity laws, more than one high-profile scientist announced publicly that they had abandoned select agent study rather than fulfill the legal requirements (11). To determine whether this phenomenon was widespread among scientists doing “live-pathogen” research on B. anthracis and Ebola virus, and to understand whether these fields have become less attractive to scientists, we developed logistic regression models for estimating the likelihood of author entry and exit. Authors were said to “enter” the field in the first year in which they published between 1992 and 2007 and to “exit” the year after their last publication within this time period.
Results of these regressions are presented separately for “all scientists” and “career scientists,” the latter being defined as those publishing in two or more years within the 16-year period of our study (Table 2). This distinction was made to highlight any effects on the core research communities. Interestingly, less than a third of the authors in the database met the career scientist criterion.
Table 2.
Effect of the biosecurity laws on the odds of US author entry and exit of the “live-pathogen” research field
| Author type | B. anthracis | Ebola virus | K. pneumoniae |
| Odds ratio of author entry after 2002 | |||
| Career scientists | 3.91*** | 2.42*** | 0.71 |
| All scientists | 9.63*** | 4.41*** | 1.11 |
| Odds ratio of author exit after 2002 | |||
| Career scientists | 0.82 | 4.81*** | 1.12 |
| All scientists | 1.87*** | 1.69** | 0.97 |
**P ≤ 0.05.
***P ≤ 0.01.
Controlling for funding, we detected an increased propensity for US authors to enter “live-pathogen” select agent research after the laws were passed. This propensity to enter the field was not observed among control organism researchers. We also observed the increased odds of Ebola virus career scientists leaving the field after 2002, which were twice their odds of entering the field.
Did the Patterns of Collaboration Change?
Institutional collaboration networks for “live-pathogen” B. anthracis and Ebola virus research§ are diagrammed in Fig. 2 and Fig. S1, respectively. Changes in the research networks in the 5 years preceding and following the passage of the biosecurity laws reveal some key features of how these communities reacted to the laws. For visual clarity, instead of author names, we plot the names of the authors’ home institutions. A link between two nodes represents papers coauthored by members of those institutions.
Fig. 2.
Schematic of the collaboration networks of research organizations working with live B. anthracis. A link between two nodes indicates a coauthorship involving members of the institutions. (A) Publication network 1997–2001. (B) Publication network 2003–2007. Red nodes indicate US educational or research institutions; blue, US government; green, US military; and yellow, foreign institutions collaborating with US institutions. CDC: Centers for Disease Control and Prevention; DUKE: Duke University; JHU: Johns Hopkins University, LANL: Los Alamos National Lab; LSU: Louisiana State University; NAU: Northern Arizona University; NIH: National Institutes of Health; USAMRIID: United States Army Medical Research Institute for Infectious Diseases; U TX: University of Texas.
Both B. anthracis and Ebola virus “live-pathogen” research networks expanded over the study period in terms of the number of organizations, the B. anthracis network expanding by a factor of more than 6 and the Ebola virus network by a factor of almost 3. In contrast, the number of institutions involved in K. pneumoniae “live-pathogen” research grew by just 30% (Table 3).
Table 3.
Indicators of changes in the research collaboration networks
| “Live-pathogen” research |
“Non-pathogen” research |
|||||
| 1997–2001 | 2003–2007 | Change | 1997–2001 | 2003–2007 | Change | |
| Total number of institutions in network | ||||||
| B. anthracis | 24 | 163 | 579% | 54 | 281 | 420% |
| Ebola virus | 29 | 82 | 183% | 22 | 59 | 168% |
| K. pneumoniae | 268 | 347 | 29% | 28 | 30 | 7% |
| Fraction of institutions belonging to the largest subgraph | ||||||
| B. anthracis | 54% | 84% | 56%*** | 43% | 84% | 95%*** |
| Ebola virus | 93% | 96% | 3% | 73% | 75% | 3% |
| K. pneumoniae | 69% | 73% | 6% | 94% | 93% | −1% |
| Share of papers involving only one US institution | ||||||
| B. anthracis | 45% | 24% | −48%** | 54% | 49% | −10% |
| Ebola virus | 35% | 18% | −47%** | 5% | 36% | 614%† |
| K. pneumoniae | 43% | 37% | −15% | 56% | 42% | −25% |
| Network centralization | ||||||
| B. anthracis | 9% | 7% | −20% | 12% | 5% | −60% |
| Ebola virus | 20% | 5% | −78% | 14% | 5% | −69% |
| K. pneumoniae | 5% | 1% | −74% | 21% | 5% | −74% |
*P ≤ 0.10, **P ≤ 0.05, ***P ≤ 0.01.
†Significance test impossible due to small sample size.
The overall level of connectivity in the select agent research communities, as measured by the fraction of institutions belonging to the largest subgraph (a connected group of nodes), increased significantly for B. anthracis research after the laws, but not for the other two organisms. This is true for both “live-pathogen” and “non-pathogen” research networks. The Ebola “live-pathogen” research network was already highly connected before the laws and remained so afterward. There were fewer single-institution papers on “live-pathogen” select agent research after the laws, but no significant change in the control group.
Was US–International Collaboration Inhibited?
As measured by the fraction of papers coauthored by US and international partners, international collaboration in research on viable B. anthracis and K. pneumoniae held roughly steady over the study period (Table 4). The international fraction of “live-pathogen” Ebola virus papers actually increased after 2002. Because many of these papers dealt with outbreaks around the world, it is not surprising to see international collaboration. In addition, the importation of B. anthracis strains became exceedingly difficult post-2002, so many international collaborations became virtual rather than physical.
Table 4.
Indicators of international collaboration
| “Live-pathogen” research |
“Non-pathogen” research |
|||||
| 1997–2001 | 2003–2007 | Change | 1997–2001 | 2003–2007 | Change | |
| Fraction of papers involving at least one US and one non-US institution | ||||||
| B. anthracis | 16% | 20% | 22% | 21% | 11% | −48%** |
| Ebola virus | 23% | 38% | 63%* | 50% | 36% | −29% |
| K. pneumoniae | 26% | 33% | 25%* | 24% | 53% | 124%** |
| Share of degree centrality of all non-US institutions | ||||||
| B. anthracis | 18% | 26% | 44% | 26% | 26% | 0% |
| Ebola virus | 32% | 28% | −13% | 24% | 39% | 63% |
| K. pneumoniae | 37% | 39% | 6% | NS | 50 | NS |
| Share of papers involving exclusively US collaborations | ||||||
| B. anthracis | 39% | 57% | 46%* | 25% | 40% | 62%*** |
| Ebola virus | 42% | 44% | 4% | 45% | 29% | −37% |
| K. pneumoniae | 30% | 30% | −1% | 21% | 5% | −74%† |
NS, indicates an insufficient number of institutions to calculate share of degree centrality. *P ≤ 0.10, **P ≤ 0.05, ***P ≤ 0.01.
†Significance test impossible due to small sample size.
Perhaps more interesting is the decline in international “nonpathogen” select agent work. Avirulent and subcellular B. anthracis research papers outnumbered “live-pathogen” papers by a factor of more than 3 to 1, so the statistically significant decline here is an important indicator of the state of the broader field. The control organism did not demonstrate a similar decline in international cooperation. Coupling this observation with the increased fraction of exclusively US papers on B. anthracis, a pattern of decline in international collaboration on B. anthracis research emerges.
Another measure of international collaboration in network terms is the share of degree centrality (DC) of the coauthorship graph attributed to non-US institutions. The degree centrality of a single node in a network is measured by the number of links emanating from it. The share of degree centrality for a class of nodes is the proportion of the total number of links in a network emanating from all members of that class of nodes (12). Changes in the share of DC for non-US institutions after 2002 appear to be minimal (Table 4). For Ebola virus, the share of papers resulting from international collaborations on “live-pathogen” work increased whereas their share of degree centrality decreased. This does not necessarily imply any contradiction because only the latter is scaled to the network in Fig. S1, which is composed of links between institutions. The fraction of international interinstitutional collaborations in the Ebola virus network decreased even though the fraction of all papers with international collaborators increased. This indicates a winnowing of non-US institutions. Collaborations with some non-US groups were dropped whereas others intensified (Table 4 and Table S1).
Did Select Agent Research Networks Become Centered on a Few Institutions?
Another concern was that the research communities would become dependent on a few gatekeeper institutions. Such a change would be captured by network centralization (13). This measure compares the actual network to a “star” network of equivalent size in which one central node is connected to all other nodes (the extreme case of centralization). Network centralization decreased over the study period for “live-pathogen” research on both select agents. The same pattern was seen in K. pneumoniae research and in non-pathogen research on the select agents (Table 3). This suggests that the observed decentralization was a secular trend unrelated to the passage of the biosecurity laws.
A similar question can be asked for institutional type: Did military or governmental institutions become more central in the network? We calculated the share of degree centrality for four categories of institutions: US government, US military, US academic and commercial,§ and non-US institutions (Table S1). The most striking observation is a significant increase in the role of the military laboratories in “live-pathogen” select agent research and the relative decline in the centrality of the civilian government laboratories. In contrast, the participation of academic institutions and foreign collaborators remained remarkably unchanged. Thus, although the select agent networks became less centralized after the laws were enacted, military institutions became more collaborative in “live-pathogen” B. anthracis and Ebola virus research.
Did the Key Institutions Change After the Legislation?
In terms of the numbers of papers published, the top two institutions retained their positions throughout the study period, although there was a shift in the later period to institutions with higher biosecurity-level laboratories and government agencies such as the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) (Table S2).
Regarding “live-pathogen” B. anthracis research, before the laws, the institutions with the most coauthorships with other institutions were Northern Arizona University (NAU), Louisiana State University (LSU), and the Los Alamos National Laboratory (LANL). After the laws, NAU remained a key player, and the US Army Medical Research Institute for Infectious Diseases (USAMRIID) attained the highest share of DC (Table S3). In contrast, USAMRIID was not even a member of the main B. anthracis subgraph during 1997–2001, its researchers being prolific but not very collaborative.
The Centers for Disease Control and Prevention (CDC) and the National Institutes of Health (NIH) were key players in “live-pathogen” Ebola virus research both before and after the laws (Table S2). In the period 2003–2007, CDC collaborations declined from accounting for nearly 19% of the degree centrality of the network to 6%, whereas USAMRIID and the NIH became more central (Table S3). Furthermore, USAMRIID ranked highest in terms of productivity (for “live-pathogen” research on both select agents) after 2002, as measured by the number of papers involving researchers from that institution (Table S2). Thus, USAMRIID saw the most growth in prominence for “live-pathogen” work on the two select agents after the laws came into force.
Were Detected Trends Consistent with Individual Experience?
Phone interviews were conducted with 13 authors to determine whether our findings were consistent with their individual experiences. Eleven of the scientists were selected because they had a large number of publications in the cleaned dataset. Two worked with select agents other than B. anthracis and Ebola virus. All agreed to be interviewed after reading the list of Institutional Review Board-approved questions. Their responses were not statistically analyzed because the purpose of the interviews was to provide anecdotal information (SI Methods).
The group did not report witnessing an exodus of select agent scientists. To the contrary, several mentioned that the increased US funding led to an influx of new scientists to the select agent field, but that many did not stay. None of the scientists reported having to sacrifice research partnerships, and most of them perceived increased collaboration and diversity of expertise within the field after 2002. However, they pointed out that the process of collaborating was made significantly slower and more tedious due to the restrictions placed on organism transfer and laboratory access. Most of the scientists with whom we spoke did not work with foreign partners, and those who did alluded to difficulties in sharing cultures. Nearly all authors complained of the increased paperwork that they were legally obligated to fill out, one of them estimating that it took twice as long to do any project as a direct result of the bureaucratic overhead. One author commented that the FBI background checks took so long that they interfered with hiring students and technicians, especially non-US citizens. In general, the interviews confirmed the bibliometric findings.
Discussion
Over the study period there were major changes not only in the biosecurity laws, but also in the size of the research communities in question and in the funding that they received. On a macro level, research involving viable virulent B. anthracis and Ebola virus does not appear to have been inhibited by the biosecurity laws, although research became less efficient. After the passage of the laws, US scientists published more papers on B. anthracis and Ebola virus research, and more scientists entered the field. Although dramatic funding increases surely influenced these phenomena, they do not completely explain them. Research collaboration increased after the laws, and US military research laboratories became more central to the research community. International partnerships with a select group of foreign institutions increased, although not necessarily physical collaborations.
This work is subject to a number of limitations. We can make no claims that the trends detected were caused by the anti-bioterrorism laws—only whether the observations were consistent with hypothesized effects. Changes not tied to the laws were certainly operative during the study period, and random effects in the early period, when research communities were very small (e.g., the retirement of a senior researcher), may have exerted a disproportionate influence. The funding and publication data were not individually linked, which added to the noise introduced by any errors in the data sorting procedures. Finally, classified research and funding are not considered.
Methods
Data Sources.
Two representative select agents were chosen for this study: the bacterium that causes anthrax, B. anthracis, and Ebola virus, both CDC “Category A” select agents. K. pneumoniae, a common pathogenic bacterium, was chosen as a control organism.
Peer-reviewed research publication records dealing with these three organisms from 1992 through 2007 were retrieved from the Institute for Scientific Information (ISI) Web of Knowledge.¶ Only the subset of papers that would be subject to the biosecurity laws, namely research involving manipulation of viable virulent strains and certain genetic materials conducted in the United States, were retained. This excludes reviews, editorials, letters, in silico studies, numerical modeling, meta-analyses, articles with no US authors, and most clinical reports.
The remaining articles were manually classified as “live-pathogen” if the research required possession of viable, virulent organisms or as “non-pathogen” if the research involved only avirulent strains or subcellular fractions of virulent strains obtained without possession of the pathogen (Tables S4 and S5). Publication data for the control microorganism were identically classified, that is, as if K. pneumoniae were a select agent.
At the time of this study, two searchable sources of annual US funding were available on the web: the NIH CRISP database, which covered only NIH grants and did not include the award amounts, and the RAND Corporation's RaDiUS database, which compiled estimated average annual US research funding data over all federal agencies. The results from using either of the databases were comparable, so for this paper we reported results derived from the RAND database because it was more comprehensive and reported dollars spent rather than number of grants (Table S6).
Using the microbe names as keywords, yearly funding data for research on each of the three microorganisms were downloaded (14). Most of the research records included abstracts so it was possible to remove nonresearch grants (e.g., funding for building construction or certain training grants) manually, but it was not possible to reliably sort the funded research into “live-pathogen” and “non-pathogen” categories from the information provided.
It is important to note that the data above naturally excluded classified and forensic research and funding, which we recognize might be non-negligible. Yet we believe that classified research has a different nature and objectives and is therefore beyond the scope of this work.
Data Classification.
Determining which years of data to consider having occurred after the laws were enacted and which years before was not trivial because all possible solutions introduce some error. The laws were passed at the end of 2001 and in the middle of 2002, but the regulations implementing them continued to be issued for the next 3 years (12) and are still being scrutinized. Sensitivity analysis supported the decision to define 2002, the first year in which both laws were in effect, as the boundary year and to discard all papers with a 2002 publication date. (SI Methods and Tables S7—S8) All papers published from 1992 to 2001 were considered to be written before the laws were enacted and those published from 2003 to 2007 as after the laws took effect.
Paper publication dates also need to be linked to funding dates, that is, to possible one- or multiyear lags between the funding award and the publication of results from the supported research. We conducted a sensitivity analysis on the length of the lag between funding award and publication and determined that 1- and 2-year lags are good predictors of future publications, with the key results consistent regardless of the lag used. We report all results in the paper with a 1-year lag. Results with other lags are presented in Tables S9—S11.
Data Analysis.
Binary logistic regressions were performed using STATA data analysis and statistical software (STATA http://www.stata.com/). Eq. 1 represents the regression model used to analyze whether there was a shift from “live-pathogen” research to “non-pathogen” research after 2002:
 |
where i = 1, 2, … , n papers; t = 1992, 1993, … , 2007;  and
and
 |
In the model represented by Eq. 1, if α2 is significantly different from zero, the propensity to publish on “live-pathogen” research was sensitive to the timing of the biosecurity laws. The odds ratio of publishing a paper on “live pathogen” research after 2002 (compared to before 2002) is exp(α2). An odds ratio of less than one means that, controlling for funding, papers after 2002 were more likely to describe “non-pathogen” studies.
The model for determining whether the laws influenced whether scientists entered select agent research predicts the probability that a given author entered in a given year, P(Entryjt = 1), where Entryjt = 1 indicates that author j appeared in the dataset in year t (Eq. 2). As in Eq. 1, the model controls for funding level for the previous year and whether the biosecurity laws were in effect at the time of publication. The exit model is identical to the entry model except that it predicts P(Exitjt = 1) for author-year pairs:
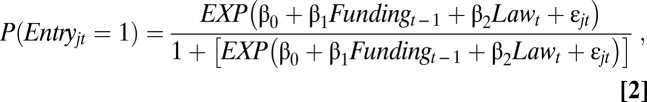 |
where j = 1, 2, …, m authors, t = 1992, 1993,…, 2007 and
 |
Network statistics and figures were generated in UCINET 6 (12). For institutional-level analyses, variants in workplace names were harmonized and classified by institutional type manually.
Supplementary Material
Acknowledgments
This research was funded by grants from the Richard A. Lounsbery Foundation, the John D. and Katherine T. MacArthur Foundation, Consejo Nacional de Ciencia y Technología (Mexico) Fellowship 168868, and National Science Foundation Grant NSF-SBE-0738182.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0915002107/-/DCSupplemental.
*Associated rules found in 42 CFR 73, 9 CFR 121, and 7 CFR 331; HHS and USDA Select Agents and Toxins, 7 CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73. http://www.cdc.gov/od/sap/docs/salist.pdf and http://www.cdc.gov/od/sap/. Interim rules were first issued in 2003, and final rules came into effect in 2005.
†The list of categories of excluded individuals is in actuality much broader by virtue of the databases that the FBI consults for its security risk assessments of personnel.
‡These select agents were chosen in an attempt to cover a range of regulatory conditions because B. anthracis can be studied in BLS 2+ and BLS 3 labs, whereas Ebola virus work has always been conducted with the highest level of biosecurity.
§Universities, hospitals, nonprofit institutes, and commercial laboratories are included in this category—i.e., all institutions not falling in the military or government categories.
¶ISI Web of Knowledge, Web of Science, Copyright © 2008 The Thomson Corporation: http://portal.isiknowledge.com/portal.cgi?DestApp=WOS&Func=Frame.
References
- 1.107th US Congress. Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism (USA PATRIOT) Act of 2001, PL 107-56 (United States of America) 2001 [Google Scholar]
- 2.107th US Congress. Public Health Security and Bioterrorism Preparedness and Response Act of 2002, PL 107-188 (United States of America) 2002 [Google Scholar]
- 3.Enserink M. US Courts. ‘Disappointed’ Butler exhausts appeals. Science. 2006;312:1120. doi: 10.1126/science.312.5777.1120b. [DOI] [PubMed] [Google Scholar]
- 4.Knezo GJ. Possible Impacts of Major Counter Terrorism Security Actions on Research, Development, and Higher Education. Washington, DC: Congressional Research Service Report to Congress, Library of Congress; 2002. [Google Scholar]
- 5.Sandia National Laboratories. Laboratory Biosecurity: A Survey of the US Bioscience Community, SAND no. 2006-1197P. 2006 [Google Scholar]
- 6.Fischer JE. Stewardship or Censorship? Balancing Biosecurity, the Public's Health, and the Benefits of Scientific Openness. Albuquerque, NM: The Henry L. Stimson Center; 2006. [Google Scholar]
- 7.Wadman M. Booming Biosafety Labs Probed. Nature. 2009;461:577. doi: 10.1038/461577a. [DOI] [PubMed] [Google Scholar]
- 8.Franz DR, Ehrlich SA, Casadevall A, Imperiale MJ, Keim PS. The “nuclearization” of biology is a threat to health and security. Biosecur Bioterror. 2009;7:243–244. doi: 10.1089/bsp.2009.0047. [DOI] [PubMed] [Google Scholar]
- 9.National Research Council. Leadership for Select Agent Research: Promoting a Culture of Responsibility. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 10.Commission on the Prevention of Weapons of Mass Destruction Proliferation and Terrorism. The Clock Is Ticking: A Progress Report on America's Preparedness to Prevent Weapons of Mass Destruction Proliferation and Terrorism. 2009 Washington, D.C. (U. S. Congress) [Google Scholar]
- 11.Gaudioso J, Salerno RM. Science and government. Biosecurity and research: Minimizing adverse impacts. Science. 2004;304:687. doi: 10.1126/science.1096911. [DOI] [PubMed] [Google Scholar]
- 12.Borgatti SP, Everett MG, Freeman LC. UCINET 6 for Windows: Software for Social Network Analysis. 2002 Analytic Technologies, Lexington, KY. [Google Scholar]
- 13.Scott JP. Social Network Analysis: A Handbook. Newbury Park, CA: Sage Publications; 1991. [Google Scholar]
- 14.RAND Corporation. RAND Database on Research and Development in the US (RADiUS) 2008 Science and Technology Policy Institute RAND, Arlington, VA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.