Abstract
The identification of biodiversity hotspots and their management for conservation have been hypothesized as effective ways to protect many species. There has been a significant effort to identify and map these areas at a global scale, but the coarse resolution of most datasets masks the small-scale patterns associated with coastal habitats or seamounts. Here we used tuna longline observer data to investigate the role of seamounts in aggregating large pelagic biodiversity and to identify which pelagic species are associated with seamounts. Our analysis indicates that seamounts are hotspots of pelagic biodiversity. Higher species richness was detected in association with seamounts than with coastal or oceanic areas. Seamounts were found to have higher species diversity within 30–40 km of the summit, whereas for sets close to coastal habitat the diversity was lower and fairly constant with distance. Higher probability of capture and higher number of fish caught were detected for some shark, billfish, tuna, and other by-catch species. The study supports hypotheses that seamounts may be areas of special interest for management for marine pelagic predators.
Keywords: by-catch, fisheries, longline, pelagic predators, seamount conservation
For the last decade there has been considerable debate about the status and sustainability of pelagic fisheries around the world (1–6) and their effects on the ecosystems that support them (7–10). Many species may be protected by identifying biodiversity hotspots and managing them for conservation (11). This approach is well established for terrestrial systems and marine tropical reefs (12, 13), but less so for the pelagic ecosystems of the open ocean (11). Simulation modeling has indicated that management techniques such as area closure are likely to help conserve many pelagic species (11). Accordingly, there has been a significant effort to identify and map pelagic biodiversity hotspots at a global scale, but progress has been limited. The coarse resolution of most datasets masks the small-scale patterns associated with coastal habitats or seamounts (14). Hotspots that have been identified in open ocean areas have been typically associated with particular environmental factors and mesoscale oceanographic features such as latitude, fronts, or eddies (14, 15). The dynamism of pelagic environments can significantly reduce the efficacy of conservation measures (16). To address this issue, dynamic marine reserves that move with the wildlife have been suggested (17), but such approaches may not be workable (18).
Many seamounts are important aggregating locations for highly migratory pelagic species (19–23), but their role in aggregating pelagic biodiversity is largely unknown. If seamounts are hotspots for pelagic biodiversity then they may prove to be suitable areas for conservation measures in open ocean environments. Morato et al. (23) demonstrated that seamounts aggregate some visitor species but did not demonstrate that this behavior can be generalized. Building upon this previous work, we examine the role of seamounts in aggregating pelagic biodiversity by applying ocean basin scale generalized linear models (GLMs) to location-specific fisheries catch data. In addition, we analyzed catch per unit of effort (CPUE) in relation to distance to seamounts to identify those pelagic species that are significantly associated with seamounts. The dataset comprised a time series from 1980 to 2007 of species catch data collected on tuna longline vessels by independent observers over large areas of the western and central Pacific Ocean, coupled with comprehensive data on the location of seamounts (24).
Results
Seamounts as Hotspots of Biodiversity?
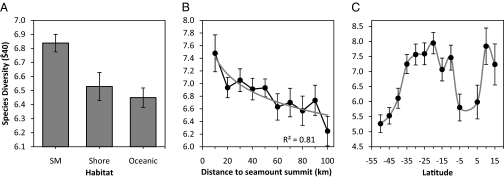
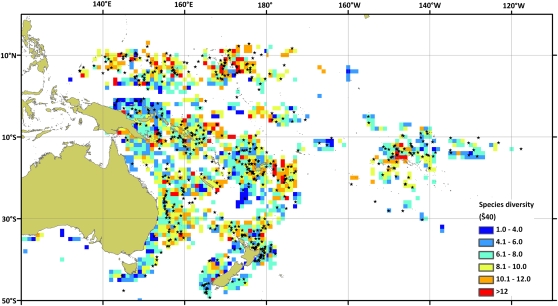
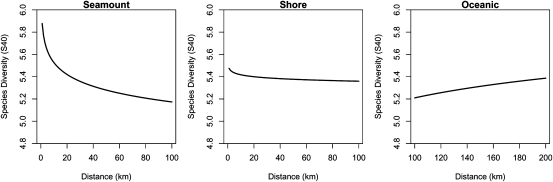
Rarefied pelagic diversity was significantly higher in seamount habitats than in coastal or oceanic waters (Fig. 1A) and was found to be nonlinearly related to the distance to seamount, with diversity higher close to the summits (Fig. 1B). Rarefied diversity was higher at intermediate latitudes (10–35 °S and 10–15 °N; Fig. 1C). Regions with higher pelagic diversity included Indonesia, Palau, Federated States of Micronesia, and Marshall Islands in the Northern Hemisphere and Tonga, New Caledonia, and Norfolk Island in the Southern Hemisphere (Fig. 2). The relationship for describing species diversity was complex with distance to features, number of hooks, and latitude the strongest predictors of species diversity (Table 1). When all variables except distance to feature were kept constant, seamounts were found to have higher rarefied diversity within 30–40 km of the summit (Fig. 3). For coastal and oceanic habitats the rarefied diversity was lower and not affected by distance to the feature (Fig. 3). A statistically significant effect of moon was not detected for rarefied diversity. The detailed GLM results are presented in Table S1.
Fig. 1.
Mean expected species diversity (±95% confidence limits) rarefied from 40 individuals (Ŝ40) as a function of (A) the main habitat [seamount (SM), shore and oceanic] where all means are significantly different at α = 0.01 (ANOVA and Tukey's honestly significant difference test), (B) distance to seamount summit where the fitted logarithmic regression is also shown (shaded line), and (C) 5 ° latitude.
Fig. 2.
Expected species diversity rarefied from 40 (Ŝ40) individuals as a function of 1 × 1 degree cells. Stars denote locations of seamounts with longline sets close to their summits.
Table 1.
Summary statistics for the GLM single-variable elimination analyses relating species diversity with habitat and other variables
| df | Deviance | AIC | P | |
| 49,748 | 54,383 | |||
| Year | 22 | 50,977 | 54,654 | <0.001 |
| Moon | 7 | 49,792 | 54,380 | 0.133 |
| Month | 11 | 49,939 | 54,410 | <0.001 |
| Lat5 | 12 | 52,747 | 55,115 | <0.001 |
| Long5 | 26 | 50,793 | 54,599 | <0.001 |
| Flag_Fleet | 29 | 51,295 | 54,720 | <0.001 |
| EEZ | 21 | 50,848 | 54,623 | <0.001 |
| Hooks (ns, df = 10) | 10 | 56,736 | 56,061 | <0.001 |
| log(dist.) × feature | 2 | 49,807 | 54,394 | <0.001 |
AIC, Akaike's information criterion; df, degrees of freedom; EEZ, Exclusive Economic Zone; ns, natural cubic splines.
Fig. 3.
The effect of the variables distance × feature on species diversity rarefied from 40 individuals (Ŝ40). One variable was predicted at a time from the results of the GLM by fixing the other variables.
Highly Migratory Pelagic Species Aggregating Around Seamounts.
To identify which species aggregate around seamounts, the relationship between CPUE and distance to seamounts was examined for individual species. There were sufficient data to analyze 37 taxa. Of these, seamount aggregation effects were detected for 41% of the taxa (15 taxa of shark, billfish, and pelagic teleost fish), although the opposite effects were detected for only 3 taxa (Table 2). For the shark taxa the probability of catching the species increased closer to seamounts for porbeagle shark (Lamna nasus), short-finned mako shark (Isurus oxyrinchus), and silky shark (Carcharhinus falciformis) and decreased for pelagic stingray (Pteroplatytrygon violacea). The average number caught per set was also higher closer to seamounts for silky sharks. We did not detect an effect of seamount on the probability of being caught for blue shark (Prionace glauca), but observed that in sets that caught the taxa the average number caught was higher closer to seamounts. For the billfishes and tunas the probability of catching the species increased closer to seamounts for yellowfin tuna (Thunnus albacares), blue marlin (Makaira nigricans), and swordfish (Xiphias gladius) and decreased for albacore (Thunnus alalunga) and shortbill spearfish (Tetrapturus angustirostris). For the other pelagic teleost fish the probability of catching the species increased closer to seamounts for ribbon fish (Trachipterus trachypterus), butterfly kingfish (Gasterochisma melampus), big-scaled pomfret (Taractichthys longipinnis), Atlantic pomfret (Brama brama), and long-snouted lancetfish (Alepisaurus ferox). The average number caught per successful set was also higher closer to seamounts for butterfly kingfish but lower for ribbon fish and big-scaled pomfret. We did not detect an effect of seamount on the probability of being caught for short-snouted lancetfish (Alepisaurus brevirostris) and moonfish (Lampris guttatus), but observed that the average numbers caught per successful set were higher closer to seamounts for both species. For the other 19 species, statistically significant trends were not detected (Table S2). Seamount aggregation effects on both probability of capture and number caught were also observed for the unidentified species category.
Table 2.
Statistics for all by-catch species that were observed in >500 of 10,602 sets (n > 500), for both the binary and the lognormal components of the GLM
| Binary |
Lognormal |
||||||
| Species | N | ΔAIC | LogdistSM | SM effect | ΔAIC | LogdistSM | SM effect |
| Sharks and Rays | |||||||
| Blue shark | 7,115 | 1.84 | 0.0153 | −4.34 | −0.0431 | Higher | |
| Porbeagle shark | 1,572 | −5.40 | −0.1976 | Higher | 1.28 | 0.0262 | |
| Silky shark | 1,890 | −1.20 | −0.0954 | Higher | −1.92 | −0.0591 | Higher |
| Pelagic stingray | 2,116 | −5.18 | 0.1149 | Lower | 0.82 | 0.0241 | |
| Short-finned mako shark | 2,207 | −4.62 | −0.1111 | Higher | 0.69 | 0.0199 | |
| Billfishes and similar | |||||||
| Swordfish | 3,973 | −1.21 | −0.0668 | Higher | 0.76 | −0.0178 | |
| Blue marlin | 1,977 | −2.36 | −0.0936 | Higher | 0.63 | 0.0214 | |
| Shortbill Spearfish | 1,451 | −4.75 | 0.1405 | Lower | 2.00 | −0.0013 | |
| Tuna, bonito and mackerel | |||||||
| Albacore | 6,898 | −2.64 | 0.1164 | Lower | −12.50 | 0.0662 | Lower |
| Yellowfin | 6,420 | −3.12 | −0.1095 | Higher | 1.88 | 0.0068 | |
| Pelagic fish and others | |||||||
| Longsnouted lancetfish | 3,268 | −2.46 | −0.0859 | Higher | 1.53 | 0.0143 | |
| Atlantic pomfret | 2,365 | −2.22 | −0.1077 | Higher | 0.95 | −0.0322 | |
| Moonfish | 3,065 | 0.49 | −0.0465 | −0.24 | −0.0258 | Higher | |
| Big-scaled pomfret | 892 | −5.62 | −0.1694 | Higher | −2.46 | 0.0678 | Lower |
| Butterfly kingfish | 1,013 | −5.46 | −0.1848 | Higher | −8.94 | −0.0980 | Higher |
| Ribbon fish | 577 | −5.85 | −0.3128 | Higher | −0.94 | 0.0956 | Lower |
| Short-snouted lancetfish | 741 | 1.34 | −0.0511 | −0.37 | −0.0575 | Higher | |
| Unidentified taxa | 1,603 | −0.77 | −0.0810 | Higher | −0.53 | −0.0504 | Higher |
For each component we present the effect of including the term for distance to seamount on the AIC (ΔAIC), the parameter estimate for the relationship with log(distance to seamount), and whether the effect represents a significantly higher or lower catch rate close to seamounts (SM). Only those taxa with statistically significant trends are shown here. The complete table is shown in Table S2.
Discussion
Our analyses suggest that seamounts are hotspots of pelagic biodiversity, because they show consistently higher species richness than do shore or oceanic areas. Moreover, our study indicates that higher species diversity is likely to occur within 30–40 km of seamount summits. This study also demonstrates that many marine predators and other visitors are associated with seamounts. The GLM model did not take into account the species being targeted or the depth and time of sets as information on these variables was not contained within the database. These factors may influence the results but are unlikely to affect the overall patterns, which are robust.
Associations with seamounts have been previously described for a few species of tuna (20, 23, 25, 26), sharks (22, 27), billfishes (28, 29), some seabirds (23, 30), and some marine mammals (23, 31), mostly at an individual seamount scale. Our study suggests, however, that seamount associations are probably more common and widespread than previously anticipated. The resolution of the data collected does not provide any opportunity to identify the mechanistic explanations for why seamounts aggregate biodiversity. However, seamounts generate conditions such as increased vertical nutrient fluxes and material retention that promote productivity and fuel higher trophic levels (32–34). Seamounts also have unique “magnetic signatures” that may contribute to their use as rest stops or feeding grounds for many pelagic species such as sharks, whales, and other migrants (35, 36). It is likely that the mechanistic explanation is a combination of factors that make seamounts suitable mating, feeding, and nursery grounds for highly migratory pelagic species as well as benthic organisms (37).
Higher pelagic biodiversity has previously been noted in intermediate latitudes (11) and our analyses support this hypothesis. However, we also noted high diversity in some tropical latitudes. In previous studies, data have been scarce for the tropical latitudes between 10 °N and 10 °S, whereas the observer data used in this study provided more comprehensive coverage. Further development of observer programs to ensure comprehensive spatial and temporal coverage is encouraged.
Conserving biodiversity hotspots has been demonstrated to yield significant conservation benefits (11). Therefore, our analyses support the utility of seamounts as potential locations for offshore marine reserves. Seamount habitats are easier to conserve than ephemeral areas because they are easier to map, survey, and enforce. The establishment of a network of marine reserves on seamounts may help to conserve pelagic biodiversity and achieve sustainability of marine predator species, such as porbeagle shark, short-finned mako shark, silky shark, blue shark, yellowfin tuna, blue marlin, swordfish, ribbon fish, butterfly kingfish, big-scaled pomfret, Atlantic pomfret, long-snouted lancetfish, short-snouted lancetfish, and moonfish.
Materials and Methods
Fisheries and Seamount Data.
The Western and Central Pacific Ocean (WCPO) is by far the most important tuna fishing ground in the world, contributing ≈50% (2.4 million tons in 2007) of the global tuna catches (38) at an economic value of US$3.8 billion. The longline fishery in the WCPO has a smaller catch (~10% of the total), but its value is relatively high (30% of the total value). It targets adult bigeye (Thunnus obesus), yellowfin (T. albacares), and albacore tuna (T. alalunga), and in some cases sharks or swordfish (X. gladius), and operates with fairly standard gear configurations that comprise a main line, branch lines between floats, and float lines. The Secretariat of the Pacific Community (SPC) maintains the regional database for fisheries observer programs in the WCPO, which commenced in 1980. The study area extends from 35 °N to 50 °S in latitude and from 130 °E to 120 °W in longitude (Fig. S1). All longline sets from the period 1980–2007 were extracted from the SPC's observer dataset, which include trips conducted onboard industrial and semi-industrial vessels from the Pacific Island Countries and Territories and from distance-water fishing nations. Depth of fishing was not recorded but is known to vary according to setting strategies. The database contains 23,546 longline sets with the number of hooks per set ranging from a few hundred to several thousand, averaging ~2,000 hooks per set. Observer quality was assumed to be consistent across all sets. The dataset contains catch data for 352 taxa, but only 50 taxa were recorded in >500 longline sets (Table S3). The number of recorded species as a function of fishing effort reached an asymptote (at ≈10–20 million hooks), indicating that the sample size obtained from the observer programs was sufficient to perform the biodiversity analyses (Fig. S2).
Catch by species was returned as number, size, and weight. Date and geographic location of the set, number of hooks, and flag and fleet of the fishing boat were also extracted. The distance of each longline set to the closest seamount was estimated using the simple spherical law of cosines and a Pacific seamount dataset containing 7,741 features (24, 39). Additionally, distances of longline sets to the closest shore were estimated using a land shapefile (including atolls). Longline sets were then categorized by habitat as seamount sets (distance to seamount < distance to shore and < 100 km), coastal sets (distance to shore < distance to seamount and < 100 km), or oceanic sets (distance to shore and distance to seamount >100 km). The longline dataset contained 10,602 seamount sets, 5,164 coastal sets, and 7,780 oceanic sets.
Biodiversity Analyses.
Species richness is known to increase with sample size, and differences in richness may be caused by differences in sample size. To solve this problem, we used rarefaction techniques to account for differences in fishing effort (number of hooks) among longline sets (40, 41). The expected number of species (Ŝ40), standardized to 1,000 hooks per longline set, was rarefied for subsamples of 40 individuals from the total number of individuals in the sample. This methodology has been extensively used to compare the species richness obtained from longline fishing fleets (11, 14, 42). The effects of habitat type and distance to habitat feature were analyzed for the estimated rarefied richness.
GLM techniques were used to standardize rarefied richness and to evaluate whether the presence of habitat features and the distance to the feature were significant explanatory variables. The explanatory variables included in the model were year as a proxy for temporal variability, moon phase as the relationship between lunar periodicity and catch rates as has been demonstrated for a wide variety of commercially exploited species (e.g., ref. 43), geographical area, fleet type, distance to the closest feature, and fishing effort. Akaike's information criterion (AIC) was used to compare the model fits using different relationships with distance to feature, with log-transformed having the better fit. The model used was
 |
Years included in the standardization were 1982 and 1987–2007 because Ŝ40 estimates were not available for other years. Moon phase was divided into eight categories from new to full. The geographical areas used in the standardization were squares of 5 ° latitude and longitude because they originate better fit than many other approaches. Vessels were categorized on the basis of a combination of their flag and fleet type. Effort was measured as the natural cubic splines (ns) of the number of hooks in each longline set. The species being targeted and the depth and time of a set can influence the nontarget species caught. Information on these variables was not contained within the database and fleet type and number of hooks were used as a proxy measures for these variables.
Analyses of the GLMs for Each Highly Migratory Pelagic Species.
We used GLM techniques to standardize catch data for each by-catch species caught in at least 500 sets of the 10,602 sets for which a seamount was the closest feature and was within 100 km (Fig. S3). We modeled the data in two parts using a δ-lognormal GLM (44). In the first (binomial) part we modeled the probability of catching any of the species in a set. In the second (lognormal) part we modeled the number caught in sets where at least one animal was caught. We used AIC to test for effects of distance to seamount by modeling the data with and without a seamount term. The explanatory variables included in the models were the same as in the biodiversity analyses. The models adopted to standardize data were
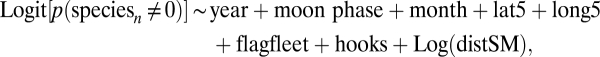 |
where speciesn ≠ 0, and
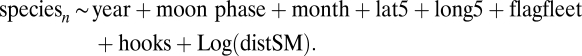 |
We examined the residuals to check that the assumptions were not violated for each model. By-catch species were considered to be associated with seamounts if the seamount distance effect was statistically significant and negative (i.e., higher catch rate when closer to seamount summits).
Supplementary Material
Acknowledgments
We thank Nick Davies and Michael Manning for help with the modeling and Emmanuel Schneiter, Colin Millar, and Peter Williams for help with the Observer database. We thank the two anonymous reviewers for their comments, which greatly improved the manuscript. We acknowledge the Secretariat of the Pacific Community member countries for the collection and provision of observer data. We particularly acknowledge the important work done by the national observer programs throughout the region. This research is part of the Pacific Islands Oceanic Fisheries Management Project supported by Global Environment Facility.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0910290107/-/DCSupplemental.
References
- 1.Baum JK, et al. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2003;299:389–392. doi: 10.1126/science.1079777. [DOI] [PubMed] [Google Scholar]
- 2.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 3.Hampton J, Sibert JR, Kleiber P, Maunder MN, Harley SJ. Fisheries: Decline of Pacific tuna populations exaggerated? Nature. 2005;434:E1–E2. doi: 10.1038/nature03581. [DOI] [PubMed] [Google Scholar]
- 4.Hilborn R. Faith-based fisheries. Fisheries. 2006;31:554–555. [Google Scholar]
- 5.Sibert J, Hampton J, Kleiber P, Maunder M. Biomass, size, and trophic status of top predators in the Pacific Ocean. Science. 2006;314:1773–1776. doi: 10.1126/science.1135347. [DOI] [PubMed] [Google Scholar]
- 6.Langley A, et al. Western Central Pacific Fisheries Commission, Scientific Committee, 5th Regular Session. Port Villa, Vanuatu: Western Central Pacific Fisheries Commission; 2009. Stock assessment of yellowfin tuna in the western and central Pacific Ocean. Available at: http://www.wcpfc.int/system/files/documents/meetings/scientific-committee/5th-regular-session/stock-assessment-swg/working-papers/SC5-SA-WP-03%20%5BYFT%20Assessment%20%28rev.1%29%5D.pdf. [Google Scholar]
- 7.Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- 8.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 9.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 10.Baum JK, Worm B. Cascading top-down effects of changing oceanic predator abundances. J Anim Ecol. 2009;78:699–714. doi: 10.1111/j.1365-2656.2009.01531.x. [DOI] [PubMed] [Google Scholar]
- 11.Worm B, Lotze HK, Myers RA. Predator diversity hotspots in the blue ocean. Proc Natl Acad Sci USA. 2003;100:9884–9888. doi: 10.1073/pnas.1333941100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 13.Roberts CM, et al. Priorities for tropical reefs marine biodiversity hotspots and conservation. Science. 2002;295:1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- 14.Worm B, Sandow M, Oschlies A, Lotze HK, Myers RA. Global patterns of predator diversity in the open oceans. Science. 2005;309:1365–1369. doi: 10.1126/science.1113399. [DOI] [PubMed] [Google Scholar]
- 15.Etnoyer P, Canny D, Mate B, Morgan L. Persistent pelagic habitats in the Baja California to Bering Sea (B2B) ecoregion. Oceanography (Wash DC) 2004;17:90–101. [Google Scholar]
- 16.Martell SJD, et al. Interactions of productivity, predation risk, and fishing effort in the efficacy of marine protected areas for the central Pacific. Can J Fish Aquat Sci. 2005;62:1320–1336. [Google Scholar]
- 17.Norse EA. Protecting the least-protected places on earth: The open oceans. MPA News. 2006;7:4. [Google Scholar]
- 18.Malakoff D. New tools reveal treasures at ocean hot spots. Science. 2004;304:1104–1105. doi: 10.1126/science.304.5674.1104. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto K. Oceanic biology: Spawning of eels near a seamount. Nature. 2006;439:929. doi: 10.1038/439929a. [DOI] [PubMed] [Google Scholar]
- 20.Holland K, Grubbs D. In: Seamounts: Ecology, Fisheries and Conservation. Pitcher TJ, et al., editors. Oxford, UK: Blackwell Science; 2007. pp. 189–201. [Google Scholar]
- 21.Kaschner K. In: Seamounts: Ecology, Fisheries and Conservation. Pitcher TJ, et al., editors. Oxford, UK: Blackwell Science; 2007. pp. 230–238. [Google Scholar]
- 22.Litvinov F. In: Seamounts: Ecology, Fisheries and Conservation. Pitcher TJ, et al., editors. Oxford, UK: Blackwell Science; 2007. pp. 202–206. [Google Scholar]
- 23.Morato T, et al. Evidence of a seamount effect on aggregating visitors. Mar Ecol Prog Ser. 2008;357:23–32. [Google Scholar]
- 24.Allain V, et al. Enhanced seamount location database for the western and central Pacific Ocean: Screening and crosschecking of 20 existing datasets. Deep Sea Res I. 2008;55:1035–1047. [Google Scholar]
- 25.Klimley AP, Jorgensen SJ, Muhlia-Melo A, Beavers SC. The occurrence of yellowfin tuna (Thunnus albacares) at Espiritu Santo Seamount in the Gulf of California. Fish Bull (Wash DC) 2003;101:684–692. [Google Scholar]
- 26.Rodríguez-Cabello C, Sánchez F, Ortiz de Zárate V, Barreiro S. Does Le Danois Bank (El Cachucho) influence albacore catches in the Cantabrian Sea? Cont Shelf Res. 2009;29:1205–1212. [Google Scholar]
- 27.Klimley AP, Richard JE, Jorgensen SJ. The home of blue water fish. Am Sci. 2005;93:42–49. [Google Scholar]
- 28.Sedberry GR, Loefer JK. Satellite telemetry tracking of swordfish, Xiphias gladius, off the eastern United States. Mar Biol. 2001;139:355–360. [Google Scholar]
- 29.Poisson F, Fauvel C. Reproductive dynamics of swordfish (Xiphias gladius) in the southwestern Indian Ocean (Reunion Island). Part 2: Fecundity and spawning pattern. Aquat Living Resour. 2009;22:59–68. [Google Scholar]
- 30.Amorim P, et al. Spatial variability of seabird distribution associated with environmental factors: A case study of marine Important Bird Areas in the Azores. ICES J Mar Sci. 2009;66:29–40. [Google Scholar]
- 31.Parrish FA. Do monk seals exert top-down pressure in subphotic ecosystems? Mar Mamm Sci. 2009;25:91–106. [Google Scholar]
- 32.Genin A, Dayton PK, Lonsdale PF, Spiess FN. Corals on seamounts provide evidence of current acceleration over deep sea topography. Nature. 1986;322:59–61. [Google Scholar]
- 33.Lueck RG, Mudge TD. Topographically induced mixing around a shallow seamount. Science. 1997;276:1831–1833. [Google Scholar]
- 34.White M, Bashmachnikov I, Arístegui J, Martins A. In: Seamounts: Ecology, Fisheries and Conservation. Pitcher TJ, et al., editors. Oxford, UK: Blackwell Science; 2007. pp. 65–87. [Google Scholar]
- 35.Pitcher TJ, Bulman C. In: Seamounts: Ecology, Fisheries and Conservation. Pitcher TJ, et al., editors. Oxford, UK: Blackwell Science; 2007. pp. 282–295. [Google Scholar]
- 36.Morato T, Bulman C, Pitcher TJ. Modelled effects of primary and secondary production enhancement by seamounts on local fish stocks. Deep Sea Res II. 2009;56:2713–2719. [Google Scholar]
- 37.Fréon P, Dagorn L. Review of fish associative behaviour: Toward a generalisation of the meeting point hypothesis. Rev Fish Biol Fish. 2000;10:183–207. [Google Scholar]
- 38.Lawson TA. Tuna Fishery Yearbook 2007. Western and Central Pacific Fisheries Commission, Kolonia, Pohnpei, Federal States of Micronesia; 2008. [Google Scholar]
- 39.Kitchingman A, Lai S. Seamounts: Biodiversity and fisheries. In: Morato T, Pauly D, editors. Fisheries Centre Research Report 12.5. Vancouver, BC, Canada: Fisheries Centre, Univ of British Columbia; 2004. pp. 7–12. [Google Scholar]
- 40.Hurlbert SH. The nonconcept of species diversity: A critique and alternative parameters. Ecology. 1971;52:577–586. doi: 10.2307/1934145. [DOI] [PubMed] [Google Scholar]
- 41.Heck KL, van Belle G, Simberloff D. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology. 1975;56:1459–1461. [Google Scholar]
- 42.Boyce DG, Tittensor DP, Worm B. Effects of temperature on global patterns of tuna and billfish richness. Mar Ecol Prog Ser. 2008;355:267–276. [Google Scholar]
- 43.Lowry M, Williams D, Metti Y. Lunar landings—Relationship between lunar phase and catch rates for an Australian gamefish tournament fishery. Fish Res. 2007;88:15–23. [Google Scholar]
- 44.Stefansson G. Analysis of groundfish survey abundance data: Combining the GLM and delta approaches. ICES J Mar Sci. 1996;53:577–588. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.