Abstract
Mutations in the dystrophin gene cause Duchenne muscular dystrophy (DMD) most commonly through loss of protein expression. In a small subpopulation of patients, missense mutations can cause DMD, Becker muscular dystrophy, or X-linked cardiomyopathy. Nearly one-half of disease-causing missense mutations are located in actin-binding domain 1 (ABD1) of dystrophin. To test the hypothesis that ABD1 missense mutations cause disease by impairing actin-binding activity, we engineered the K18N, L54R, D165V, A168D, L172H, and Y231N mutations into the full-length dystrophin cDNA and characterized the biochemical properties of each mutant protein. The K18N and L54R mutations are associated with the most severe diseases in humans and each caused a small but significant 4-fold decrease in actin-binding affinity, while the affinities of the other four mutant proteins were not significantly different from WT dystrophin. More interestingly, WT dystrophin was observed to unfold in a single-step, highly cooperative manner. In contrast, all six mutant proteins were significantly more prone to thermal denaturation and aggregation. Our results suggest that missense mutations in ABD1 may all cause loss of dystrophin function via protein instability and aggregation rather than through loss of ligand binding function. However, more severe disease progressions may be due to the combinatorial effects of some mutations on both protein aggregation and impaired actin-binding activity.
Keywords: muscular dystrophy, thermal stability, calponin homology domain
Duchenne muscular dystrophy (DMD) is a severe muscle wasting disease that affects 1/3,500 males born and is caused by mutations in the dystrophin gene (1 and 2). Dystrophin is a 427 kDa protein that localizes to costameres, large subsarcolemmal protein assemblies that are thought to transmit force radially from the Z-line of the contractile apparatus to neighboring muscle fibers (2–6). At costameres, dystrophin interacts with the cortical actin cytoskeleton, composed primarily of γ-actin and the transmembrane heterodimer dystroglycan (6 and 7) which in turn binds to the extacellular matrix protein laminin (8 and 9). Through interactions with γ-actin, dystroglycan, and other proteins, dystrophin forms the dystrophin glycoprotein complex (DGC), which establishes a mechanically strong link between the intracellular cortical actin cytoskeleton and the extracellular matrix that provides stability to the fragile sarcolema during muscle contraction (9–11). Loss of dystrophin expression destabilizes the DGC, which results in sarcolemmal damage and muscle cell necrosis (9, 11–13).
Dystrophin is a large, multidomain protein containing a tandem calponin homology (CH) actin-binding domain (ABD1) at its amino terminus (2 and 9). The largest domain of dystrophin is composed of a series of 24 spectrin-like repeats and four hinge regions that are thought to allow dystrophin to adopt a flexible rod-like architecture (9 and 14). The rod domain was also shown to contain a second actin-binding site (ABD2) composed of a series of basic spectrin-like repeats that interact electrostatically with acidic actin filaments (15). The combined actin-binding activities of the amino-terminal tandem CH and central rod domain enable dystrophin to bind laterally along actin filaments with submicro molar affinity (7 and 16). The carboxyl terminus of dystrophin is a globular domain containing a cysteine-rich domain, a WW domain, a ZZ domain, and two EF hand modules (9, 17, 18) that interact with the cytoplasmic domain of β-dystroglycan as well as other members of the DGC (9).
Three actin-binding sequences (ABS 1–3, Fig. 1 and Fig. S1) within the dystrophin tandem CH domain were initially thought to directly interact with actin filaments (19 and 20). ABS1 and 2 were identified through analysis of peptide binding to actin filaments using NMR spectroscopy (19 and 20). ABS3 was proposed based on its homology with actin-binding sequences identified in other tandem CH domain containing proteins (21). Subsequent studies showed that only the first 90 amino acids of the dystrophin tandem CH domain were critical for actin binding in vitro (22 and 23) while a KTFT (amino acid sequence LysThrPheThr in Dystorphin ABD1) motif in ABS1 proposed to contact actin filaments directly was not required (22). Most recently, a microgene replacement study demonstrated that an intact tandem CH domain is required to rescue the phenotypes associated with dystrophin-deficiency in the mdx mouse (24).
Fig. 1.
Disease state and expression of mutant dystrophin proteins. (A) A ribbon diagram of the dystrophin tandem CH domain structure (38). Gray shaded region denotes proposed actin interacting regions (19 and 20). Residues marked in red represent disease-causing mutations not located in a proposed actin-binding region. The K18N mutation lies in ABS1 and is orange. (B) Disease phenotype and molecular location of missense mutations studied. DMD, Duchenne muscular dystrophy; BMD, Becker muscular dystrophy; XLCM, and X-linked cardiomyopathy. The molecular location of each mutation was classified as buried in a hydrophobic core or surface exposed based on the location of the residue in the crystal structure of wild-type protein (38). (C) Coomassie blue-stained SDS-polyacrylamide gels of purified WT and mutant dystrophins. Molecular weight standards are shown on the left.
While dystrophinopathies are most typically caused by mutations that lead to loss of protein expression or deletion of key ligand binding domains, missense mutations have been identified in a subset of patients (www.dmd.nl). Of the 35 disease-causing missense mutations identified to-date, 17 are located in ABD1 (www.dmd.nl). The first such mutation identified, L54R, is associated with DMD, other mutations in the tandem CH domain cause a less severe disease known as Becker muscular dystrophy (BMD) (25–27), while the K18N mutation is associated with X-linked cardiomyopathy (28). Since almost half of the missense mutations localize to ABD1, we have tested the hypothesis that one or more of these mutations would specifically disrupt the interaction between dystrophin and actin filaments. Using biochemical analysis of full-length recombinant proteins bearing six different missense mutations, we demonstrate that these ABD1 mutations had only modest effects on actin-binding activity. Instead, the most noteworthy biochemical change we observed for every mutation studied was significantly decreased protein stability and solubility. These results suggest that several different single amino acid changes in ABD1 all may cause a loss of function through protein aggregation.
Results
Expression of disease-causing dystrophin mutations: Several amino acids in the tandem CH domain of ABD1 are conserved between dystrophin and its autosomal homolog utrophin. Furthermore, these residues are also conserved from flies to humans (Fig. S1), suggesting that they are critical to protein structure and/or function. Consistent with this idea, mutations in the tandem CH domain of ABD1 account for 50% of the known disease-causing missense mutations in the dystrophin gene even though the domain only comprises 6.7% of the dystrophin sequence (Fig. S1).
The eight ABD1 missense mutations known at the initiation of this study are associated with myopathies of varying degrees of severity and are either buried in hydrophobic alpha helices or reside on solvent-exposed surface regions of alpha helices (Fig. 1 A and B). To understand how single amino acid changes cause disease in such a large protein, we introduced each mutation into the full-length dystrophin cDNA by site-directed mutagenesis and expressed each protein in Sf9 insect cells using the baculovirus system. Six mutants expressed to high levels and purified as single bands that comigrated with WT dystrophin on SDS-polyacrylamide gels (Fig. 1C).
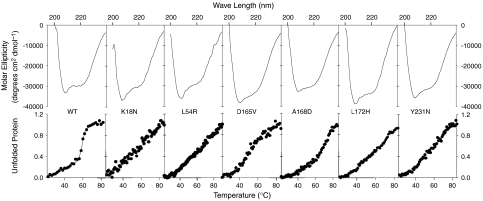
Circular Dichroism: To examine the effect of each mutation on the secondary structure of dystrophin, we analyzed WT and mutant proteins using CD spectroscopy. At concentrations between 0.2 and 1 mg/mL, WT dystrophin displayed CD spectra typical of highly alpha helical proteins (Fig. 2). Mutant dystrophin proteins over the same range of concentrations exhibited CD spectra similar to WT protein (Fig. 2). In order to determine if disease-causing mutations rendered the protein less stable, we performed a melting point analysis of WT and mutant dystrophin proteins. For WT dystrophin, we observed a sharp two state transition with a melting temperature of 59.6 °C (Fig. 2). Surprisingly, none of the disease-causing mutant proteins exhibited sharp transitions and appeared to unfold in a noncooperative fashion. The linear melting curves of the mutant proteins were consistent for multiple preparations and reproducible at multiple concentrations.
Fig. 2.
Circular Dichroism measurements of WT and mutant dystrophin. WT and mutant dystrophin proteins display characteristic alpha-helical CD signature between 200–260 nm. Full-length WT dystrophin unfolds cooperatively and has melting temperature of 59.6 °C. Mutant dystrophin proteins unfold noncooperatively.
Solubility of dystrophin mutants: To determine whether disease-causing missense mutations affected the solubility of dystrophin, we measured the fraction of insoluble WT and mutant protein when overexpressed in Sf9 insect cells. While only 5% of WT dystrophin protein was found in the insoluble fraction, K18N, L172H, and Y231N mutations all had more than 25% of the total protein associated with the insoluble fraction of insect cell lysates (Fig. S2A and Fig. 3A). The L54R, D165V, and A168D mutations caused 40–50% of dystrophin to shift to the insoluble fraction (Fig. 3A). In addition to analyzing cell lysates, we measured the solubility of purified WT and mutant dystrophin using high-speed centrifugation. Equal concentrations of WT and mutant protein were spun at 100,000 × g and protein levels determined for the supernatant and pellet. Our previous experiments showed that WT dystrophin was predominantly associated with the soluble supernatant. Therefore, the fraction of WT or mutant protein found in the pellet was considered insoluble or aggregated. For WT dystrophin, less than 15% of the protein was in the insoluble or aggregated fraction (Fig. S2B and Fig. 3B). In contrast, the K18N, L54R, A168D, and L172H mutations caused a significant increase in the amount of aggregated protein as compared to WT dystrophin (Fig. 3B). The fraction of aggregation for the D165V and Y231N mutants was greater than WT protein, but the difference was not statistically significant (Fig. 3B).
Fig. 3.
Relative solubility of cellular and purified dystrophin proteins. (A) Significantly more of each dystrophin mutant in SF9 insect cell lysates pelleted at 14,000 × g compared to WT (*, p < 0.05; n = ≥3). (B) Significantly more purified K18N, L54R, A168D, and L172H mutants pelleted at 100,000 × g compared to WT (*, p < 0.05; #, p < 0.01; n≥3). (A and B) Error bars represent S.E.M.
Actin-binding properties of missense mutant dystrophins: Despite the greater insolubility of mutant dystrophins, we were able to purify sufficient protein to characterize their actin-binding properties. We previously measured the affinity of full-length dystrophin for F-actin at 0.2 μM (16 and 29) using an actin cosedimentation assay where the concentration of dystrophin was varied against a constant actin concentration. Since a substantial fraction of each mutant protein self-pelleted under conditions used in the actin-binding assay we varied the concentration of actin (Fig. S3) and corrected all binding data by subtracting the amount of self-pelleting dystrophin measured in the absence of actin. When actin cosedimentation assays were performed with increasing concentrations of F-actin and a fixed concentration of 0.5 μM WT dystrophin, we measured a Kd value of 0.22 ± 0.081 μM (Fig. 4A), which is in good agreement with our previous studies (16 and 29). Surprisingly, D165V, A168D, L172H, and Y231N mutant dystrophins showed no significant differences in F-actin affinity compared with WT protein (Fig. 4D and Fig. S4). K18N and L54R mutant dystrophins bound F-actin with affinities of 0.76 ± 0.40 μM and 0.85 ± 0.21 μM for K18N and L54R, respectively (Fig. 4 B and C), which were 4–5-fold lower than and significantly different from WT dystrophin (p ≤ 0.5). Additionally, we observed significant increases in Bmax values for the binding of L54R, D165V, A168D, and L172H mutants to F-actin.
Fig. 4.
Actin-binding properties of WT and mutant dystrophins. F-actin-binding isotherms for 0.5 μM WT (A), K18N (B), and L54R (C) dystrophins measured by high-speed cosedimentation with increasing amounts of F-actin. Symbols represent three independent experiments plotted on the same graph and curves represent the best fit of the aggregate cosedimentation data. (D) The Kd’s obtained from fitted data for WT and each mutant protein were significantly different for K18N and L54R (p < 0.05). The Bmax values obtained from the same dataset were significantly different (p < 0.05) for L54R, D165V A168D, and L172H.
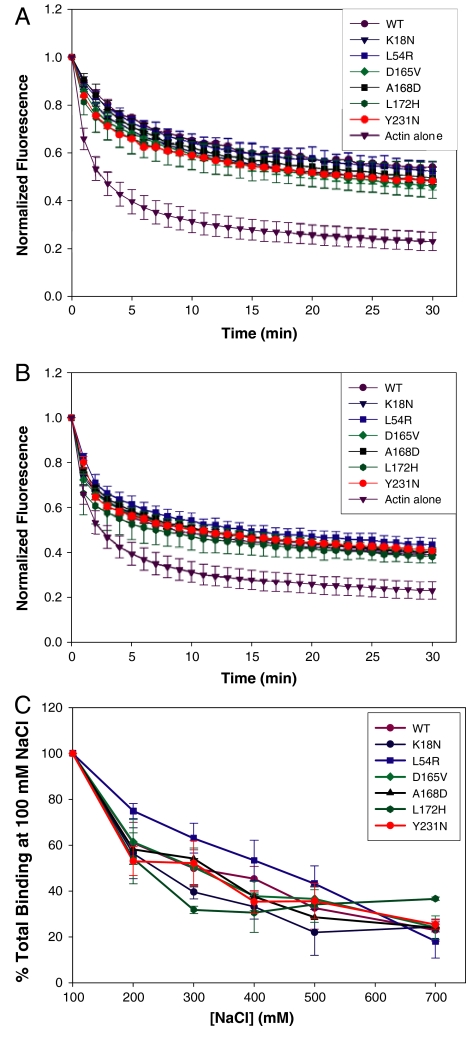
Dystrophin binds laterally along actin filaments with a stoichiometry of 1 dystrophin per 24 actin monomers and protects actin filaments from forced depolymerization in vitro (16 and 30). At dystrophin:actin molar ratios of 1∶12 and 1∶24, we found that WT dystrophin and all mutants slowed pyrene-labeled actin filaments from forced depolymerization with similar efficacies (Fig. 5 A and B). Finally, dystrophin binding to actin filaments is inhibited by increasing salt concentrations, predominantly due to the disruption of the electrostatic interaction between ABD2 and actin filaments (15 and 16). We reasoned that the effects of mutations in ABD1 may render the full-length protein even more sensitive to increasing ionic concentration. To correct for the small differences between WT and mutant dystrophins in binding affinity, experiments were performed at a fixed concentration of 0.5 μM WT or mutant dystrophin and concentrations of actin corresponding to each protein’s measured Kd. We found that binding of mutant dystrophin to actin filaments with increasing salt concentration (up to 700 mM) followed the same trend as WT, suggesting that the mutations did not affect the electrostatic binding component of dystrophin (Fig. 5C).
Fig. 5.
Effect of WT and mutant dystrophin on actin depolymerization. (A and B) WT and mutant dystrophins were coincubated with actin filaments at dystrophin:actin ratios of 1∶12 (A) and 1∶24 (B). Filament depolymerization was induced by dilution and monitored by decay of pyrene-actin fluorescence. Normalized fluorescence is plotted as a function of time (minutes). (C) Effect of NaCl concentration on WT and mutant dystrophin binding to actin. WT and mutant dystrophins at a concentration of 0.5 μM were subjected to high-speed cosedimentation with Kd concentrations of F-actin at the indicated concentration of NaCl. The data are expressed as the percent of each protein pelleted in the presence of 100 mM NaCl. (A–C) Error bars represent S.E.M.
Discussion
Genetic studies have demonstrated that a disproportionately high percentage of disease-causing missense mutations are located in ABD1 of dystrophin (www.dmd.nl). Based on the crystal structure of ABD1, Norwood et al., predicted that disease-causing missense mutations would greatly destabilize ABD1 and disrupt folding (31). Our results demonstrate that the actin-binding activity of dystrophin is remarkably robust as six different ABD1 mutations only modestly decreased actin-binding affinity. More interestingly, we observed significantly more aggregation of the mutant dystrophins compared to WT protein. Mutant dystrophin protein formed large aggregates which pelleted when centrifuged at 100,000 × g and were found in insoluble cellular extracts (Fig. 3). Additionally, the L54R, D165V, A168D, and Y231N mutants showed significantly higher Bmax values than WT protein in actin cosedimentation assays (Fig. 4). The increased Bmax values may be due to the formation of microaggregates that did not pellet at 100,000 × g but cosedimented with actin filaments. Previously, two microdystrophin constructs deleted for either CH1 or CH2 showed a greater propensity to form aggregates when expressed in dystrophin-deficient mdx muscle (24). While cellular localization studies on patient specimens bearing missense mutations are rare, immunofluorescence microscopic analysis of dystrophin localization in a biopsy from the original L54R DMD patient revealed the presence of increased cytoplasmic staining with bright puncta (25). Our results extend these previous studies by showing that six different single amino acid changes in ABD1 can dramatically induce aggregation in vitro.
Recently a study from Legardinier et al. (32) reported on the effects of two disease-causing missense mutations in a recombinant protein fragment encoding spectrin-like repeat 23 of dystrophin. Distinct from the effects of ABD1 mutations on full-length dystrophin, the mutant spectrin-like repeat fragments remained fully soluble in vitro. While mutations in the isolated spectrin-like repeat resulted in decreased thermal stability, the sharp transition from folded to denatured was primarily left-shifted compared to WT (32). In contrast, we found that ABD1 mutants of full-length dystrophin yielded CD spectra similar to WT dystrophin at room temperature, but all mutations in ABD1 abolished the sharp transition at approximately 60 °C and mutant proteins unfolded in a linear fashion as a function of increasing temperature. In order for a protein to undergo a cooperative unfolding transition, the protein must be in a compact, well-folded state. A broad or noncooperative unfolding, as seen for mutated dystrophin proteins, suggests that the protein sample is loosely folded or exists in numerous folded states. The mutated residue may disrupt the compact fold of the amino-terminal CH domain or the mutation may cause the domain to adopt multiple folding conformations. Our results suggest that disease-causing missense mutations in ABD1 of dystrophin lead to dramatic destabilization of the full-length dystrophin protein.
While 35 missense mutations and their corresponding disease phenotypes have been cataloged for the dystrophin gene, much less information is available on the tissue expression levels associated with each mutation. The L54R mutant protein was measured at 20% of normal dystrophin levels (25). A more recent study by Hamed et al. (26) reported that the L172H and R82P mutations reduced protein levels by Western blot to 20% and 50% of normal levels, respectively. Based on studies in transgenic mdx mice, 20% of WT dystrophin protein levels is sufficient to rescue the dystrophic phenotype (33). Our data on the L54R and L172H mutations suggest that both mutations cause the full-length protein to be markedly less soluble than WT dystrophin but only the L54R mutation binds to actin filaments with 4-fold lower affinity. These data fit well with previous studies (22 and 23) identifying CH1 as the predominant actin-binding module in ABD1 of dystrophin. The L172H mutation, which causes the milder BMD, exhibits a similar propensity to aggregate as the L54R mutation but displayed near WT actin affinity for actin filaments. Perhaps the L54R mutation results in a more severe disease phenotype through the combined effects of protein aggregation and decreased actin-binding activity. In light of the limited characterization of rare patients bearing disease-causing missense mutations, we cannot exclude the possibility that pathogenesis may also be driven by premature degradation or toxic gain-of-function by misfolded or aggregated protein. Mutations in many muscle proteins result in protein aggregate myopathies (PAMs) where misfolded proteins resist degradation and aggregate resulting in impaired muscle function (34). Further in vivo analysis is required to determine whether ABD1 missense mutant dystrophins also classify as PAMs.
Despite substantial thermodynamic instability and protein aggregation, disease-causing ABD1 mutants of dystrophin retained near WT affinity for actin filaments. A similar scenario has been observed for the Δ508F mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) (35). The Δ508F CFTR mutation retains function, but is improperly processed leading to premature degradation of the protein rather than trafficking to the plasma membrane (35). Pharmacological modulation of heat shock proteins by 4-phenylbutyrate was shown to increase the functional levels of the Δ508F CFTR protein in the plasma membrane (36) and trials are underway to examine how protein levels in CFTR patients can be increased by pharmacologically manipulating the folding and degradation pathway (35 and 36). Based on our results, drugs that block protein aggregation may hold promise for the treatment of DMD patients carrying missense mutations in ABD1.
Finally, our CD spectroscopic analysis of WT full-length dystrophin speaks to the potential for structural heterogeneity within the rod domain. Several studies have reported that different spectrin repeats in the rod domain display widely varying thermal stabilities when characterized in isolation (32, 37–41). These data have led to the suggestion that the dystrophin rod domain cannot function as a monolithic single unit (40). However, our studies demonstrate that full-length dystrophin melted in a highly cooperative, one-step process (Fig. 2). We conclude that the thermal stability of individual spectrin-like repeats is strongly influenced by neighboring sequences so that the entire protein functions as a single unit regardless of the different intrinsic stabilities of its isolated parts.
Materials and Methods
Site-directed mutagenesis—Disease-causing missense mutations (Fig. 1A and Fig. S1) were introduced into the first 1.2 kb of mouse dystrophin cDNA containing an amino-terminal FLAG tag in the pFastBac™ vector (Invitrogen) using overlapping primers in (Fig. S5) and the quick-change mutagenesis kit (Stratagene) following the manufacturer’s instructions. Mutant full-length FLAG-dystrophin cDNAs were engineered into pFastBac™ vector by ligating the remaining 12.5 kb of the dystrophin cDNA into the vector containing the mutant 1.2 kb construct using a SpeI site common to the dystrophin sequence and pFastBac™.
Protein expression and purification—WT and mutant dystrophin proteins were expressed using the Bac-to-Bac Baculovirus System (Invitrogen) in Sf9 insect cells as previously described (42). After verifying protein expression, P1 viruses were amplified and used to infect Sf9 cells at 1-L scale by Kinnakeet Biotechnology. WT and mutant proteins were purified by FLAG affinity chromatography (Sigma-Aldrich) and dialyzed against two changes of phosphate-buffered saline pH 7.5 (PBS). Proteins were concentrated using Amicon Ultra centrifuge-based cartridge concentrators (Millipore) and protein concentration determined with the BioRad Dc Protein Assay using a BSA standard curve (BioRad).
Solubility assay—Purified protein was centrifuged at 100,000 × g for 25 min and the amount present in the supernatant and pelleted fractions was quantified by densitometric analysis of Coomassie blue-stained SDS-polyacrylamide gels. To measure protein solubility in live cells, Sf9 insect cells infected with baculoviruses encoding WT or mutant dystrophins were lysed and total protein levels were compared to protein remaining in the supernatant after centrifugation at 16,000 × g for 15 min. At least three experiments for each condition were averaged and compared to the WT values using student’s t-test.
Circular dichroism measurements—WT and mutant full-length proteins were prepared for CD measurements in an identical fashion as all other experiments except 0.5% Triton X100 was omitted from the elution buffer. Proteins were centrifuged at 16,000 × g to remove large light scattering particles. CD spectra were acquired on a J-815 spectopolarimeter (JASCO Inc.) fitted with a Peltier temperature regulator. Spectrum for WT and mutant dystrophins were acquired from 260 nanometer (nm) to 200 nm at 20 ºC and at a range of concentrations between 0.5 and 0.2 mg/mL at pH 7.5. The melt curves for full-length WT dystrophin and dystrophin mutants were collected by varying the temperature from 20 ºC to 85 ºC collecting a spectrum from 235 nm to 200 nm every 1 ° Celsius. Data from the 222 nm reading was fit by regression analysis in Sigma Plot (Systat Software, Inc.) using an equation for a two state unfolding model reported by Legardinier et al (32).
Actin-binding assay—The binding of WT and mutant dystrophins to actin filaments was measured using a previously described high-speed cosedimentation assay (16) except that the actin concentration was varied between 0.2 μM and 15 μM around a fixed concentration of 0.5 μM dystrophin. Regression analysis was performed using Sigma Plot software using the equation
corresponding to one site saturation kinetics. F-actin binding in the presence of increasing NaCl concentration (100–700 mM) was also measured using the high-speed cosedimentation assay at a fixed concentration of 0.5 μM dystrophin and an actin concentration corresponding to WT or each mutant’s measured Kd (dissociation constant Kd).
Actin depolymerization assay—WT or mutant dystrophins were incubated for 30 min with 2 μM pyrene-labeled actin (Cytoskeleton, Inc.) filaments at ratios of 1∶12 or 1∶24 dystrophin molecules bound per actin monomer. Actin filaments were induced to depolymerize by dilution below critical concentration in low salt buffer (G-buffer: 5 mM Tris pH 8.0, 0.2 mM CaCl2 0.2 mM ATP and 0.5 mM DTT) and the fluorescence decay was monitored every minute for 30 min using a Gemini plate reader system (Molecular Devices). Fluorescence was normalized to initial fluorescent signal for each condition and plotted as a function of time.
Supplementary Material
Acknowledgments.
We thank Dr. Elisabeth Le Rumeur for providing regression equations used in CD unfolding experiments. We also thank Bin Li for excellent technical support and Dr. David D. Thomas for access to his CD spectrometer and fluorescence plate reader. Additionally, we thank Dr. Ben Perrin and Dr. Michele Jaeger-Padilla for helpful comments during manuscript preparation. This work was supported by the National Institutes of Health (NIH) Training Program in Muscle Research (AR007612) and National Institutes of Health Grant AR042423.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001517107/-/DCSupplemental.
References
- 1.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- 3.Zubrzycka-Gaarn EE, et al. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988;333:466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]
- 4.Porter GA, Dmytrenko GM, Winkelmann JC, Bloch RJ. Dystrophin colocalizes with β-spectrin in distinct subsarcolemmal domains in mammalian skeletal muscle. J Cell Biol. 1992;117:997–1005. doi: 10.1083/jcb.117.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straub V, Bittner RE, Léger JJ, Voit T. Direct visualization of the dystrophin network on skeletal muscle fiber membrane. J Cell Biol. 1992;119:1183–1191. doi: 10.1083/jcb.119.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ervasti JM. Costameres: the Achilles’ heel of herculean muscle. J Biol Chem. 2003;278:13591–13594. doi: 10.1074/jbc.R200021200. [DOI] [PubMed] [Google Scholar]
- 7.Rybakova IN, Patel JR, Ervasti JM. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J Cell Biol. 2000;150:1209–1214. doi: 10.1083/jcb.150.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta. 2007;1772:108–117. doi: 10.1016/j.bbadis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Mokri B, Engel AG. Duchenne dystrophy: Electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology. 1975;25:1111–1120. doi: 10.1212/wnl.25.12.1111. [DOI] [PubMed] [Google Scholar]
- 11.Menke A, Jockusch H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature. 1991;349:69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- 12.Weller B, Karpati G, Carpenter S. Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J Neurol Sci. 1990;100:9–13. doi: 10.1016/0022-510x(90)90005-8. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda R, Nishikawa A, Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with evans blue: Evidence of apoptosis in dystrophin- deficient muscle. J Biochem-Tokyo. 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- 14.Koenig M, Kunkel LM. Detailed analysis of the repeat domain of dystrophin reveals four potential hinge segments that may confer flexibility. J Biol Chem. 1990;265:4560–4566. [PubMed] [Google Scholar]
- 15.Amann KJ, Renley BA, Ervasti JM. A cluster of basic repeats in the dystrophin rod domain binds F-actin through an electrostatic interaction. J Biol Chem. 1998;273:28419–28423. doi: 10.1074/jbc.273.43.28419. [DOI] [PubMed] [Google Scholar]
- 16.Rybakova IN, Humston JM, Sonnemann KJ, Ervasti JM. Dystrophin and utrophin bind actin filaments through distinct modes of contact. J Biol Chem. 2006;281:9996–10001. doi: 10.1074/jbc.M513121200. [DOI] [PubMed] [Google Scholar]
- 17.Bork P, Sudol M. The WW domain: a signalling site in dystrophin? Trends Biochem Sci. 1994;19:531–533. doi: 10.1016/0968-0004(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 18.Tinsley JM, et al. Primary structure of dystrophin-related protein. Nature. 1992;360:591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- 19.Levine BA, Moir AJG, Patchell VB, Perry SV. The interaction of actin with dystrophin. FEBS Letters. 1990;263:159–162. doi: 10.1016/0014-5793(90)80728-2. [DOI] [PubMed] [Google Scholar]
- 20.Levine BA, Moir AJG, Patchell VB, Perry SV. Binding sites involved in the interaction of actin with the N-terminal region of dystrophin. FEBS Letters. 1992;298:44–48. doi: 10.1016/0014-5793(92)80019-d. [DOI] [PubMed] [Google Scholar]
- 21.Bresnick AR, Warren V, Condeelis J. Identification of a short sequence essential for actin binding by Dictyostelium ABP-120. J Biol Chem. 1990;265:9236–9240. [PubMed] [Google Scholar]
- 22.Corrado K, Mills PL, Chamberlain JS. Deletion analysis of the dystrophin-actin binding domain. FEBS Lett. 1994;344:255–260. doi: 10.1016/0014-5793(94)00397-1. [DOI] [PubMed] [Google Scholar]
- 23.Fabbrizio E, Bonet-Kerrache A, Leger JJ, Mornet D. Actin-dystrophin interface. Biochemistry. 1993;32:10457–10463. doi: 10.1021/bi00090a023. [DOI] [PubMed] [Google Scholar]
- 24.Banks GB, Gregorevic P, Allen JM, Finn EE, Chamberlain JS. Functional capacity of dystrophins carrying deletions in the N-terminal actin-binding domain. Hum Mol Genet. 2007;16:2105–2113. doi: 10.1093/hmg/ddm158. [DOI] [PubMed] [Google Scholar]
- 25.Prior TW, et al. A missense mutation in the dystrophin gene in a Duchenne muscular dystrophy patient. Nat Genet. 1993;4:357–360. doi: 10.1038/ng0893-357. [DOI] [PubMed] [Google Scholar]
- 26.Hamed S, Sutherland-Smith A, Gorospe J, Kendrick-Jones J, Hoffman E. DNA sequence analysis for structure/function and mutation studies in Becker muscular dystrophy. Clin Genet. 2005;68:69–79. doi: 10.1111/j.1399-0004.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- 27.Roberts RG, Gardner RJ, Bobrow M. Searching for the 1 in 2,400,000: a review of dystrophin gene point mutations. Hum Mutat. 1994;4:1–11. doi: 10.1002/humu.1380040102. [DOI] [PubMed] [Google Scholar]
- 28.Feng J, Yan J, Buzin CH, Towbin JA, Sommer SS. Mutations in the dystrophin gene are associated with sporadic dilated cardiomyopathy. Mol Genet Metab. 2002;77:119–126. doi: 10.1016/s1096-7192(02)00153-1. [DOI] [PubMed] [Google Scholar]
- 29.Rybakova IN, Amann KJ, Ervasti JM. A new model for the interaction of dystrophin with F-actin. J Cell Biol. 1996;135:661–672. doi: 10.1083/jcb.135.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rybakova IN, Ervasti JM. Dystrophin-glycoprotein complex is monomeric and stabilizes actin filaments in vitro through a lateral association. J Biol Chem. 1997;272:28771–28778. doi: 10.1074/jbc.272.45.28771. [DOI] [PubMed] [Google Scholar]
- 31.Norwood FL, Sutherland-Smith AJ, Keep NH, Kendrick-Jones J. The structure of the N-terminal actin-binding domain of human dystrophin and how mutations in this domain may cause Duchenne or Becker muscular dystrophy. Structure. 2000;8:481–491. doi: 10.1016/s0969-2126(00)00132-5. [DOI] [PubMed] [Google Scholar]
- 32.Legardinier S, et al. A two-amino acid mutation encountered in Duchenne muscular dystrophy decreases stability of the rod domain 23 (R23) spectrin-like repeat of dystrophin. J Biol Chem. 2009;284:8822–8832. doi: 10.1074/jbc.M805846200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phelps SF, et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet. 1995;4:1251–1258. doi: 10.1093/hmg/4.8.1251. [DOI] [PubMed] [Google Scholar]
- 34.Vrabie A, Goebel HH. In: Protein misfolding, aggregation, and conformational diseases part B: molecular mechanisms of conformational diseases. Uversky VN, Fink AL, editors. Vol 6. New York: Springer; 2007. pp. 365–389. [Google Scholar]
- 35.Lukacs GL, Durie PR. Pharmacologic approaches to correcting the basic defect in cystic fibrosis. N Engl J Med. 2003;349:1401–1404. doi: 10.1056/NEJMp038113. [DOI] [PubMed] [Google Scholar]
- 36.Andersson C, Roomans GM. Activation of deltaF508 CFTR in a cystic fibrosis respiratory epithelial cell line by 4-phenylbutyrate, genistein and CPX. Eur Respir J. 2000;15:937–941. doi: 10.1034/j.1399-3003.2000.15e21.x. [DOI] [PubMed] [Google Scholar]
- 37.Saadat L, Pittman L, Menhart N. Structural cooperativity in spectrin type repeats motifs of dystrophin. Biochim Biophys Acta. 2006;1764:943–954. doi: 10.1016/j.bbapap.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Menhart N. Hybrid spectrin type repeats produced by exon-skipping in dystrophin. Biochim Biophys Acta. 2006;1764:993–999. doi: 10.1016/j.bbapap.2006.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legardinier S, et al. Sub-domains of the dystrophin rod domain display contrasting lipid-binding and stability properties. Biochim Biophys Acta. 2008;1784:672–682. doi: 10.1016/j.bbapap.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Mirza A, Menhart N. Stability of dystrophin STR fragments in relation to junction helicity. Biochim Biophys Acta. 2008;1784:1301–1309. doi: 10.1016/j.bbapap.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruszczak C, Mirza A, Menhart N. Differential stabilities of alternative exon-skipped rod motifs of dystrophin. Biochim Biophys Acta. 2009;1794:921–928. doi: 10.1016/j.bbapap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rybakova IN, Patel JR, Davies KE, Yurchenco PD, Ervasti JM. Utrophin binds laterally along actin filaments and can couple costameric actin with the sarcolemma when overexpressed in dystrophin-deficient muscle. Mol Biol Cell. 2002;13:1512–1521. doi: 10.1091/mbc.01-09-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.