Abstract
The apparent rarity of contingent cooperation in animals has convinced many investigators that such reciprocity is unimportant, stimulating consideration of alternative explanations for cooperation, such as by-product mutualism and biological markets motivated by the likelihood of immediate reward. Nevertheless, there is also limited evidence that animals do sometimes rely on memory of recent interactions when behaving altruistically toward others. Here we describe a playback experiment conducted on wild female baboons, suggesting that contingent cooperation may occur among unrelated individuals, even when there is a temporal delay between the two cooperative acts. Hearing the recruitment call of an unrelated recent grooming partner caused subjects to move in the direction of the loudspeaker and approach their former partner, particularly when this partner had an infant. When the subject and her partner were close kin no such effect was observed. Subjects’ responses were not influenced by any type of recent interaction, because prior grooming and prior aggression influenced their behavior in qualitatively different ways. Similarly, their responses were not prompted only by the motivation to resume friendly interactions, because prior grooming alone did not elicit approach. Instead, subjects were most likely to approach their former grooming partner when they had also heard her recruitment call. Results suggest that at least some forms of cooperation in animals may be based on memory of specific recent interactions.
During the last decade there has been increasing skepticism about the relevance of contingent cooperation in the social behavior of animals. Because most cooperative behavior occurs among long-term partners (often kin) for whom any single altruistic act may be relatively insignificant, many investigators are now convinced that the sort of contingent reciprocity first proposed by Trivers (1) is both rare and fragile in nature (e.g., refs. 2–3). Although there is limited experimental (e.g., refs. 4–8) and correlational (e.g., refs. 9–11) evidence that unrelated animals may sometimes rely on memory of recent interactions when behaving altruistically toward others, interpretation has been complicated by a paucity of convincing examples, the absence of important controls in some early tests (e.g., ref. 4), and several experimental studies of captive apes, suggesting a general insensitivity to contingency in cooperative tasks (e.g., refs. 12–13). These concerns have stimulated consideration of alternative explanations for cooperation among unrelated partners, such as by-product mutualism and biological markets motivated by the likelihood of immediate reward (e.g., refs. 14–16). The change in focus has also been prompted by doubts that animals possess the requisite cognitive abilities to sustain contingent cooperation. These include the ability to delay reward, to quantify past cooperative acts, to plan and anticipate future outcomes, and to detect and punish cheaters (e.g., refs. 17–20).
Although these objections have some validity, the lack of compelling evidence for contingent cooperation can also be traced to methodological confounds. Because it is almost impossible to demonstrate under natural conditions that supportive behavior is causally dependent upon a specific prior interaction, most investigations of contingent cooperation have been conducted either in settings that lack ecological validity, are complicated by food rewards, and/or demand the capture and restraint of subjects (e.g., refs. 5–6, 8, 12–13). Playback experiments that simulate conflicts offer one, if imperfect, alternative because they provide a noninvasive means of investigating whether individuals’ responsiveness to a recent partner may be influenced by a prior interaction.
Here we describe a playback experiment designed to test whether wild female baboons’ (Papio hamadryas ursinus) willingness to attend to another individual's recruitment call is influenced by the nature of their recent interaction with that individual. The experiment extends an earlier investigation of contingent cooperation in vervet monkeys (Chlorocebus aethiops) (4). In the test condition (groom+call), a subject was played the threat grunts of a lower-ranking female at least 10 min after she and that female had groomed and then separated without any further interactions (see Materials and Methods for additional information). This playback was designed to mimic a context in which the former grooming partner was threatening another individual and soliciting aid. Female baboons give threat grunts primarily when threatening lower-ranking individuals, and the calls function at least in part to recruit allies (21). We used two dependent measures when scoring responses: whether the subject's first move following playback was toward the loudspeaker; and whether, in the next half hour, the subject approached to within 2 m of her former partner without threatening her (a “friendly approach”).
We also introduced two control conditions. The first control (groom only) was similarly conducted after the subject and the same female had groomed and then separated for at least 10 min. In this case, however, we did not conduct a playback experiment but only observed the subject's behavior. This control was designed to test whether a recent grooming interaction might simply motivate females to approach their partner again, even in the absence of any solicitation for support. In the second control (threat+call), we played the same female's threat grunts to the same subject at least 10 min after the subject had threatened that female. This control was designed to test whether subjects’ responses to a recruitment call were primed by any prior interaction, not just a friendly one.
We predicted that, if recent friendly behavior increases an individual's willingness to support another, females should respond more strongly in the groom+call condition than in either of the two control conditions. Importantly, such stronger responses should occur even when there had been a time delay between the grooming interaction and the subsequent solicitation for support. On the assumption that a recent cooperative interaction would exert a stronger influence on females with weaker social bonds than those with stronger social bonds (7, 11), we also predicted that nonkin would be more likely than kin to show different responses across conditions. In this baboon population, as in others, females form the strongest and most enduring bonds with kin (22–24). Kin selection theory also predicts that contingency-based altruism should be less common among kin than among nonkin. Indeed, in one study of captive Japanese macaques (Macaca fuscata), kin were never observed to support each other in the half hour after grooming, even when they had the opportunity to do so (25).
Results
Because the data set contained incomplete values (not all dyads could be tested in all conditions) and because different individuals appeared a different number of times as subjects or partners, we analyzed our results using generalized linear mixed models (GLMM; ref. 26) in which subject and partner identity, as well as the identity of each unique dyad, were entered as random factors (e.g., ref. 27).
First Move.
We examined subjects’ behavior immediately following playback using as the response measure whether or not subjects’ first move was toward the speaker (a binary variable). We compared responses under two conditions: groom+call and threat+call. We used a GLMM with experimental condition, dyad relatedness (kin vs. nonkin), their interaction, and caller's reproductive state (lactating or not) as predictor variables. Dyad relatedness and the interaction between experimental condition and dyad relatedness were entered as predictors because we expected that the responses of kin would not be contingent upon recent events. Caller's reproductive state was entered because several studies have shown that female baboons are strongly attracted to infants (e.g., ref. 28), and some investigators have argued that grooming is motivated primarily by short-term benefits, such as the opportunity to handle another's infant (20, 29).
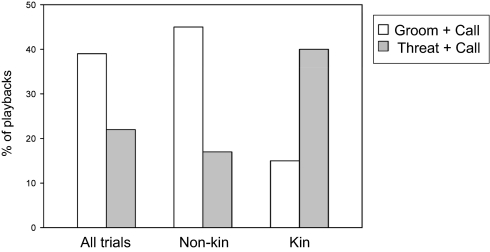
Experimental condition (P = 0.008) and dyad relatedness (P = 0.022) were significant predictors of subjects’ first move, but caller's reproductive state was not (Table 1). There was also a significant interaction between these two variables (P = 0.014), indicating that the manner in which each main effect acted on behavior depended on the value of the other main effect (Fig. 1). Specifically, subjects in nonkin dyads were more likely to move toward the speaker after grooming than after aggression, whereas subjects in kin dyads were not. Separate post hoc tests on kin and nonkin dyads revealed that experimental condition was a highly significant predictor of responses in nonkin dyads (n = 92, z = −2.90, P = 0.004), but not in kin dyads (n = 23, z = 0.34, P = 0.733). The absence of a statistically significant difference between conditions among kin dyads may have resulted at least in part from small sample size.
Table 1.
Results of a GLMM analysis in which the dependent variable was whether the subject's first move was toward the speaker
| Predictor variables | Estimate | SE | z value | Pr(>|z|) |
| Experimental condition | −0.754 | 0.283 | −2.666 | 0.008** |
| Kin vs. nonkin | −3.134 | 1.372 | −2.284 | 0.022* |
| Caller reproductive state | 0.038 | 0.459 | 0.083 | 0.934 |
| Experimental condition × kin vs. nonkin | 1.453 | 0.593 | 2.449 | 0.014* |
Predictor variables were experimental condition (groom+call vs. threat+call), dyad relatedness (kin vs. nonkin), and caller's reproductive state (lactating or not). N = 92 nonkin dyads and 23 kin dyads.
*P < 0.05; **P < 0.001
Fig. 1.
The frequency that subjects’ first move was toward the speaker in the groom+call and threat+call conditions. In groom+call, all dyads = 57 (44 nonkin dyads, 13 kin dyads); in threat+call, all dyads = 58 (48 nonkin dyads, 10 kin dyads). Frequencies were similar for the 31 dyads that appeared in both conditions (groom+call: nonkin dyads = 0.45, kin dyads = 0.20; threat+call: nonkin dyads = 0.19; kin dyads = 0.40).
Friendly Approach.
We examined longer-term changes in subjects’ behavior using as a response measure the occurrence (or lack) of a friendly approach by the subject to within 2 m of her former partner (a binary variable) in the half hour following playback, or, in the groom only condition, in the 10–40 min after grooming had ended. Because these experiments were conducted on wild animals that had often become widely separated by the time that trials were conducted, most subjects did not come into proximity of their former partners again after their initial interaction. Nonetheless, those subjects that did approach their former partners were significantly more likely to do so in the groom+call condition than in either the groom only or threat+call conditions.
We first used a likelihood ratio test to compare a full model that included all predictors against a null model that included only the random effects (30:109). The GLMM in which partner's reproductive state, dyad relatedness, and experimental condition were entered as predictors of a friendly approach fit the data better than the null model that included only random effects (χ2 = 9.182, df = 3, P = 0.027).
Given this result, we ran a single model with experimental condition, dyad relatedness, and caller's reproductive state as predictors, including all possible interactions among predictors. The only significant or near-significant predictors were the partner's reproductive state (z = 2.102, P = 0.035) and the interaction between partner's reproductive state and experimental condition (z = −1.833, P = 0.066). A significantly larger number of subjects made a friendly approach toward their former partner when she was lactating than when she was not; however, experimental condition also affected their behavior.
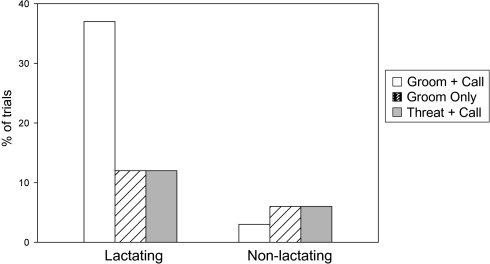
Because the effect of experimental condition was apparent only in tests involving lactating female partners, we conducted post hoc tests on these dyads only (n = 93). Experimental condition and dyad relatedness were predictors. Overall, experimental condition was a significant predictor of subjects’ response (P = 0.015; Table 2). Pairwise comparisons among the three conditions (Table 2 and Fig. 2) showed that subjects were significantly more likely to approach their former partner in the groom+call condition than in the threat+call condition (Table 2 and Fig. 2), indicating that the quality of the prior interaction affected their response to the recruitment call. Importantly, however, females’ propensity to approach their former partner did not depend only on prior grooming, because subjects were also significantly more likely to approach their partner in the groom+call condition than in the groom only condition (Table 2 and Fig. 2). By contrast, in the comparison between the groom only and threat+call conditions, where we had no a priori prediction, we found no significant effect of experimental condition (Table 2 and Fig. 2). The small number of kin dyads in the lactating category precluded a definitive comparison of kin and nonkin dyads.
Table 2.
Results of post hoc GLMM analyses involving only lactating females as partners, in which the dependent variable was whether the subject made a friendly approach toward her partner
| Estimate | SE | z | Prob(>|z|) | |
| Predictor variables: experimental condition, kin vs. nonkin | ||||
| Experimental condition | −1.029 | 0.452 | −2.277 | 0.023* |
| Kin vs. nonkin | 0.625 | 2.024 | 0.309 | 0.816 |
| Predictor variables: experimental condition 1 (groom+call) vs. experimental condition 2 (groom only) | ||||
| Condition | −1.609 | 0.662 | −2.431 | 0.015* |
| Experimental condition 1 (groom+call) vs. experimental condition 3 (threat+call) | ||||
| Condition | −0.795 | 0.365 | −2.179 | 0.029* |
| Experimental condition 2 (groom only) vs. experimental condition 3 (threat+call) | ||||
| Condition | 0.021 | 0.808 | 0.025 | 0.980 |
Definition of predictor variables as in Table 1, with the inclusion of groom only as a third experimental condition. N = 144 nonkin dyads and 39 kin dyads.
Fig. 2.
The frequency that subjects approached their former partner in the groom+call, groom only, and threat+call conditions. In groom+call, lactating dyads = 27, nonlactating dyads = 30; in groom only, lactating dyads = 38, nonlactating dyads = 30; in threat+call, lactating dyads = 28, nonlactating dyads = 30. Frequencies were similar for the 23 dyads that appeared in all three conditions (lactating: groom+call = 0.38; call only = 0.05; threat+call = 0.07. Nonlactating: groom+call = 0; groom only = 0.11; threat+call = 0.07).
In sum, whereas subjects appeared to be more motivated to approach a recent partner when she had an infant than when she did not, both the quality of recent interactions (grooming or aggression) and the presence or absence of a recruitment call also significantly affected their behavior. Subjects were most likely to approach lactating females after they had both groomed with them and heard their recruitment call.
Effect of Grooming Direction on Responses.
Although most of the grooming bouts (93%) preceding experimental trials were bidirectional, they were not usually evenly balanced. However, the strength of subjects’ responses was not influenced by the relative amounts of grooming given and received during their previous interaction (cf. ref. 6). There were nine females who appeared as subjects at least twice in the groom+call condition. Three females were more likely to approach the speaker after hearing the caller from whom they had received the higher proportion of grooming, three were less likely to do so, and three were equally likely.
Discussion
These results support earlier experiments (4, 6, 7) and observations (9–11) in suggesting that contingent cooperation may occur in nonhuman primates, even when there is a temporal delay between the first cooperative act and subsequent solicitation for support. Hearing an unrelated grooming partner's recruitment call caused subjects to move toward the speaker and to approach their former partner. Because these playback experiments only simulated an aggressive interaction, it was by definition impossible to determine whether subjects’ approaches were motivated specifically by the willingness to support their former partners. A number of alternative explanations, however, can be ruled out. First, subjects’ responses were not influenced by any type of recent interaction, because prior aggression and prior grooming affected their behavior in qualitatively different ways. Second, their responses were not prompted only by the motivation to resume a friendly interaction, because grooming alone did not cause females to approach their former partner. Females were most likely to approach their former grooming partner when they had also heard her recruitment call. Finally, females’ tendency to approach others was not motivated only by the prospect of immediate reward, such as the opportunity to handle an infant (20, 29). Although subjects were more likely to approach a former partner if she had an infant, they were significantly more likely to do so after they had heard her recruitment call in combination with a recent grooming interaction than after grooming only or after aggression. Taken together, results suggest that females’ propensity to approach unrelated recent grooming partners was at least partly motivated by the opportunity to provide support.
In recent years, there has been considerable debate about whether grooming is best regarded as a mutualistic behavior that is traded as a commodity for immediate benefit (14–16), or as a form of reciprocal altruism in which individuals derive future benefits despite incurring short-term costs. These hypotheses are not mutually exclusive. Although nonhuman primates do appear to derive some immediate benefits from grooming (e.g., refs. 29, 31–32), grooming often occurs in the absence of an immediate reward, such as access to an infant, and it is seldom evenly balanced between partners within single bouts. Supporting the view that grooming also has long-term adaptive consequences, baboons, chimpanzees, and other nonhuman primates form the strongest bonds with those individuals with whom they have the most balanced and reciprocal grooming interactions over extended periods of time (22–25, 27, 33–35). Furthermore, female baboons with the strongest social bonds enjoy higher offspring survival (36, 37). Thus, grooming appears to be a low-cost altruistic activity in which partners are willing to forego short-term inequities for future benefits, including those in different currencies, such as alliance support (10, 11). Such long-term reciprocal exchanges may be particularly important when animals live in large social groups composed of individuals of varying degrees of genetic relatedness, and in which cooperation with both kin and nonkin is essential.
It has been argued that the cognitive abilities demanded by contingent cooperation limit its occurrence in animals (see Introduction). Although animals may be less adept than humans at keeping track of favors given and returned, they nonetheless have the capacity to recognize noncooperators (38, 39) and to remember specific events. Several experiments have now demonstrated, for example, that baboons’ behavior is strongly influenced by the memory of specific interactions involving particular individuals or their relatives (21, 40–42). The paucity of convincing evidence for contingent cooperation in tests with captive animals may be due in part to the stringent standards set by these experiments, which typically require proof of equal back-and-forth exchanges of single currency food rewards (e.g., refs. 12, 13). These requirements may have set the bar unrealistically high, leading investigators to underestimate the extent to which a recent cooperative interaction may motivate animals to cooperate again. Indeed, many forms of contingent cooperation may be mediated by relatively simple proximate mechanisms based on the memory of previous interactions rather than the expectation of future reward (11). Thus, reciprocity may be maintained by a kind of partner-specific “emotional bookkeeping” (11, 43) that permits long-term tracking of multiple partners and facilitates cooperation in different behavioral currencies. The resulting bonds that develop between preferred partners may motivate future positive interactions, without the need for explicit tabulation of favors given and returned, or calculations of anticipated benefits (11, 43–45). For unrelated females who interact at low rates, a single grooming bout may temporarily elevate a female's positive emotions toward her partner sufficiently above baseline to influence her immediate interactions with her. In contrast, grooming and support among females with close bonds (who are also usually kin) should be less subject to immediate contingencies and less influenced by single interactions. Indeed, previous research has suggested that the support of kin is less contingency based than that of nonkin (4, 7, 25). In these experiments, too, we found no significant differences across conditions in the likelihood that kin would move toward or away from the speaker upon hearing a relative's recruitment call.
In sum, several factors may interact to motivate contingent cooperation in nonhuman primates under natural conditions: the strength of the partners’ social relationship (or kinship), the nature of their recent interactions, and the opportunity to reengage in some form of cooperative behavior. Contingent cooperation is doubtless relatively rare in animals compared with the high rates of cooperation that occur among long-term partners and kin. Moreover, given the methodological difficulties inherent in demonstrating that a potentially altruistic act has been elicited by a specific recent interaction, it is unlikely that any single test can ever conclusively demonstrate its occurrence under natural conditions. Nonetheless, the results presented here suggest that under certain conditions animals’ motivation to support a recent partner is mediated, at least in part, by the nature of a specific recent interaction.
Materials and Methods
Study Site and Behavioral Observations.
The study was conducted on a group of wild baboons living in the Moremi Game Reserve, in the Okavango Delta of Botswana (46, 47). The group had been observed since 1978, and all animals were fully habituated to human observers on foot. Maternal relatedness for all natal animals was known. In these experiments, kin were defined as mothers, daughters, maternal sisters, and maternal aunts, and nonkin as animals less closely related than maternal first cousins. During the period of this study (June 2006–July 2007), group size ranged from 62 to 73 individuals. Subjects included 28 females aged at least 5 years. Females were defined as lactating if they had not yet resumed cycling following the birth of an infant that was still alive at the time a given trial was conducted. Almost all lactating female partners (13/15) were mothers of older infants (>3 months). Although infant attractiveness wanes with age (28), the mothers of older infants continue to receive attention from other females. During this study, lactating females with older infants received friendly grunts from other females at 5.5 times the rate of cycling and pregnant females.
Observational data, including grooming interactions and alliance support, were collected almost daily, using focal animal sampling (48). Focal animal observations lasted 10 min, and each female was sampled three to four times a week. We defined a female as supporting another in an alliance when she joined that female in a dispute against a third individual by head-bobbing, lunging at, chasing, or biting the target of that female's aggression.
During the period when experiments were conducted, females formed alliances in 6.6% of aggressive interactions that targeted other females, not including vocal alliances. This frequency is comparable to that previously reported for this study site (21) and for other species of Old World monkeys (e.g., refs. 25, 49–52). As in other studies, all alliances targeted lower-ranking individuals. Females formed the majority of alliances (69%) with nonkin (see also ref. 21). When corrected for the number of kin and nonkin, females formed more alliances than expected with kin.
Playback Experiments.
Trials were conducted opportunistically over an 11-month period. We tested as many dyads as possible in each condition (groom+call, groom only, and threat+call). We were able to test 23 nonkin and 5 kin dyads in all three conditions. An additional 23 nonkin and 8 kin dyads appeared in two conditions, whereas 29 nonkin and 8 kin dyads appeared in only one condition. Trials included 23 unique subjects and 21 unique partners.
All trials were initiated at least 10 min after the subject and her partner had ceased interacting and had separated. We chose 10 min as the minimum time delay (rather than 30 min, as in ref. 4), because we were concerned that a longer delay would increase the likelihood that subject and partner would come into proximity of one another, thus limiting our opportunities to conduct a trial. The mean time delay between cessation of the grooming bout or threat and the playback experiment or control follow was 22 min (range: 10–55). There was no evidence that results were affected by the time delay between cessation of a grooming bout or threat and the playback experiment.
In trials involving playbacks, we used as stimuli threat grunts that had been recorded within the previous year using Sennheiser ME88 microphones and Nomad and Marantz digital recorders. Calls were analyzed using Cool Edit software (Syntrillium) to ensure that they were of high quality, without vocalizations from other baboons or background noise. All sequences were selected to match the amplitude and bout length (2–3 s) of naturally occurring calls. Calls were broadcast from a Bose Roommate II speaker concealed in vegetation at least 5 m from the subject and in the same direction as the caller's actual location.
Trials were initiated when the subject was out of sight and earshot of the individual whose call was being played and either sitting or feeding with her body oriented at a 90°–135° angle from the speaker. No individual was the subject of more than one playback per week, and a maximum of one playback occurred each day.
We used two dependent measures when scoring trials (i): whether the subject's first move after playback was toward the speaker; and (ii) whether, in the half hour after playback, the subject approached to within 2 m of her former grooming partner without threatening or supplanting her (a “friendly approach”). Given that the playback only mimicked an aggressive interaction, a dependent measure based on actual alliance support was by definition impossible. Numerous playback experiments on birds and primates have used movement toward or away from the source of a vocalization as a proxy measure of an individual's willingness to engage in an interaction or avoid one (e.g., refs. 21, 40–42, 53–55).
Statistical Analysis.
All statistical analyses were conducted with R statistical software (version 2.9.0, R Foundation for Statistical Computing, R Development Core Team, 2009). In analyses where we found a significant interaction between two effects, we split the data set and carried out post hoc comparisons to explore the nature of the interaction in more detail. GLMMs were calculated using the function ‘lmer’ of the R package ‘lme4’ (56; R package version 0.999375–31). Models were fitted using binomial error structure and logit link.
Acknowledgments
We thank the Office of the President of the Republic of Botswana and the Botswana Department of Wildlife and National Parks for permission to conduct research in the Moremi Reserve. We also thank Alec Mokopi, Chantelle Shaw, and Werner Smith for assistance with data collection and logistical support in the field. We are grateful to Joan Silk and two anonymous reviewers for their comments and to Art Dunham and Peter Petraitis for their statistical advice. Research was supported by National Institutes of Health Grant MH62249 and approved by the Animal Care and Use Committee of the University of Pennsylvania (Protocol 19001).
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
References
- 1.Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- 2.Hammerstein P. In: Genetic and Cultural Evolution of Cooperation. Hammerstein P, editor. Cambridge: MIT Press; 2003. pp. 83–94. [Google Scholar]
- 3.Clutton-Brock TH. Cooperation between non-kin in animal societies. Nature. 2009;462:51–57. doi: 10.1038/nature08366. [DOI] [PubMed] [Google Scholar]
- 4.Seyfarth RM, Cheney DL. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature. 1984;308:541–543. doi: 10.1038/308541a0. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson GS. Reciprocal food sharing in vampire bats. Nature. 1984;308:181–184. [Google Scholar]
- 6.Hemelrijk CK. Support for being groomed in long-tailed macaques, Macaca fascicularis. Anim Behav. 1994;48:479–481. [Google Scholar]
- 7.de Waal FBM. The chimpanzee's service economy: Food for grooming. Evol Hum Behav. 1997;18:375–386. [Google Scholar]
- 8.Krams I, Krama T, Igaune K, Mand R. Experimental evidence of reciprocal altruism in the pied flycatcher. Behav Ecol Sociobiol. 2008;62:599–605. [Google Scholar]
- 9.Mitani J. In: Cooperation in Primates and Humans: Mechanisms and Evolution. Kappeler PM, van Schaik CP, editors. Berlin: Springer Verlag; 2006. pp. 101–113. [Google Scholar]
- 10.Schino G. Grooming and agonistic support: A meta-analysis of primate reciprocal altruism. Behav Ecol. 2007;18:115–120. [Google Scholar]
- 11.Schino G, Aureli F. Reciprocal altruism in primates: Partner choice, cognition, and emotions. Adv Study Behav. 2009;39:45–69. [Google Scholar]
- 12.Melis AP, Hare B, Tomasello M. Do chimpanzees reciprocate favours? Anim Behav. 2008;76:951–962. [Google Scholar]
- 13.Brosnan SF, et al. Chimpanzees (Pan troglodytes) do not develop contingent reciprocity in an experimental task. Anim Cogn. 2009;12:587–597. doi: 10.1007/s10071-009-0218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noe R, Hammerstein P. Biological markets: Supply and demand determine the effect of partner choice in cooperation, mutualism, and mating. Behav Ecol Sociobiol. 1994;35:1–11. [Google Scholar]
- 15.Noë R. In: Economics in Nature. Noë R, van Hooff JARAM, Hammerstein P, editors. Cambridge: Cambridge Univ Press; 2001. pp. 93–118. [Google Scholar]
- 16.Barrett L, Henzi P. In: Cooperation in Primates and Humans: Mechanisms and Evolution. Kappeler PM, van Schaik CP, editors. Berlin: Springer; 2006. pp. 209–232. [Google Scholar]
- 17.Stevens JR, Hauser MD. Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn Sci. 2004;8:60–65. doi: 10.1016/j.tics.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Stevens JR, Cushman FA, Hauser MD. Evolving the psychological mechanisms for cooperation. Annu Rev Ecol Evol Syst. 2005;36:499–518. [Google Scholar]
- 19.Barrett L, Henzi P, Rendall D. Social brains, simple minds: Does social complexity really require cognitive complexity? Philos Trans R Soc Lond B Biol Sci. 2007;362:561–575. doi: 10.1098/rstb.2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henzi P, Barrett L. Coexistence in female-bonded primate groups. Adv Study Behav. 2007;37:107–132. [Google Scholar]
- 21.Wittig RM, Crockford C, Seyfarth RM, Cheney DL. Vocal alliances in chacma baboons Papio hamadryas ursinus. Behav Ecol Sociobiol. 2007;61:899–909. [Google Scholar]
- 22.Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behav Ecol Sociobiol. 2006;61:183–195. [Google Scholar]
- 23.Silk JB, Alberts SC, Altmann J. Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behav Ecol Sociobiol. 2006;61:197–204. [Google Scholar]
- 24.Silk JB, et al. Female chacma baboons form strong, equitable, and enduring bonds. Behav Ecol Sociobiol. 2010 doi: 10.1007/s00265-010-0986-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schino G, di Sorrentino EP, Tiddi B. Grooming and coalitions in Japanese macaques (Macaca fuscata): Partner choice and the time frame reciprocation. J Comp Psychol. 2007;121:181–188. doi: 10.1037/0735-7036.121.2.181. [DOI] [PubMed] [Google Scholar]
- 26.Baayen RH. Analyzing Linguistic Data: A Practical Introduction to Statistics Using R. New York: Cambridge Univ Press; 2008. [Google Scholar]
- 27.Gomes CM, Mundry R, Boesch C. Long-term reciprocation of grooming in wild West African chimpanzees. Proc Biol Sci. 2009;276:699–706. doi: 10.1098/rspb.2008.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silk JB, Rendall D, Cheney DL, Seyfarth RM. Natal attraction in adult female baboons (Papio cynocephalus ursinus) in the Moremi Reserve Botswana. Ethology. 2003;109:627–644. [Google Scholar]
- 29.Henzi SP, Barrett L. Infants as a commodity in a baboon market. Anim Behav. 2002;63:915–921. [Google Scholar]
- 30.Crawley MJ. Statistics: An Introduction Using R. New York: John Wiley; 2008. [Google Scholar]
- 31.Barrett L, Henzi SP, Weingrill A, Lycett JE, Hill R. Market forces predict grooming reciprocity in female baboons. Proc Biol Sci. 1999;266:665–670. [Google Scholar]
- 32.Fruteau C, Voelkl B, van Damme E, Noe R. Supply and demand determine the market value of food providers in wild vervet monkeys. Proc Natl Acad Sci USA. 2009;106:12007–12012. doi: 10.1073/pnas.0812280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitani JC. Male chimpanzees form enduring and equitable social bonds. Anim Behav. 2009;77:633–640. [Google Scholar]
- 34.Langergraber K, Mitani J, Vigilant L. Kinship and social bonds in female chimpanzees (Pan troglodytes) Am J Primatol. 2009;71:840–851. doi: 10.1002/ajp.20711. [DOI] [PubMed] [Google Scholar]
- 35.Schino G, Di Giuseppe F, Visalberghi E. Grooming, rank, and agonistic support in tufted capuchin monkeys. Am J Primatol. 2009;71:101–105. doi: 10.1002/ajp.20627. [DOI] [PubMed] [Google Scholar]
- 36.Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1331–1334. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- 37.Silk JB, et al. The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proc R Soc Lond Ser B. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melis AP, Hare B, Tomasello M. Chimpanzees recruit the best collaborators. Science. 2006;311:1297–1300. doi: 10.1126/science.1123007. [DOI] [PubMed] [Google Scholar]
- 39.Melis AP, Hare B, Tomasello M. Engineering cooperation in chimpanzees: Tolerance constraints on cooperation. Anim Behav. 2006;72:275–286. [Google Scholar]
- 40.Cheney DL, Seyfarth RM. Reconciliatory grunts by dominant female baboons influence victims’ behaviour. Anim Behav. 1997;54:409–418. doi: 10.1006/anbe.1996.0438. [DOI] [PubMed] [Google Scholar]
- 41.Engh AE, Hoffmeier RR, Cheney DL, Seyfarth RM. Who me? Can baboons infer the target of vocalisations? Anim Behav. 2006;71:381–387. [Google Scholar]
- 42.Wittig RM, Crockford C, Wikberg E, Seyfarth RM, Cheney DL. Kin-mediated reconciliation substitutes for direct reconciliation in female baboons. Proc Biol Sci. 2007;274:1109–1115. doi: 10.1098/rspb.2006.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Waal FBM de Waal FB. Attitudinal reciprocity in food sharing among brown capuchin monkeys. Anim Behav. 2000;60:253–261. doi: 10.1006/anbe.2000.1471. [DOI] [PubMed] [Google Scholar]
- 44.Aureli F, Schaffner CM. Relationship assessment through emotional mediation. Behaviour. 2002;139:393–420. [Google Scholar]
- 45.Aureli F, Whiten A. In: Primate Psychology. Maestripieri D, editor. Cambridge: Cambridge Univ Press; 2003. pp. 289–323. [Google Scholar]
- 46.Cheney DL, et al. Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. Int J Primatol. 2004;25:401–428. [Google Scholar]
- 47.Cheney DL, Seyfarth RM. Baboon Metaphysics: The Evolution of a Social Mind. Chicago: University of Chicago Press; 2007. [Google Scholar]
- 48.Altmann J. Observational study of behavior: Sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein IS, Ehardt CL. Agonistic aiding: Kinship, rank, age, and sex influences. Am J Primatol. 1985;8:37–52. doi: 10.1002/ajp.1350080105. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan JR, Chikazawa DK, Manuck SB. Aspects of fight interference in free-ranging and compound-dwelling rhesus macaques (Macaca mulatta) Am J Primatol. 1987;12:287–298. doi: 10.1002/ajp.1350120306. [DOI] [PubMed] [Google Scholar]
- 51.Cheney DL, Seyfarth RM. How Monkeys See the World: Inside the Mind of Another Species. Chicago: University of Chicago Press; 1990. [Google Scholar]
- 52.Silk JB, Alberts SC, Altmann J. Patterns of coalition formation by adult female baboons in Amboseli, Kenya. Anim Behav. 2004;67:573–582. [Google Scholar]
- 53.Burt JM, Campbell E, Beecher MD. Song type matching as threat: A test using interactive playbacks. Anim Behav. 2001;62:1163–1170. [Google Scholar]
- 54.Peake TM, Terry AMR, McGregor PK, Dabelsteen T. Do great tits assess rivals by combining direct experience with information gathered by eavesdropping? Proc Biol Sci. 2002;269:1925–1929. doi: 10.1098/rspb.2002.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Kort SR, Bohman ER, Cramer ERA, Vehrencamp SL. The deterrent effect of bird song in territory defense. Behav Ecol. 2009;20:200–206. doi: 10.1093/beheco/arn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bates D, Maechler M lme4: Linear mixed-effects models using S4 classes R package version 0999375-31. 2009. Available at http://CRANR-projectorg/package=lme4. Accessed on April 17, 2009.