Abstract
Background
A breakthrough in the understanding of centriole assembly was provided by the characterization of the UNI3 gene in Chlamydomonas. Deletion of this gene, found to encode a novel member of the tubulin superfamily, delta-tubulin, results in the loss of the C-tubule, in the nine microtubule triplets which are the hallmark of centrioles and basal bodies. Delta-tubulin homologs have been identified in the genomes of mammals and protozoa, but their phylogenetic relationships are unclear and their function is not yet known.
Results
Using the method of gene-specific silencing, we have inactivated the Paramecium delta-tubulin gene, which was recently identified. This inactivation leads to loss of the C-tubule in all basal bodies, without any effect on ciliogenesis. This deficiency does not directly affect basal body duplication, but perturbs the cortical cytoskeleton, progressively leading to mislocalization and loss of basal bodies and to altered cell size and shape. Furthermore, additional loss of B- and even A-tubules at one or more triplet sites are observed: around these incomplete cylinders, the remaining doublets are nevertheless positioned according to the native ninefold symmetry.
Conclusions
The fact that in two distinct phyla, delta-tubulin plays a similar role provides a new basis for interpreting phylogenetic relationships among delta-tubulins. The role of delta-tubulin in C-tubule assembly reveals that tubulins contribute subtle specificities at microtubule nucleation sites. Our observations also demonstrate the existence of a prepattern for the ninefold symmetry of the organelle which is maintained even if less than 9 triplets develop.
Background
In addition to the alpha-, beta- and gamma-tubulins, essential for microtubule assembly in all eukaryotes, several new tubulin subfamilies have been recently identified in a cascade of discoveries [1]. Complementation cloning of the UNI3 mutation in Chlamydomonas led to the characterization of delta-tubulin, an unexpected fourth member of the tubulin subfamily, involved in assembly of basal bodies [2]. Genome search for delta-tubulin led to identify not only deltas in mammals and protozoa, but also to disclose further new divergent tubulins, epsilon and zeta [3,4,5]. A sixth subfamily, eta, was characterized in Paramecium [6] by complementation cloning of the sm19 mutation affecting basal body duplication [7]. In contrast to alpha-, beta- and gamma-tubulins, the new tubulins, of which a few sequences only are available, do not seem to be present in all eukaryotes and their function might concern such elaborate microtubule arrays as centrioles and basal bodies.
For each of the new subfamilies, sequence conservation is weak and, as only a few sequences are presently available, their phylogenetic relationships are unclear [1,6]. It is therefore important to ascertain if the members of a given presumed subfamily have the same function. For the delta-tubulin subfamily, the function is known only for Chlamydomonas: deletion of this gene results in the loss of the C-tubule, in each of the nine microtubule triplets which are the hallmark of centrioles and basal bodies. In the absence of a functional assay, a similar role of the delta-tubulin homologs identified in mammals [3,4] and protozoa [5,6] had not yet been demonstrated. We report here a functionnal analysis of delta-tubulin in Paramecium, a favourable organism because of the high number of its basal bodies and of their differentiated duplication pattern.
Results
In the course of random sequencing (see http://caroll.vjf.inserm.fr/pt/) of clones from the recently described indexed genomic library of Paramecium [8], a likely δ-tubulin homolog was characterized [6]. Southern blots (not shown) demonstrate that this sequence corresponds to a unique gene, designated as δPT1. The deduced polypeptide, 397 amino acids long, is shorter than tubulins; alignment with the known δ-tubulin sequences shows (Figure 1) that 43% of its residues are identical to the corresponding residue in at least one of the other sequences. After those of Chlamydomonas [2], man [4], mouse [3] and Trypanosoma [5], δPT1 is the fifth δ or putative δ-tubulin identified to date.
Figure 1.
Comparison of the predicted δPT1 sequence - Paramecium tetraurelia (pt) - with the the δ-tubulins from mouse (mm), man (hs), C. reinhardtii (cr) and T.brucei (tb). The aligned sequences are: H. sapiens δ-tubulin: NP07345C; M.musculus δ-tubulin: AF081568; C. reinhardtii δ-tubulin: AAB 71840;P. tetraurelia δ-tubulin: AJ401299; T. brucei δ-tubulin: AAF 32301. The sequences were aligned using the Clustal W1.8 program [21] and adjusted manually.
To ascertain whether δPT1 had a similar function, we took advantage of the phenomenon of homology-dependent gene silencing operating in Paramecium, previously discribed [9] and used to demonstrate the role of δ-tubulin in basal body duplication [10]. Microinjection of the coding sequence of a gene, at high copy number, into the macronucleus results in the inactivation of the corresponding endogenous gene.
The effect of δPT1 inactivation was studied on the individual progenies of 132 microinjected cells from two independent series of 33 and 99 cells respectively. In both sets of clones, growth rate, cell morphology and cytoskeletal organization remained normal over the four first divisions after injection. Then, over the next 2-3 divisions, the same syndrome progressively developed in a large majority of the clones (25/33 and 86/99 respectively for the two experiments): disorganisation of the basal body complex forming the oral apparatus, disorders in the cortical pattern with abnormal positioning of basal bodies and orientation of striated and microtubule rootlets, presence of intra-cytoplasmic basal bodies and erratic microtubule bundles suggesting occurence of microtubule nucleation at abnormal sites, possibly in relation to the intracytoplasmic basal bodies (Figure 2). All such transformed cells reached a terminal phenotype: rounded shape, smaller cell size with reduced number of basal bodies in the oral apparatus and on the cortex and after 7-8 divisions died of starvation as their oral apparatus became non-functional.
Figure 2.
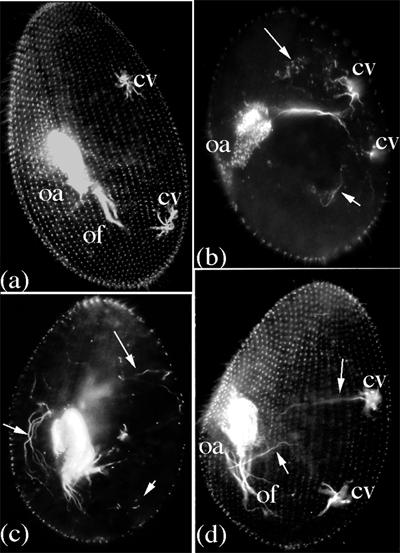
Cytological abnormalities induced by inactivation of the δPT1 gene: cellular level. (a): surface view of the dorsal side of a control cell, showing the basal body pattern, the outline of the oral apparatus (oa) and post-oral fibers (of) and the contractile vacuole pores and rootlets (cv). (b), (c), and (d): transformed cells from the pool presented in Table 1. (b), (c): sub-surface view of two transformed cells, showing disorders in the organization of the oral apparatus and postoral fibers, and the presence of erratic microtubule bundles (short arrows) and groups of intracytoplasmic basal bodies (arrows). (d): dorso-lateral view of a transformed cell showing erratic microtubule bundles (short arrows) and altered organization of oral apparatus and post-oral fibers. Note that the three transformed cells display a less constrained shape than the control. Bar for 10 μM : 0.5cm long.
Table 1.
Cytoskeletal abnormalities induced by binactivation of the δPT1 gene
| Normal cortex | Normal cortex | Abnormal | Abnormal | Total number of | |
| cortex | cortex | cells observed | |||
| Normal oral | Abnormal oral | ||||
| apparatus | apparatus | Normal oral | Abnormal oral | ||
| apparatus | apparatus | ||||
| Number of cells | 6 | 40 | 9 | 87 | 142 |
| With erratic | 0 | 3 | 4 | 12 | 19 |
| microtubules | |||||
| With internal | 0 | 0 | 6 | 30 | 36 |
| basal bodies |
48 hours after microinjection, i.e. after microinjected cells had undergone 5-6 divisions, a random sample was taken from 26 transformed clones, pooled and processed for immunofluorescence. Labelling with the anti-tubulin ID5 [19] reveals the basal body pattern on the cortex and in the oral apparatus allowing detection of even small pattern abnormalities and of "internal basal bodies", not inserted in the cortex or in the oral structure, never observed in normal cells. In addition to basal bodies, the monoclonal ID5 stains the thick post-oral fibers and the proximal part of the microtubule rootlets of the vacuole pore system (see Figure 2), but no other microtubule of the internal microtubule network. Cells were classified according to cell size, to defects in either cortex or oral apparatus or both and to the presence/absence of internal basal bodies and erratic intracytoplasmic microtubules (see text and Figure 2). The figures indicate the number of cells in each category.
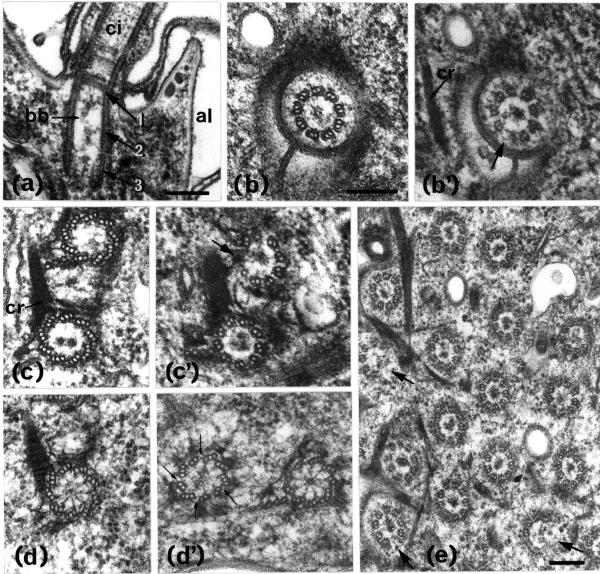
Basal body ultrastructure was examined on cells just beginning to display cortical disorders and shape changes, around the 5th division. At this early stage of expression of the transformant phenotype, a striking defect, lack of the C-tubule, was found in practically all basal bodies (156 out of 171 unambiguous basal body cross-sections), corresponding to at least 20 different cells from several transformed clones (Figure 3). It must be pointed out that this effect is highly specific: loss of the C-tubule was never observed in other experimental or genetical conditions interfering with basal body properties or duplication, such as γ-tubulin or centrin inactivation [10, 11; Garreau de Loubresse, unpublished] or in basal body defective mutants such as sm19 cells, mutated in η-tubulin [6], or sm2 cells [7].
Figure 3.
Cytological abnormalities induced by inactivation of the δPT1 gene: ultrastructural level. (a) Longitudinal section through a basal body (bb) and its cilium (ci) anchored in the surface between subpellicular alveoli (al). (b),(b'),(c),(c') and (d), (d') compare cross-sections at levels 1, 2 and 3, illustrated in (a), from control (b,c,d) and inactivated cells (b', c', d'). All sections are similarly oriented with respect to basal body and cell polarities and shown as seen from the cell center. Ciliary rootlets (cr) run upwards and to the left of the observer; newly formed basal bodies position anterior to the parent. (b), (b'): near the terminal plate (level 1), the C-tubules, visible in b are absent in b'. (c), (c'): level 2 section through mother and daughter basal bodies; in c', the mother organelle (posterior) shows a ring of 8 doublets and 1 triplet; the daughter basal body shows a more defective structure with one, possibly two singlets; the basis of the ciliary rootlet appears narrower and less dense in c'. (d), (d'): cross-section through level 3, showing the cartwheel; in (d'),both basal bodies show doublets and triplets (small arrows). (e): level 2 section through a field ("anarchic field" destined to generate a new oral apparatus at their next division) of basal bodies near the oral apparatus of a transformed cell showing rings of 9 doublets: note the incomplete rings (arrows), with empty triplet sites, as in (b') and (c'). Bars: 0.2 μM; same magnification in (b), (b'), (c), (c'), (d) and (d').
In some basal bodies, both triplets and doublets coexisted (Figure 3c', e'), suggesting, as demonstrated in the case of other genes [9], that inactivation of the δPT1 gene was not total. In several instances, a mother "all doublets"basal body had produced a daughter basal body with more pronounced tubule deficiencies (Figure 3c'; Figure 4). Absence of the C-tubule clearly does not prevent duplication but may lead over successive duplication cycles to more severe ultrastructural defects accounting for abortive structures. Positioning and nucleation properties are also affected: intracytoplasmic basal bodies were found (not shown), in agreement with immunocytological observations (Figure 2b,c). Another recurrent anomaly concerned the ciliary rootlet. This thick striated fiber is normally nucleated and anchored on the three anterior right triplets (Figure 3c); on basal bodies composed of doublets (Figure 3c'), the rootlet arises from a narrower region covering only two doublets and it appears fuzzier and less tapered than in control cells. The meshes of the infraciliary lattice, a contractile meshwork whose pattern depends on the basal body pattern [11] were also distorted. Cross-sections of cilia were essentially normal, in agreement with the fact that axonemal outer doublets originate from the A- and B-tubules of the basal body.
Figure 4.
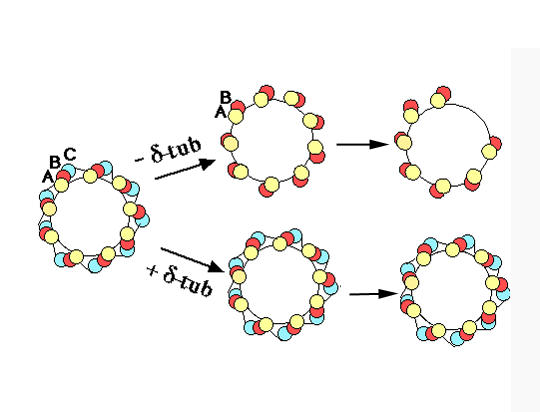
Schematic representation of the effect of δPT1 inactivation on basal body organisation. In addition to microtubules, fibrous links of unknown molecular nature are represented: linkers between C-tubule of a triplet and the A-tubule of the adjacent triplet [15] (see Figure 3, d-d'), and the dense material lining the inside of the cylinder, visible on favorable sections above the cartwheel region. Note that the loss of the C-tubule suppresses the A-C linker between adjacent triplets. Upon δ-tubulin depletion, duplication reproduces the doublet condition, but can generate further microtubule loss, as illustrated in Figure 3 (b'), (c'), (e) (arrows).
Discussion
Inactivation of the δPT1 gene results in loss of the C-tubule, specific of the centriolar structure, as previously observed in the UNI3 mutant of Chlamydomonas, carrying a total deletion of the δ-tubulin gene [2]. In Paramecium, further loss, namely of B- and even A-tubules, is also occasionally observed. The latter observation may imply either that the function of δ-tubulin is not restricted to C-tubule assembly, or that absence of the C-tubule destabilizes B- and, in turn, A-tubules. While δ-tubulin function remains to be characterized, the available observations in the two organisms at least delineate likely functions of the C-tubule. First, in both organisms, the C-tubule plays a role in anchoring, development and positioning of basal body appendages which form the cortical cytoskeleton; in Paramecium, these defects also correlate with mislocalization and internalization of basal bodies, yielding cortical disorders and reduction of basal body number and cell size. Second, the C-tubule does not play a direct role in ciliogenesis: axoneme structure and cilary activity seem normal in Paramecium ; in Chlamydomonas, doublet basal bodies can form flagella, although after some maturation delay [2]. Third, the C-tubule is not directly required for basal body duplication: doublet basal bodies duplicate normally in the algae and in the ciliate; however, in Paramecium, further tubule loss may appear (Figure 3c', Figure 4). This difference may be due to the fact that in Chlamydomonas, basal bodies duplicate once per cell cycle, while in Paramecium, more than half of the ca 3500 cortical basal bodies, and all those destined to the neo-formed oral apparatus, undergo two or three fast cycles of duplication at each division [12,13]: the C-tubule might then contribute to stability of the microtubule scaffold (see Figure 4) and in turn to the efficiency of the "duplication complex" assumed to relay the structural information from mother to daughter basal body [14,15]. Conversely, the striking fact that absence of one or more triplets does not alter the underlying ninefold symmetry of the organelle, as also observed in Chlamydomonas mutants [16], provides an experimental argument in favour of the existence of a complex providing pattern information.
Finally, considering that addition of the C-tubule (a hemi-tubule, like the B-tubule) is the last step in basal body/centriole assembly [17,18], its absence upon depletion of δ-tubulin reveals not only the existence of subtle specificities at microtubule nucleation sites, but also a role of tubulin isotype diversity in generating microtubule arrays, well documented in Drosophila [19,20].
Material and Methods
Strain and Culture conditions
The wild type strain used was stock d4-2 of Paramecium tetraurelia. Cells were grown at 27°C as previously discribed [9].
Gene inactivation
As previously demonstrated, a specific gene can be silenced by microinjecting large quantities of its coding sequence in the Paramecium macronucleus [9]. This results in a significant decrease in the amount of the corresponding mRNA or of the corresponding polypeptide measured by immunological reactivity on Western blots or in situ [9,10]. Furthermore, this inactivation is highly sequence-specific, and within multigenic families, effective only within a sub-family sharing at least 85% identity, as in the case of the two γ-tubulin genes [10]. In order to inactivate δ-tubulin gene expression, PCR amplification of macronuclear genomic DNA, as previously discribed[10] was carried out using the primers δ-ATG (5'-ATGTCTTTAGGTTTTATTTAATTAGGATAATGTGG-3') and δ-TGA (5'-TCATTAAATTAATTATTCATAATC-3'), resulting in amplification of the gene precisely limited by its initiation and stop codons. After primer removal, the DNA was concentrated and microinjected in the macronucleus. Previous experiments allowed us to verify that the efficiency of gene silencing obtained after microinjection of PCR amplified genes was equivalent to that obtained with plasmid cloned genes and that silencing efficiency was correlated with the amount of microinjected DNA, which unavoidably varies from cell to cell.
Cytological methods
Immunolabeling of whole cells was performed as previously described [6,10] using an anti-tubulin antibody ID5 [21] which allows precise observation of the pattern of basal bodies both on the cell cortex and in the oral apparatus. This antibody also decorates the post-oral fibers, a massive microtubule bundle nucleated on the right side of the oral apparatus.
For electron microscopy, pooled cells from transformed clones were fixed in 2% glutaraldehyde in 0.05 M cacodylate buffer, pH 7.2, for 90 min at 4°C. After washing in the same buffer, the samples were postfixed in 1% osmium tetroxide in 0.05 M cacodylate buffer, for 60 min at 4°C. After dehydratation, thin sections were contrasted with ethanolic uranyl acetate and lead citrate, and examined with a Philips 410 electron microscope.
Acknowledgments
Acknowledgments
We thank André Adoutte, Jean Cohen, France Koll, Linda Sperling and Michel Wright for discussions and critical reading of the manuscript and Annie Le Berre for excellent technical assistance. This work was supported by the Centre National de la Recherche Scientifique and the Association pour la Recherche sur le Cancer (Contrat #5425).
Contributor Information
Nicole Garreau de Loubresse, Email: garreau@cgm.cnrs-gif.fr.
Françoise Ruiz, Email: ruiz@cgm.cnrs-gif.fr.
Janine Beisson, Email: Beisson@cgm.cnrs-gif.fr.
Catherine Klotz, Email: klotz@cgm.cnrs-gif.fr.
References
- Dutcher SK. The tubulin fraternity: alpha to eta. Curr Opin Cell Biol. 2001;13:49–54. doi: 10.1016/S0955-0674(00)00173-3. [DOI] [PubMed] [Google Scholar]
- Dutcher SK, Trabuco EC. The UNI3 gene is required for assembly of basal bodies of Chlamydomonas and encodes δ-tubulin, a new member of the tubulin superfamily. Mol Biol Cell. 1998;9:1293–1308. doi: 10.1091/mbc.9.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrzka O, Delgehyr N, Bornens M. Tissue-specific expression and subcellular localization of mammalian δ-tubulin. Curr Biol. 2000;10:413–416. doi: 10.1016/S0960-9822(00)00418-8. [DOI] [PubMed] [Google Scholar]
- Chang P, Stearns T. δ-tubulin and ε-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nature Cell Biology. 2000;2:30–35. doi: 10.1038/71350. [DOI] [PubMed] [Google Scholar]
- Vaughan S, Attwood T, Navarro M, Scott V, McKean P, Gull K. New tubulins in protozoal parasites. Curr Biol. 2000;10:R258. doi: 10.1016/S0960-9822(00)00414-0. [DOI] [PubMed] [Google Scholar]
- Ruiz F, Krzywicka A, Klotz C, Keller AM, Cohen J, Koll F, Balavoine G, Beisson J. The SM19 gene, required for duplication of basal bodies in Paramecium, encodes "η-tubulin", a new member of the tubulin superfamily. Curr Biol. 2000;10:1451–1454. doi: 10.1016/S0960-9822(00)00804-6. [DOI] [PubMed] [Google Scholar]
- Ruiz F, Garreau de Loubresse N, Beisson J. A mutation affecting basal body duplication and cell shape in Paramecium. J Cell Biol. 1987;104:417–430. doi: 10.1083/jcb.104.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AM, Cohen J. An indexed genomic library for Paramecium complementation cloning. J Eukaryot Microbiol. 2000;47:1–6. doi: 10.1111/j.1550-7408.2000.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Ruiz F, Vayssié L, Klotz C, Sperling L, Madeddu L. Homology-dependent gene silencing in Paramecium. Mol Biol Cell. 1998;9:931–943. doi: 10.1091/mbc.9.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz F, Beisson J, Rossier J, Dupuis-Williams P. Basal body duplication in Paramecium requires γ-tubulin. Curr Biol. 1999;9:43–46. doi: 10.1016/S0960-9822(99)80045-1. [DOI] [PubMed] [Google Scholar]
- Klotz C, Garreau de Loubresse N, Ruiz F, Beisson J. Genetic evidence for a role of centrin-associated proteins in the organization and dynamics of the infraciliary lattice in Paramecium. Cell Motil Cytoskeleton. 1997;38:172–86. doi: 10.1002/(SICI)1097-0169(1997)38:2<172::AID-CM6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Iftode F, Cohen J, Ruiz F, Torrès-Rueda A, Chen-Shan L, Adoutte A, Beisson J. Development of surface pattern in Paramecium. Mapping of duplication and reorganization of cortical cytoskeletal structures in the wild type. Development. 1989;105:191–211. [Google Scholar]
- Iftode F, Fleury A, Adoutte A. Development of surface pattern during division in Paramecium III. Study of stomatogenesis in the wild type using anti-tubulin antibodies and confocal microscopy. Europ J Protozool. 1997;33:145–167. [Google Scholar]
- Mignot JP. Nouvelles hypothèses sur la duplication des centrioles et des corps basaux. C R Acad Sci III. 1996;319:1093–1099. [PubMed] [Google Scholar]
- Paoletti A, Bornens M. Organisation and functional regulation of centrosomes in animal cells. Progress in Cell Cycle Research. 1997;3:285–299. doi: 10.1007/978-1-4615-5371-7_23. [DOI] [PubMed] [Google Scholar]
- Preble AM, Giddings TH, Jr., Dutcher SK. Extragenic bypass suppressors of mutations in the essential gene BLD2 promote assembly of basal bodies with abnormal microtubules in Chlamydomonas reinhardtii. Genetics. 2001;157:163–181. doi: 10.1093/genetics/157.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippell RV. The development of basal bodies in Paramecium. Proc Natl Acad Sci. 1968;61:461–468. doi: 10.1073/pnas.61.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins VI, Porter KR. Centriole replication during ciliogenesis in the chick tracheal epithelium. Z Zellforsch Mikrosk Anat. 1969;100:1–30. doi: 10.1007/BF00343818. [DOI] [PubMed] [Google Scholar]
- Raff EC, Fackenthal JD, Hutchens JA, Hoyle HD, Turner FR. Microtubule architecture specified by a beta-tubulin isoform. Science. 1997;275:70–3. doi: 10.1126/science.275.5296.70. [DOI] [PubMed] [Google Scholar]
- Raff EC, Hutchens JA, Hoyle HD, Nielsen MG, Turner FR. Conserved axoneme symmetry altered by a component beta-tubulin. Curr Biol. 2000;101:391–4. doi: 10.1016/s0960-9822(00)00784-3. [DOI] [PubMed] [Google Scholar]
- Wehland J, Weber K. Turnover of the carboxy-terminal tyrosine of α-tubulin and means of reaching elevated levels of detyrosination in living cells. J Cell Sci. 1987;88:185–203. doi: 10.1242/jcs.88.2.185. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]