Abstract
Metabolic adaptation to the host niche is a defining feature of the pathogenicity of Mycobacterium tuberculosis (Mtb). In vitro, Mtb is able to grow on a variety of carbon sources, but mounting evidence has implicated fatty acids as the major source of carbon and energy for Mtb during infection. When bacterial metabolism is primarily fueled by fatty acids, biosynthesis of sugars from intermediates of the tricarboxylic acid cycle is essential for growth. The role of gluconeogenesis in the pathogenesis of Mtb however remains unaddressed. Phosphoenolpyruvate carboxykinase (PEPCK) catalyzes the first committed step of gluconeogenesis. We applied genetic analyses and 13C carbon tracing to confirm that PEPCK is essential for growth of Mtb on fatty acids and catalyzes carbon flow from tricarboxylic acid cycle–derived metabolites to gluconeogenic intermediates. We further show that PEPCK is required for growth of Mtb in isolated bone marrow–derived murine macrophages and in mice. Importantly, Mtb lacking PEPCK not only failed to replicate in mouse lungs but also failed to survive, and PEPCK depletion during the chronic phase of infection resulted in mycobacterial clearance. Mtb thus relies on gluconeogenesis throughout the infection. PEPCK depletion also attenuated Mtb in IFNγ-deficient mice, suggesting that this enzyme represents an attractive target for chemotherapy.
Keywords: carbon metabolism, gluconeogenesis, metabolomics, microbial pathogenesis, phosphoenolpyruvate carboxykinase
Carbon metabolism is a significant determinant of the ability of Mycobacterium tuberculosis (Mtb) to replicate and persist in the host. Knowledge of the metabolic pathways used by Mtb during infection is therefore important for understanding its pathogenicity, and can also guide the development of new drug therapies. New drugs are urgently needed to control infections with Mtb, which kills ≈2 million people annually (1, 2). Mounting evidence suggests that Mtb preferentially uses fatty acids during in vivo growth (3, 4). The enzymes required for fatty acid metabolism in Mtb, however, remain incompletely defined.
Fatty acids are substrates for both the tricarboxylic acid (TCA) cycle and gluconeogenesis, which serve energy and biomass production, respectively (5). When biomass production solely relies on fatty acids, cells must avoid carbon loss in the form of CO2 during oxidation in the TCA cycle. This carbon preservation need is fulfilled by the glyoxylate cycle, which diverts isocitrate to succinate and glyoxylate through the joint activities of isocitrate lyase and malate synthase. Most Mtb strains, including Mtb Erdman, express two isoforms of isocitrate lyase (ICL) encoded by icl1 and icl2. Mtb missing both icl1 and icl2 was unable to grow using fatty acid substrates and was rapidly eliminated from lungs of infected mice (6). These findings are in agreement with other observations (7 –11), which suggested that Mtb relies on fatty acid metabolism through the glyoxylate cycle for in vivo growth. However, ICLs of Mtb also function as methylisocitrate lyase (MCL), which is involved in the metabolism of propionyl-CoA through the methylcitrate cycle yielding pyruvate (12, 13). Beta-oxidation of odd-chain fatty acids generates propionyl-CoA, and incomplete metabolism of propionyl-CoA due to the absence of methylcitrate lyase activity can cause accumulation of toxic intermediates (14). It has therefore been suggested that the marked attenuation of the MtbΔicl1/Δicl2 double mutant in mice might be due to impaired propionyl-CoA detoxification instead of defective fatty acid catabolism and gluconeogenesis, or may be a result of both (14).
We investigated the specific role of gluconeogenesis in Mtb pathogenesis by studying phosphoenolpyruvate carboxykinase (PEPCK), which catalyzes the first committed step in gluconeogenesis. PEPCK catalyzes the guanosine or adenosine mononucleotide–dependent reversible conversion of oxaloacetate (OAA) and phosphoenolpyruvate (PEP) (15, 16). PEPCK from Mycobacterium smegmatis is GTP-dependent and preferentially catalyzes the gluconeogenic direction, whereby PEP is formed from OAA (17). The gene encoding PEPCK, pckA, is induced by fatty acids in vitro and during growth of Mtb in mice, suggesting a demand for gluconeogenesis during infection (9 –11). PEPCK was first implicated in mycobacterial pathogenesis because M. bovis deficient in PEPCK did not cause spleen lesions in guinea pigs following s.c. injection (18). In addition, M. bovis bacillus Calmette–Guérin lacking pckA was killed almost 10-fold more than wild-type (WT) bacillus Calmette–Guérin in mouse spleens between day 20 and 35 after i.v. infection, although both strains survived similarly at later time points (19).
Here, we demonstrate that PEPCK plays a pivotal role in the pathogenesis of tuberculosis, as it is essential for growth and survival of Mtb during infections in mice. Our data indicate that Mtb relies primarily on gluconeogenic substrates for in vivo growth and persistence. This work also points to PEPCK as potential target for anti-TB chemotherapy.
Results
PEPCK Is Required for Growth of Mtb on Fatty Acids.
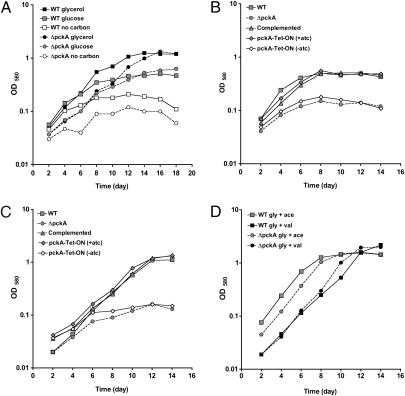
We constructed an Mtb knockout strain, ΔpckA, in which the entire pckA ORF was deleted and replaced with a hygromycin resistance cassette (Fig. S1A); deletion of pckA was confirmed by Southern blot (Fig. S1B) and immunoblot (Fig. S1C). In vitro growth of WT Mtb and ΔpckA was similar with glycerol or glucose as the sole carbon source (Fig. 1A), but ΔpckA failed to grow more than in the absence of an external carbon source when provided the fatty acids acetate, valerate, or butyrate as the sole carbon source (Fig. 1 B and C). This growth defect was restored when ΔpckA was transformed with a plasmid that expressed pckA from its native promoter. Growth on fatty acids was also complemented when pckA was expressed from a tetracycline repressor (TetR)–regulated promoter (20, 21), and growth of this TetR-regulated mutant strain (pckA-TetON) in gluconeogenic substrates was dependent on anhydrotetracycline (atc), the inducer of TetR-regulated gene expression (Fig. 1 B and C). Growth of ΔpckA in media containing fatty acids could also be restored by adding glycerol (Fig. 1D), demonstrating that fatty acids are not toxic to ΔpckA in the presence of an alternative carbon source.
Fig. 1.
PEPCK is necessary for growth with a fatty acid as sole carbon source. Growth in carbon-defined media of WT Mtb (□), ΔpckA (●), ΔpckA complemented using its native promoter (△), and complemented under TetR control (pckA-TetON, ⋄). (A) Growth in media with 0.1% glycerol (black), glucose (gray), or no carbon (white). (B) Growth in media with 0.1% acetate or (C) 0.1% valeric acid. (D) Growth in media with 0.1% glycerol and 0.1% acetate (gray) or 0.1% glycerol and 0.1% valeric acid (black). Data represent one of three independent experiments.
Together these results demonstrate that PEPCK is essential for growth of Mtb on fatty acids as the sole carbon source.
Absence of PEPCK Blocks Gluconeogenic Carbon Flow of TCA Cycle Intermediates.
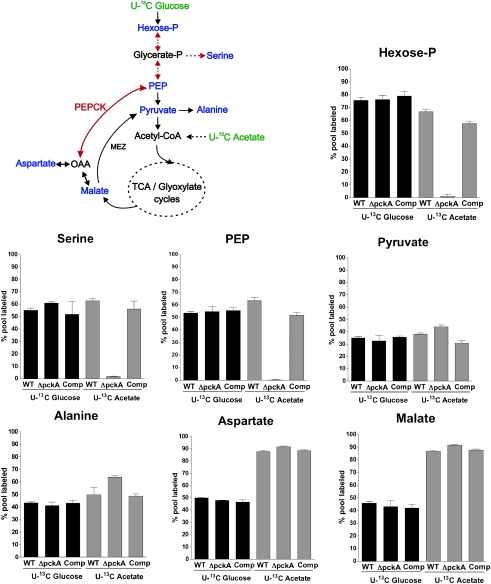
We used liquid chromatography–mass spectrometry (LC-MS) to examine how carbon flux of uniformly (U) 13C-labeled glucose and acetate into key metabolites of glycolysis and the TCA cycle was altered by the deletion of pckA, and to identify the specific metabolic defect associated with the failure of ΔpckA to grow on fatty acids (Fig. 2). We grew strains on a combination of fatty acid and carbohydrates before labeling (to provide cell biomass) and then exposed them to U-13C glucose or U-13C acetate for 16 h, a period sufficient to achieve an isotopic steady state (Fig. S2). We examined 13C label incorporation into the metabolites hexose-phosphate, serine, alanine, pyruvate, phosphoenolpyruvate (PEP), aspartate, and malate to follow carbon flow through glycolysis and gluconeogenesis (4). We could not measure OAA directly because of its instability (22), and instead measured aspartate the direct product and reporter of OAA (23). Metabolism of U-13C glucose was indistinguishable between WT and ΔpckA (Fig. 2), confirming that PEPCK is dispensable for glycolysis. Metabolism of U-13C acetate into the TCA cycle–derived intermediates malate and aspartate was also unaffected in ΔpckA. In contrast, metabolism of U-13C acetate into glycolytic intermediates was blocked in the absence of PEPCK, reflected by the lack of detectable incorporation from U-13C acetate–derived carbon into PEP, serine, and hexose-phosphate (Fig. 2). This block in gluconeogenic carbon flow was alleviated in the complemented mutant. Interestingly, the PEPCK mutant could still incorporate carbon from U-13C acetate into pyruvate and alanine, indicating the presence of a PEPCK-independent pathway for carbon flow from the TCA cycle to pyruvate, such as that catalyzed by the malic enzyme (MEZ) (Fig. 2). Moreover, the lack of acetate-derived 13C incorporation into serine suggests that pyruvate can neither serve as a direct precursor for the biosynthesis of this amino acid, nor through its conversion into PEP for subsequent biosynthesis of phosphoserine via 3-phosphoglycerate. In summary, these metabolomic analyses establish PEPCK as the sole enzyme in Mtb capable of driving TCA cycle derived carbons for the biosynthesis of glycolytic and gluconeogenic precursors.
Fig. 2.
Gluconeogensis is blocked in the absence of PEPCK. Schematic illustration of metabolic pathways studied using carbon tracing analysis and relative extent of incorporation of U-13C acetate or U-13C glucose into the intracellular pool of selected metabolites in WT, ΔpckA, and complemented mutant. Strains were grown to log-phase on permissive media and then exposed to U-13C acetate or U-13C glucose for 16 h. U-13C–labeled carbon sources are indicated in green, and analyzed metabolites are highlighted in blue. Dashed arrows represent more than one enzymatic step. PEPCK, phoshoenolpyruvate carboxykinase; MEZ, malic enzyme; OAA, oxaloacetate; and PEP, phoshoenolpyruvate. Each bar represents the mean of three sample replicates; error bars indicate the SD. Data are representative of two independent experiments.
Replication of Mtb in Murine Macrophages Requires PEPCK.
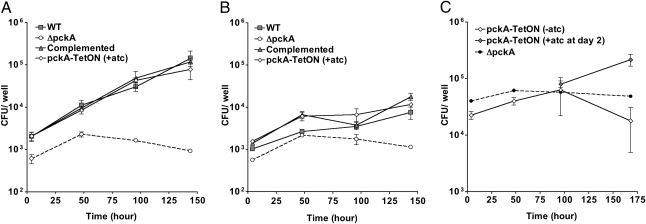
To investigate whether Mtb relies on gluconeogenic substrates for intracellular growth, we measured replication of the pckA mutants within murine bone marrow–derived macrophages. In contrast to WT Mtb, ΔpckA was unable to replicate within resting macrophages (Fig. 3A). IFNγ-activated macrophages controlled replication of WT Mtb but did not reduce survival of ΔpckA in activated macrophages more than naive macrophages (Fig. 3B). Introduction of a copy of the pckA gene restored replication of ΔpckA in macrophages to WT levels (Fig. 3A). The pckA-TetON mutant replicated in the presence, but not in the absence, of atc (Fig. 3 A and C), indicating that pckA expression was efficiently regulated by TetR within macrophages. In fact, induction of pckA expression at day 2 postinfection with atc rescued replication of the pckA-TetON mutant (Fig. 3C). These results thus demonstrate that PEPCK is required for replication of Mtb in macrophages.
Fig. 3.
PEPCK is necessary for replication in macrophages. CFU of WT (□), ΔpckA (●), complemented mutant (△), and pckA-TetON mutant (⋄) in bone marrow–derived macrophages. (A) Infection of resting macrophages. (B) Infection of IFNγ-activated macrophages. (C) Infection of resting macrophages with ΔpckA and pckA-TetON. One set of macrophages infected with pckA-TetON received atc at day 2. Data represent the mean of triplicate cultures; error bars indicate the SD. Data shown in A and B are representative of three independent experiments.
PEPCK Is Essential for Growth and Survival of Mtb During Both the Acute and Chronic Phases of Infection in the Mouse.
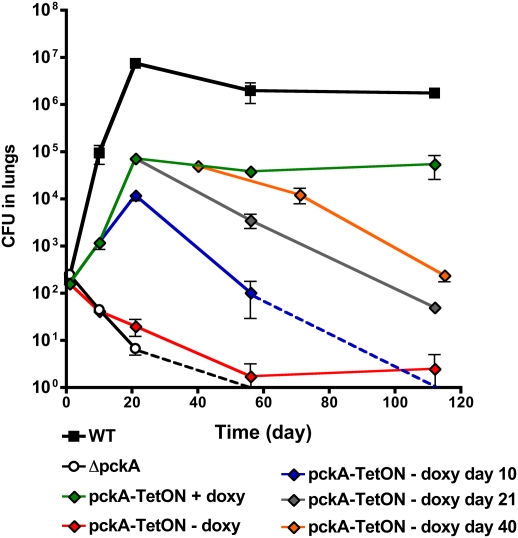
To determine the role of PEPCK in a model of pulmonary tuberculosis, we infected immune-competent mice by aerosol with WT, ΔpckA, and the complemented strains. The ΔpckA strain failed to replicate during the acute phase of infection and was cleared from lungs by day 56 (Fig. 4). Introduction of pckA expressed from its native promoter (Fig. S3) or the TetR-regulated promoter (Fig. 4) restored survival and replication, although the bacterial load in the lungs did not reach WT level. In contrast, growth of ΔpckA on fatty acids in vitro and in macrophages was fully restored to WT levels in the complemented strains (Figs. 1 B and C and 3A). PEPCK protein levels were also found to be similar in WT and complemented strains (Fig. S1C). This discrepancy in complementation led us to investigate whether the attenuation of the complemented strains might be due to a deficiency of the cell wall–associated lipid phthiocerol dimycocerosate (PDIM). Loss of PDIM results in impaired replication of Mtb in mouse lungs (24, 25), and mutations that abolish synthesis of this lipid have been show to occur spontaneously (26 –28). Lipid profile analysis revealed that both WT and ΔpckA strains expressed similar levels of PDIM. However, this lipid was nearly absent in both complemented strains (Fig. S3A), suggesting that these strains were less virulent than WT due to the absence of PDIM. To directly assess the impact of loss of PDIM on virulence of our WT strain, we transformed it with an empty plasmid and recovered PDIM-negative and PDIM-positive strains (Fig. S3A). We compared growth in mouse lungs of these isogenic WT strains that differed in their PDIM status. This confirmed that the absence of PDIM resulted in a 2 log10 reduction in CFU in mouse lungs at day 21 postinfection (Fig. S3B). In addition, the WT PDIM-negative and the PDIM-negative complemented ΔpckA strains achieved similar bacterial loads in the lung. These data suggest that loss of PDIM caused the remaining growth defect of the complemented ΔpckA strain in mouse lungs.
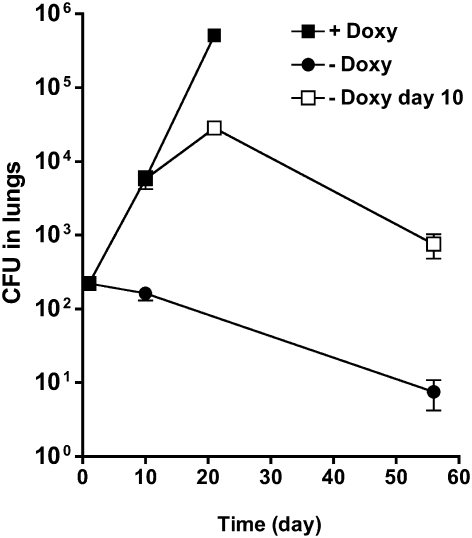
Fig. 4.
PEPEC is essential for growth and survival in mice. Bacterial loads in lungs from mice infected with WT (□), ΔpckA (●), or pckA-TetON (⋄). PckA expression in pckA-TetON was induced by feeding mice the inducer doxycycline (doxy) and silenced by removal of doxy from food. Mice infected with pckA-TetON were fed with doxy for the entire experiment (green diamond), for 10 days (blue diamond), for 21 days (gray diamond), or for 40 days (orange diamond). In mice kept without doxy food (red diamonds), bacteria could be detected only in two (day 56) or one (day 112) of four animals (entire lungs were plated for CFU). Dashed lines indicate that no CFU were detected in whole lungs at the next time point. Data represents the mean of four mice per group; error bars indicate the SD.
We previously demonstrated that the activity of TetR-controlled promoters can be regulated during infections in mice (21, 29). In Mtb-containing WT TetR, a TetR-controlled promoter is active in mice that receive doxycycline (doxy)–containing food and is repressed in mice that receive doxy-free food. Accordingly, growth and survival of pckA-TetON was restored only in mice fed with doxy throughout the infection (Fig. 4). In mice not receiving doxy, the pckA-TetON mutant was cleared similar to ΔpckA. When doxy was provided only for the first 10 days of infection, growth of pckA-TetON was impaired between day 10 and day 21; subsequently, bacterial titers progressively declined and reached undetectable levels by day 112 postinfection. When expression of pckA was silenced beginning at day 21 and day 40 postinfection, this resulted in a 3-log10 and 2-log10 decrease, respectively, in bacterial titers in the lungs at day 112 (Fig. 4). These experiments demonstrate that PEPCK is not only essential for Mtb to establish an infection and to grow during the acute phase of infection, but is equally important for Mtb survival during the chronic phase of infection.
ΔpckA Is Killed in Vivo Independently of IFN-γ–Mediated Immune Responses.
To identify stresses that might be responsible for killing of ΔpckA in vivo, we measured survival of the knockout under various in vitro conditions. ΔpckA was not significantly more sensitive than WT to stresses likely to be encountered inside the host, such as low pH, hydrogen peroxide, nitric oxide (NO), and starvation (Fig. S4). Moreover, IFNγ-activated macrophages did not kill ΔpckA more than resting macrophages, and ΔpckA was killed in vivo even before the onset of the adaptive immune response. Thus, the in vivo killing of ΔpckA is unlikely to depend on IFNγ-dependent elements of the host immune response. To test this hypothesis, we infected IFNγ-deficient mice with the pckA-TetON mutant and determined the impact of pckA silencing. PckA-TetON replicated in IFNγ−/− mice with similar kinetics as in WT mice, when mice were fed doxy during the entire experiment (Fig. 5). In contrast, silencing pckA expression throughout the infection resulted in loss of survival of the bacteria, so that 90% of the inoculum was killed by day 56. Silencing pckA expression at day 10 postinfection, when the bacteria were actively replicating, resulted in a 1-log10 reduction in CFU from day 21 to day 56 postinfection. Thus, Mtb requires PEPCK for growth and survival in immunecompromised mice.
Fig. 5.
In vivo killing of ΔpckA occurs independently of IFNγ-mediated immune responses. Bacterial loads in lungs from IFNγ−/− mice infected with pckA-TetON. PckA expression was induced (■), silenced during the entire experiment (●) or silenced at day 10 (□). Data represent the mean of data from four mice per group; error bars indicate the SD.
Discussion
Mtb can use a variety of carbon substrates via multiple pathways including glycolysis, pentose phosphate pathway, and the TCA, glyoxylate and methylcitrate cycles. Analyses of the Mtb icl mutants suggested that fatty acids are an important carbon and energy source for Mtb during infection (3, 4). However, fatty acids are toxic to MtbΔicl1/2 even in the presence of carbohydrates, complicating the mechanistic interpretation of the attenuation of this mutant in mice. The failure of the ICL mutant to establish an infection in mice could be the result of defective replenishment of TCA cycle intermediates (anaplerosis), defective gluconeogenesis, or impaired propionyl-CoA metabolism through the methylcitrate cycle (13, 14, 30). Furthermore, ICL is important for essential intracellular ATP level reduction in nonreplicating, carbon-starved Mtb (31).
Similar to the glycoxylate cycle, gluconeogenesis is a biosynthetic pathway that is dispensable for growth of many bacteria in media containing carbohydrates, but is essential for growth on fatty acids. If fatty acids are the primary carbon source for Mtb during infections, mutations that inactivate gluconeogenesis should drastically impair in vivo growth. From previous work, however, it is not clear whether Mtb requires gluconeogenesis to grow during infections. The work described here demonstrates that gluconeogenesis is critical for the pathogenicity of Mtb during both active and latent forms of disease.
PEPCK, encoded by pckA, catalyzes the first committed step in gluconeogenesis; accordingly, ΔpckA could not grow using gluconeogenic carbon substrates, such as fatty acids, as sole carbon source. U-13C tracing analysis of glycolytic (glucose) and gluconeogenic (acetate) carbon substrates confirmed that PEPCK is the sole gluconeogenic enzyme in Mtb that can convert TCA cycle intermediates to PEP. Metabolism of U-13C glucose–derived carbons to TCA cycle intermediates was unaffected by the absence of PEPCK, suggesting that in Mtb PEPCK is not required for the conversion of PEP to OAA under the tested experimental conditions. Carbon from U-13C acetate was not incorporated into PEP, serine, and hexose-P, indicating that gluconeogenic carbon flux of acetate was blocked in ΔpckA. Carbon flux through the TCA cycle was, however, unimpaired, suggesting that anaplerosis occurs independently of PEPCK. U-13C acetate–derived carbons were metabolized into pyruvate and alanine, indicating activity of the malic enzyme that converts malate into pyruvate, the direct precursor of alanine. However, pyruvate could not serve as precursor for the biosynthesis of PEP. Thus, in Mtb, PEPCK is required for and predominantly catalyzes the formation of PEP and does not appear to catalyze the reverse reaction to OAA during metabolism of glucose. These findings are in accordance with the enzymatic properties reported for PEPCK from M. smegmatis (17).
ΔpckA was unable to grow in macrophages and mice and was susceptible to killing early during infection in mice. These phenotypes are reminiscent of Mtb lacking both ICLs (6). However, in contrast to the ICL mutant, Mtb lacking PEPCK was not susceptible to death induced by incomplete propionyl-CoA metabolism in vitro, as its growth with glycolytic substrates was not inhibited by the addition of odd-chain fatty acids such as valerate and propionate (Fig. 1D and Fig. S5) and radiolabeled propionate was incorporated into the cell wall lipid PIDIM (Fig. S3A). Fatty acid toxicity is therefore unlikely the reason for the in vivo growth defect of ΔpckA. The strong attenuation of this mutant strongly argues that sugars are not available to Mtb during infections in mice, and that growth and persistence depends on gluconeogenic carbon sources.
Unexpectedly, PEPCK was not only important for growth in vivo but was also required for persistence of Mtb in mice, as silencing pckA expression during the chronic infection phase resulted in effective mycobacterial killing. Thus, Mtb appears to rely on gluconeogenesis and to be metabolically active throughout the infection. In contrast, the nonpathogenic M. bovis bacillus Calmette–Guérin lacking pckA survived with reduced titers compared with WT bacillus Calmette–Guérin for 8 weeks in mouse spleens (19), suggesting that the metabolic pathways of Mtb and M. bovis might be different.
The inability to use gluconeogenic substrates for energy and biomass production explains the failure of ΔpckA to grow in vivo, but it is currently unclear how the host kills this mutant. In vivo killing occurred independently of the stage of infection and did not require IFNγ-dependent host immune responses. However, ΔpckA was not significantly more susceptible than WT Mtb to various in vitro stress conditions, including oxidative and nitrosative stress, acid, and prolonged carbon starvation (Fig. S4). Mtb pathogenesis is an intricate process involving a wide array of host–microbe interactions, any of which could be disrupted due to the absence of PEP formation in ΔpckA during infection. The cell wall of Mtb serves as an important virulence factor, and changes in this complex structure likely facilitate clearance of Mtb by its host (32, 33); however, ΔpckA did not seem to have a compromised cell wall, as it was not hypersensitive to detergents (Fig. S6). In addition, loss of PEPCK did not affect production of PDIM during culture in vitro. It is possible that the defect in gluconeogenesis sensitized ΔpckA to the antimicrobial mechanisms in the in vivo environment.
In summary, this work supports the view that Mtb preferentially metabolizes gluconeogenic carbon substrates such as fatty acids and/or amino acids, and therefore requires PEPCK for growth and survival in vivo. Clearance of ΔpckA in mice did not require IFNγ-dependent immune responses. Thus, PEPCK might represent a promising new target for effective eradication of Mtb infection in immune-competent and immune-compromised patients.
Materials and Methods
Bacterial Strains and Media.
Mtb (Erdman) strains were grown at 37 °C in Middlebrook 7H9 liquid medium (Difco) containing 0.2% glycerol, 0.5% BSA, 0.2% dextrose, 0.085% NaCl, and 0.05% Tween 80, or on Middlebrook 7H10 agar plates containing 10% OADC supplement (Becton Dickinson) and 0.5% glycerol. For growth with defined carbon sources, 7H9 medium with 0.05% Tyloxapol and a carbon substrate at 0.1% (wt/vol) was used. Hygromycin B (50 μg/mL) and kanamycin (25 μg/mL) were included when required for selection. Anhydrotetracycline (Sigma) was used at 200 ng/mL and replenished every 4 days in liquid culture. For metabolomic profiling, Mtb was cultivated on filters according to Brauer et al. (34). Mtb was seeded on 0.22-μM nitrocellulose filters and grown on 7H10 agar plates containing 10% OADC supplement (Becton Dickinson) and 0.5% glycerol for 5 days. Filters were then transferred to 7H10 plates containing 0.5% BSA, 0.085% NaCl, and 0.2% U-13C acetate or 0.2% U-13C glucose (Cambridge Isotope Laboratories) for ≈0.75 generation times (16 h). Preliminary studies established that isotopic steady state for the tested metabolites was achieved by 16 h following transfer of filter-laden bacteria in the logarithmic phase of growth to fresh 13C-containing media (Fig. S3). Bacteria were metabolically quenched by immersion into acetonitrile:methanol:H2O (40:40:20) precooled to −40 °C, and metabolites were extracted by mechanical lysis followed by clarification and filtration across a 0.22-μm filter. Bacterial biomass of individual samples was determined by measuring residual protein content.
Mutant Construction.
ΔpckA was constructed via allelic exchange using specialized transducing phage phAE87 (35). Briefly, ~500-bp fragments containing the upstream and downstream region of the pckA gene were amplified by PCR and cloned into pJSC284-loxP to flank the hygromycin resistance gene. pJSC284-loxP is a derivative of pJSC284 (gift from Jeff S. Cox) containing loxP sites flanking the hygromycin cassette. The plasmid was digested with PacI and packaged into the unique PacI site of the temperature-sensitive phage phAE87. The phage was amplified in M. smegmatis at 30 °C and used to infect Mtb as previously described (36). The ΔpckA::hyg knockout was confirmed using Southern blot (Fig. S1A).
Complementation plasmids were constructed using Gateway Cloning Technology (Invitrogen). The putative native pckA promoter was amplified by PCR. The Pmyc1tetO promoter (21) and WT TetR (20) were used to generate the pckA-TetON mutant. Primer sequences are available upon request.
Mouse and Macrophage Infections.
C57BL/6 mice and IFNγ−/− mice on a C57BL/6 genetic background (Jackson Laboratory) were infected with Mtb by aerosol as described (37). When indicated, mice received doxycycline-containing mouse chow (2,000 ppm; Research Diets). Bacterial numbers were determined by plating homogenized organs for CFU. Bone marrow–derived mouse macrophages were isolated and infected with Mtb as described (37). The Institutional Animal Care and Use Committee of Weill Cornell Medical College approved murine experimental procedures.
Liquid Chromatography–Mass Spectrometry.
Metabolites were separated on a Cogent Diamond Hydride Type C column (Microsolve Technologies) (38). The mass spectrometer used was Agilent Accurate Mass 6220 TOF coupled to an Agilent 1200 LC system. Dynamic mass axis calibration was achieved by continuous infusion of a reference mass solution using an isocratic pump with a 100:1 splitter. This configuration achieved mass errors of ≈5 parts per million (ppm), mass resolution ranging from 10,000–25,000 (over m/z 121–955 amu), and 5 log10 dynamic range. Detected ions were deemed metabolites on the basis of unique accurate mass retention time (AMRT) identifiers for masses exhibiting the expected distribution of accompanying isotopomers (Table S1). Metabolite identities were established by querying against a prepopulated AMRT library of metabolite standards and demonstrating chromatographic coelution of candidate metabolites with pure chemical standards spiked into representative biological samples.
Isotopomer Data Analysis.
The extent of isotopic labeling for each metabolite was determined by dividing the summed peak height ion intensities of all labeled species by the ion intensity of both labeled and unlabeled species, expressed in percent. Label-specific ion counts were corrected for naturally occurring 13C species (i.e., [M+1] and [M+2]). The relative abundance of each isotopically labeled species was determined by dividing the peak height ion intensity of each isotopic form (corrected for naturally occurring 13C species as above) by the summed peak height ion intensity of all labeled species.
Supplementary Material
Acknowledgments
We thank E. Hwang, T. Odaira, and S. Puckett for excellent technical support. We thank Luiz Pedro S. de Carvalho for helpful discussions, G. Lin and C. Nathan for PrcB-specific antiserum, and J.S. Cox for plasmid pJSC284. This work was supported by National Institutes of Health (NIH) Grant R01AI63446 (to S.E), a Bill and Melinda Gates Foundation Grand Challenges Exploration Grant (to K.Y.R.), and a Burroughs Wellcome Career Award in the Biomedical Sciences (to K.Y.R.). J.M. was supported by NIH Grant T32 AI007621 and R01AI63446-03S1. The Department of Microbiology and Immunology acknowledges the support of the William Randolph Hearst Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1000715107/-/DCSupplemental.
References
- 1.Harries AD, Dye C. Tuberculosis. Ann Trop Med Parasitol. 2006;100:415–431. doi: 10.1179/136485906X91477. [DOI] [PubMed] [Google Scholar]
- 2.Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boshoff HI, Barry CE., 3rd Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Microbiol. 2005;3:70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz-Elías EJ, McKinney JD. Carbon metabolism of intracellular bacteria. Cell Microbiol. 2006;8:10–22. doi: 10.1111/j.1462-5822.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 5.Sauer U, Eikmanns BJ. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol Rev. 2005;29:765–794. doi: 10.1016/j.femsre.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz-Elías EJ, McKinney JD. Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat Med. 2005;11:638–644. doi: 10.1038/nm1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloch H, Segal W. Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J Bacteriol. 1956;72:132–141. doi: 10.1128/jb.72.2.132-141.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole ST, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Schnappinger D, et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: Insights into the phagosomal environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubnau E, Smith I. Mycobacterium tuberculosis gene expression in macrophages. Microbes Infect. 2003;5:629–637. doi: 10.1016/s1286-4579(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 11.Timm J, et al. Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc Natl Acad Sci USA. 2003;100:14321–14326. doi: 10.1073/pnas.2436197100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gould TA, van de Langemheen H, Muñoz-Elías EJ, McKinney JD, Sacchettini JC. Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Mol Microbiol. 2006;61:940–947. doi: 10.1111/j.1365-2958.2006.05297.x. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz-Elías EJ, Upton AM, Cherian J, McKinney JD. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol. 2006;60:1109–1122. doi: 10.1111/j.1365-2958.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- 14.Upton AM, McKinney JD. Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology. 2007;153:3973–3982. doi: 10.1099/mic.0.2007/011726-0. [DOI] [PubMed] [Google Scholar]
- 15.Matte A, Tari LW, Goldie H, Delbaere LT. Structure and mechanism of phosphoenolpyruvate carboxykinase. J Biol Chem. 1997;272:8105–8108. doi: 10.1074/jbc.272.13.8105. [DOI] [PubMed] [Google Scholar]
- 16.Hanson RW. Thematic minireview series: A perspective on the biology of phosphoenolpyruvate carboxykinase 55 years after its discovery. J Biol Chem. 2009;284:27021–27023. doi: 10.1074/jbc.R109.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukhopadhyay B, Concar EM, Wolfe RS. A GTP-dependent vertebrate-type phosphoenolpyruvate carboxykinase from Mycobacterium smegmatis. J Biol Chem. 2001;276:16137–16145. doi: 10.1074/jbc.M008960200. [DOI] [PubMed] [Google Scholar]
- 18.Collins DM, et al. Production of avirulent mutants of Mycobacterium bovis with vaccine properties by the use of illegitimate recombination and screening of stationary-phase cultures. Microbiology. 2002;148:3019–3027. doi: 10.1099/00221287-148-10-3019. [DOI] [PubMed] [Google Scholar]
- 19.Liu KY, Yu JZ, Russell DG. pckA-deficient Mycobacterium bovis BCG shows attenuated virulence in mice and in macrophages. Microbiology. 2003;149:1829–1835. doi: 10.1099/mic.0.26234-0. [DOI] [PubMed] [Google Scholar]
- 20.Klotzsche M, Ehrt S, Schnappinger D. Improved tetracycline repressors for gene silencing in mycobacteria. Nucleic Acids Res. 2009;37:1778–1788. doi: 10.1093/nar/gkp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrt S, et al. Controlling gene expression in mycobacteria with anhydrotetracycline and Tet repressor. Nucleic Acids Res. 2005;33:e21. doi: 10.1093/nar/gni013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rej R. Oxaloacetate, UV-method. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Weinheim 3rd Ed, Vol VII: Verlag Chemie; 1985. pp. 59–67. [Google Scholar]
- 23.Petersen S, et al. Metabolic consequences of altered phosphoenolpyruvate carboxykinase activity in Corynebacterium glutamicum reveal anaplerotic regulation mechanisms in vivo. Metab Eng. 2001;3:344–361. doi: 10.1006/mben.2001.0198. [DOI] [PubMed] [Google Scholar]
- 24.Camacho LR, Ensergueix D, Perez E, Gicquel B, Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol. 1999;34:257–267. doi: 10.1046/j.1365-2958.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 25.Cox JS, Chen B, McNeil M, Jacobs WR., Jr. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 26.Domenech P, Reed MB. Rapid and spontaneous loss of phthiocerol dimycocerosate (PDIM) from Mycobacterium tuberculosis grown in vitro: Implications for virulence studies. Microbiology. 2009;155:3532–3543. doi: 10.1099/mic.0.029199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kana BD, et al. The resuscitation-promoting factors of Mycobacterium tuberculosis are required for virulence and resuscitation from dormancy but are collectively dispensable for growth in vitro. Mol Microbiol. 2008;67:672–684. doi: 10.1111/j.1365-2958.2007.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjunatha UH, et al. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103:431–436. doi: 10.1073/pnas.0508392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandotra S, Schnappinger D, Monteleone M, Hillen W, Ehrt S. In vivo gene silencing identifies the Mycobacterium tuberculosis proteasome as essential for the bacteria to persist in mice. Nat Med. 2007;13:1515–1520. doi: 10.1038/nm1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savvi S, et al. Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: Implications for propionate metabolism during growth on fatty acids. J Bacteriol. 2008;190:3886–3895. doi: 10.1128/JB.01767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gengenbacher M, Rao SP, Pethe K, Dick T. Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology. 2009;156:81–87. doi: 10.1099/mic.0.033084-0. [DOI] [PubMed] [Google Scholar]
- 32.Barry CE., 3rd Interpreting cell wall ‘virulence factors’ of Mycobacterium tuberculosis. Trends Microbiol. 2001;9:237–241. doi: 10.1016/s0966-842x(01)02018-2. [DOI] [PubMed] [Google Scholar]
- 33.Brennan PJ, Nikaido H. The envelope of mycobacteria. Annu Rev Biochem. 1995;64:29–63. doi: 10.1146/annurev.bi.64.070195.000333. [DOI] [PubMed] [Google Scholar]
- 34.Brauer MJ, et al. Conservation of the metabolomic response to starvation across two divergent microbes. Proc Natl Acad Sci USA. 2006;103:19302–19307. doi: 10.1073/pnas.0609508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bardarov S, et al. Conditionally replicating mycobacteriophages: A system for transposon delivery to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1997;94:10961–10966. doi: 10.1073/pnas.94.20.10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glickman MS, Cox JS, Jacobs WR., Jr A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol Cell. 2000;5:717–727. doi: 10.1016/s1097-2765(00)80250-6. [DOI] [PubMed] [Google Scholar]
- 37.Vandal OH, Nathan CF, Ehrt S. Acid resistance in Mycobacterium tuberculosis. J Bacteriol. 2009;191:4714–4721. doi: 10.1128/JB.00305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pesek JJ, Matyska MT, Fischer SM, Sana TR. Analysis of hydrophilic metabolites by high-performance liquid chromatography-mass spectrometry using a silica hydride-based stationary phase. J Chromatogr A. 2008;1204:48–55. doi: 10.1016/j.chroma.2008.07.077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.