Abstract
Objectives:
To study the antidiabetic activity of Barleria prionitis Linn in normal and alloxan-induced diabetic rats.
Materials and Methods:
Alcoholic extract of leaf and root of B. prionitis was tested for their antidiabetic activity. Albino rats were divided into six groups of six animals each. In three groups, diabetes was induced using alloxan monohydrate (150 mg/kg b.w., i.p.) and all the rats were given different treatments consisting of vehicle, alcoholic extract of leaves, and alcoholic extract roots of B. prionitis Linn (200 mg/kg) for 14 days. The same treatment was given to the other three groups, comprising non-diabetic (normal) animals. Blood glucose level, glycosylated hemoglobin, liver glycogen, serum insulin, and body weight were estimated in normal and alloxan-induced diabetic rats, before and 2 weeks after administration of drugs.
Results:
Animals treated with the alcoholic extract of leaves of B. prionitis Linn showed a significant decrease in blood glucose level (P<0.01) and glycosylated hemoglobin (P<0.01). A significant increase was observed in serum insulin level (P<0.01) and liver glycogen level (P<0.05), whereas the decrease in the body weight was arrested by administration of leaf extract to the animals. The alcoholic extract of roots showed a moderate but non-significant antidiabetic activity in experimental animals.
Conclusion:
The study reveals that the alcoholic leaf extract of B. prionitis could be added in the list of herbal preparations beneficial in diabetes mellitus.
Keywords: Barleria prionitis, alloxan monohydrate, alcoholic extract
Introduction
Diabetes mellitus is the most important non-infective epidemic to hit the globe in the present millennium. By the year 2025, India shall have the maximum number of diabetics in the world making it, the “Diabetic capital of the world.”[1] Despite the great strides, made in understanding and management of diabetes, the disease and disease-related complications are increasing unabated due to multiple defects, in its pathophysiology.[2] Parallel, to this, the holistic approach of herbs has accelerated the global efforts to harness and harvest medicinal plants having multiple beneficial effects.[3] Some of them have been evaluated and active principles isolated; however, the search for novel antidiabetic drugs continues.[4]
Barleria prionitis Linn (Acanthaceae) is a well-known plant in Ayurveda. It is distributed throughout India, Ceylon, and South Asia.[5] The plant is said to be rich in potassium and valued as diuretic.[6] Flavonoids, iridoid glucosides, and fatty acids have also been reported.[7‐9] The extract of plant rich in iridoid glycosides is a potent hepatoprotective agent[8] and useful in respiratory infections,[10] whooping cough, and tuberculosis.[11] The juice of leaves is useful in fungal infections,[12] wound healing, bleeding teeth, toothache, and joint pain.[13,14] The roots are used in fever and glandular swelling and have been shown to 100% antifertility activity.[15] The plant has many uses but the antidiabetic potential of the plant is yet to be explored, so B. prionitis was selected for the present study.
Materials and Methods
Plant Material and Extract Preparation
Fresh leaves and roots of healthy mature plants of B. prionitis after authentification and verification (RUBL20108) were collected from the medicinal garden of Lal Bahadur Shastri College of Pharmacy, Jaipur, where it grows. The leaves and roots were dried under shade, coarsely powdered and were packed separately in airtight containers.
Alcoholic Extract
The dried plant material was coarsely powdered. The powdered mass of each part was defatted with petroleum ether (60-80°C) followed by extraction with alcohol (95% v/v) and water. The yield was found 2.16% in roots and16.64% in leaves.
The dried alcoholic extract was formulated as suspension using distilled water and the strength of the suspension adjusted according to the dose administered (i.e. 200 mg/kg).
Both the alcoholic extract of roots and leaves drug were administered orally twice a day, for 2 weeks, in a dose of 200 mg/kg body weight, with the help of a gastric catheter.
Preliminary Phytochemical Screening
The chromatographic analysis of the root and leaf extract did not show the presence of the alkaloids as per monograph in the 3rd volume of Ayurvedic pharmacopoeia. Beta-sitosterol, saponins, tannins, and flavonoids were found present in both the root and the leaf alcoholic extract.[16]
Animals
Adult albino rats of either sex weighing between 180 and 200 g were acclimatized for a period of 7 days at room temperature (25±2°C) and 50±15% relative humidity. They were housed in a standard cage and maintained on standard pellets and water at libitum. The animals described as “fasted” were deprived of food for 18 h, but had free access to water. The study was carried out in the Toxicology Lab of Zoology Department, University of Rajasthan, and the study protocol was approved by the Institutional Ethics Committee.
Toxicity Studies
Adult albino rats were used for this study. The alcoholic extract of roots and leaves of B. prionitis Linn at different dose levels i.e. 1, 1.5, 2, 2.5 g/kg body weight was administered orally. The control group received distilled water. The animals were observed for 14 days, for mortality and general behavior. No death was observed upto the end of the study. The test samples were found safe up to 2.5 g/kg.
Induction of Diabetes
A single dose (150 mg/kg b.w., i.p.) of alloxan monohydrate (Sigma Ltd, USA) dissolved in normal saline was used for induction of Type II diabetes in rats after overnight fasting. After 1 h of alloxan administration, the animals were fed standard pellets and water ad libitum. The animals were stabilized for a week and animals showing blood glucose level (estimated by GOD-POD method) more than 200 mg/dl were selected for the study.
Experimental Design
The fasted rats were divided into six groups of six animals each (three group of normal animals and three groups for induction of diabetes). No standard of comparison was used.
Group I- Served as normal control rats and received distilled water .
Group II- Diabetic rats served as diabetic control and received distilled water.
Group III- Diabetic rats received alcoholic extract of leaves (200 mg/kg b.w.) using an intra gastric tube for 2 weeks.
Group IV- Normal rats received alcoholic extract of leaves (200 mg/kg b.w.) using an intra gastric tube for 2 weeks.
Group V- Diabetic rats received alcoholic extract of roots (200 mg/kg b.w.) using an intra gastric tube for 2 weeks.
GroupVI- Normal rats received alcoholic extract of roots (200 mg/kg b.w.) using an intra gastric tube for 2 weeks.
The drug treatment was carried out every day morning (200 mg/kg b.w.) and evening (200 mg/kg b.w.) with the help of intragastric tube for 2 weeks.
After 2 weeks, body weights were determined and the animals were sacrificed under the influence of anesthetic ether. The blood was collected by heart puncture and the liver was excised and chilled in ice cold 0.9% sodium chloride.
Methods
The blood sample withdrawn from the sacrificed animals was centrifuged at 3000 rpm for 10 min.[17] Blood glucose,[18] glycosylated hemoglobin,[19] and serum insulin (RIA using a kit from BARC, Mumbai, India Ltd) were estimated on the 15th day. The excised liver tissue was processed and liver glycogen was estimated.[20]
Statistical Analysis
All the values were expressed as mean ± SEM. The data obtained through careful observation were analyzed using Student's t-test. Wherever required ANOVA followed by Dunnets multiple 't'-test was used. A “P” Value of less than 0.05% was considered statistically significant.
Results
Effect on Blood Glucose
Analysis of data shows a decrease in the blood glucose level on treatment with the alcoholic extract of leaves and roots (200 mg/kg, orally for 2 weeks). The alcoholic leaf extract exhibited a statistically significant decrease (P<0.01) in the blood glucose level when comparison was done with the diabetic control group and between before and after treatment. But the decrease with the alcoholic root extract was statistically non-significant [Figures 1 and 2]. Both the test drugs i.e. alcoholic extract of leaves and roots did not affect the blood glucose in normal rats [Figure 3].
Figure 1.
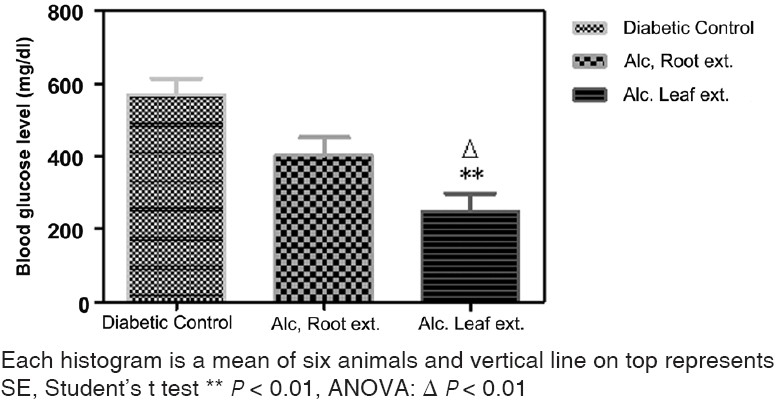
Effect of leaf and root extract of Barleria prionitis Linn. On blood glucose level in alloxan induced diabetic rats.
Figure 2.
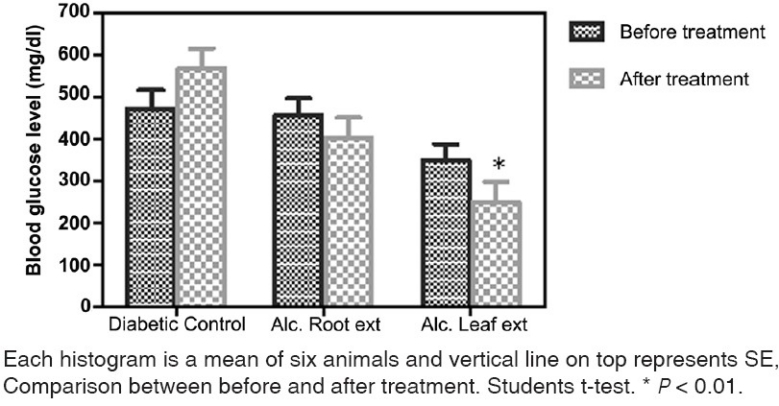
Efeect of leaf and root extract of Barleria prionitis Linn. On blood glucose level in alloxan induced diabetic rats
Figure 3.
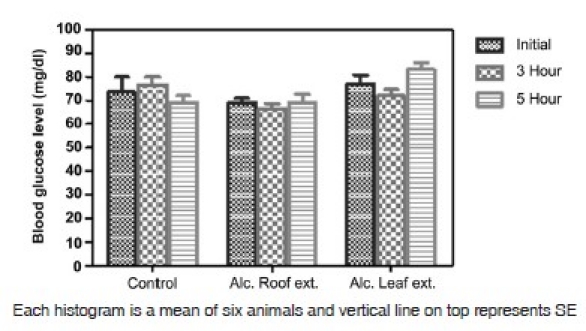
Effect of leaf and root extract of Barleria prionitis linn. On blood glucose level in normal rats
Effect on Serum Insulin Level
Insulin level was found to be decreased in the alloxan-induced diabetic rats. On administration of both leaf extract (P<0.01) and root extract (P<0.05), there was an increase in serum insulin level which was statistically significant [Table 1].
Table 1.
Effect of alcoholic extract of leaves and roots of Barleria prionitis Linn. on serum insulin, liver glycogen, and glycosylated hemoglobin
| Parameters | Normal control | Diabetic control | Root treated | % Change | Leaf treated | % Change |
|---|---|---|---|---|---|---|
| Serum insulin | 43.40 ± 2.71 | 20.00 ± 1.53 | 26.00 ± 2.61* | ↑30.00@ | 46.30 ± 4.54** | ↑130.00@ |
| Liver glycogen | 4.20 ± 0.27 | 1.81 ± 0.17 | 2.65 ± 0.62 | ↑46.40@ | 3.56 ± 0.64* | ↑96.68@ |
| Glycosylated hemoglobin | 6.00 ± 0.81 | 09.00 ± 0.64** | 08.00 ± 0.89 | ↓11.00@ | 7.00 ± 0.71** | ↓22.00@ |
Each value represents the mean ± SEM of six observations, Student's paired 't'-test :
P < 0.05,
P < 0.01
-Decrease
- Increase
- comparison to the diabetic control group
Effect on Glycosylated Hemoglobin
A statistically significant increase (P<0.01) was seen in the level of the glycosylated hemoglobin in the diabetic control group. The alcoholic leaf extract significantly decreased (P<0.01) the glycosylated hemoglobin level, but a moderate and non-significant decrease was seen with alcoholic root extract [Table 1].
Effect on Liver Glycogen
Depletion of liver glycogen content was seen in the diabetic control group. A significant increase (P<0.05) in the glycogen content of liver was observed after administration of alcoholic leaf extract, but increase with alcoholic root extract was statistically not significant [Table 1].
Effect on Body Weight
Body weight of alloxan-induced diabetic rat was found to be statistically less compared to the normal rats at basal level (before treatment) (P<0.01). Weight gain was not observed after the treatment with either of the test drugs, but the decrease in body weight in alcoholic leaf extract group was found to be very negligible, however statistically non-significant [Figure 4].
Figure 4.
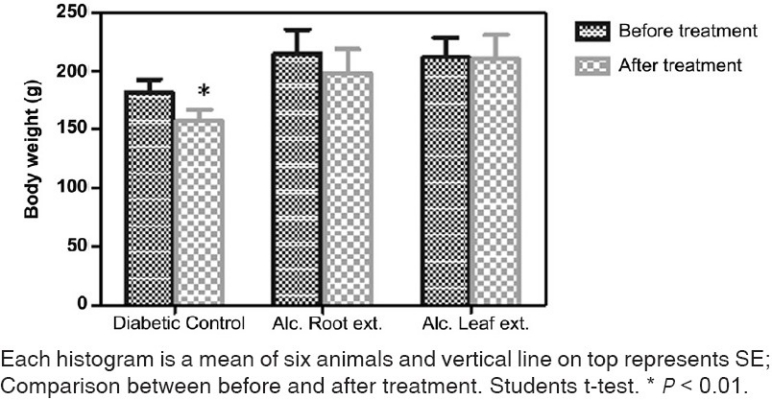
Effect of leaf and root extract of Barleria prionitis linn. On body weight in alloxan induced diabetic rats.
Discussion
The study reports the antidiabetic activity of alcoholic extract of leaves of B. prionitis Linn, which is a well-known herb in Ayurveda. Phytochemical analysis of B. prionitis shows the presence of sterols, saponins, tannins, and flavonoids. Flavonoids, sterols/triterpenoids, tannins, and phenolics are known bioactive antidiabetic principles.[2,3,21] Flavonoids are also known to regenerate the damaged beta cells in the alloxan diabetic rats.[22]
In the present study, alloxan was used as a diabetogen. It induces diabetes by destroying β-cells of the pancreas partially, through production of reactive oxygen species.[23] Alcoholic extract of leaves and roots were assessed for their antidiabetic activity. The alcoholic leaf extract exhibited a significant decrease in the blood glucose level in alloxan-induced diabetic animals. A non-significant decrease was seen with the alcoholic root extract. But both the treatments did not produce hypoglycemia in normal rats, which is a therapeutic advantage.
Insulin level was found decreased in alloxan-induced diabetic rats. Reversal of this effect was seen on treatment by the leaf extract This may be indicative of regeneration of the islet cells more by the leaf extract, and possibly, attenuation of the alloxan initiated degenerative changes more prominently in the leaf extract-treated group as compared to the root extract-treated group. The same treatment, however, did not increase the serum insulin level in normoglycemic rats, so it can be concluded that the extract has the potential to enhance the glucose-dependent insulin release from the pancreatic beta cells and thereby decrease the blood glucose level only in alloxan-induced diabetic rats. The action of extract is very similar to biguanides, which are also termed as “Euglycemics.” Biguanides brings the elevated blood sugar level to its normal value and do not produce hypoglycemia. Biguanides promote peripheral uptake and utilization of blood glucose and they also exhibit a favorable effect on lipid profile i.e. a decrease in the TGs.[24] In a study conducted, B. prionitis has been reported as a potent hepatoprotective, showing a highly significant decrease in TGs.[8] This suggests that the mechanism of studied herb is similar to that of biguanides.
In diabetes, glycogen content decreases due to enhanced glycogenolysis and the normal capacity of the liver to synthesize glycogen is impaired, which is due to insulin deficiency.[25] The liver glycogen was found depleted in the diabetic control group. A significant increase in the liver glycogen level on administration of alcoholic leaf extract was observed which may be due to an increase in the insulin level by it. Reversal of the depletion indicates attenuation of severity of diabetes and can be considered as an index of the presence of antidiabetic activity in the test drug.
Protein can universally bind non-enzymatically with glucose or other sugars present in the vicinity. The degree of glycation is directly proportional to the concentration of the sugar present in the surrounding medium. Therefore, estimation of glycosylated hemoglobin (HbA1c) gives an accurate reflection of mean plasma glucose concentration over this period and correlates best with the degree of the glycemia.[26] A change in HbA1c of 1% would reflect a blood glucose alteration of about 30 mg%. A significant decrease with leaf extract (P<0.01) was observed in the treated rats as compared to alloxan-induced diabetic rats. On treatment with roots, the decrease was moderate. This is indicative of a better glycemic control for a longer period by the leaf sample.
A significant reduction in the body weight was observed in the alloxan-induced diabetic rats. The decrease in the weight in diabetes is due to continuous excretion of glucose and decrease in peripheral uptake of glucose and glycogen synthesis.[27] The decrease in weight was arrested on administration of alcoholic leaf extract to a greater extent as compared to root extract. All the above observations suggest that the test drug i.e. alcoholic leaf extract can be a promising antidiabetic.
Conclusion
Studies revealed that alcoholic leaf extract of B. prionitis can be considered as an important addition to the therapeutic armamentarium for the treatment of diabetes. Further studies can be undertaken at the cellular and molecular level, which may further elucidate its mechanism in detail.
Acknowledgments
The authors are thankful to Emeritus Professor Dr. V N Sharma and Professor Rakesh Gupta for providing necessary guidance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hillary K, Ronald EA, William HH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates and projections. Diabetes Care. 1998;21:141–3. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Ivorra MD, Payá M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27:243–75. doi: 10.1016/0378-8741(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 3.Kameswara Rao, Giri, R, Kesavulu MM, Apparao Herbal medicine in the treatment of diabetes mellitus. Manphar Vaidhya Patrika. 1997;1:33–5. [Google Scholar]
- 4.Nagarajan S, Jain HC, Aulakh GS. Indigenous plants used in the control of Diabetes, Publication and Information Directorate. New Delhi: CSIR; 1987. p. 586. [Google Scholar]
- 5.Burkill HM. The useful plants of West Tropical Africa. UK: Royal Botanic Garden Kew; 1985. p. 960. [Google Scholar]
- 6.Gujral ML, Saxena PN, Mishra SS. An experimental study of the comparative activity of indigenous diuretics. J Indian Med Assoc. 1995;25:49. [PubMed] [Google Scholar]
- 7.Nagarjuna S, Barnabas CGG. Antimicrobial activity of Flavanoids of Plant extracts. Department of Chemistry, Bharatidasan University T.N : 1986. [Google Scholar]
- 8.Singh B, Chandan BK, Prabhakar A, Taneja SC, Singh J, Qazi GN. Chemistry and hepatoprotective activity of an active fraction from Barleria prionitis Linn.in experimental animals. Phytother Res. 2005;18:391–404. doi: 10.1002/ptr.1509. [DOI] [PubMed] [Google Scholar]
- 9.Gupta HM, Saxena VK. Chemical examination of the glyceride contents of the Roots of B.Prionitis Linn: Bulletin of Medico-Ethno Botanical Research. New Delhi: CCRAS; 1986. pp. 178–83. [Google Scholar]
- 10.Chen JL, Blanc P, Stoddart CA, Bogan M, Rozhon EJ, Parkinson N, et al. New iridoids from the medicinal plant Barleria prionitis with potent activity against respiratory syncytial virus. J Nat Prod. 1998;612:1295–7. doi: 10.1021/np980086y. [DOI] [PubMed] [Google Scholar]
- 11.Oomachan, MM Ethno-botanical and conservation aspects of Medicinal Plants of Madhya Pradesh. In J of Pure and Appli Sci. 1991;6:39. [Google Scholar]
- 12.Panwar HS, Nauriyal MM, Joshi HC. In vitro screening of certain indigenous plants for their antimycotic activity. Veterinary Res Bull. 1979;2:164–7. [Google Scholar]
- 13.Parrotta, JA . Healing plants of Peninsular India. Wellington, UK & New York: CABI Publishing; 2001. p. 917. [Google Scholar]
- 14.Kakrani HN, Saliya AK. Traditional treatment through herbs in Kutch district, Gujarat State, India Part-II: Analgesic, anti-inflammatory, antirheumatic, anti-arthritic plants Fitoterapia. 1994;5:427. [Google Scholar]
- 15.Gupta RS, Kumar P, Dixit VP, Dobhal MP. Antifertility studies of the root extract of the Barleria prionitis Linn in male albino rats with special reference to testicular cell population dynamics. J Ethnopharmacol. 2000;70:111–7. doi: 10.1016/s0378-8741(99)00150-6. [DOI] [PubMed] [Google Scholar]
- 16.Pharmacopoeial standards for Ayurvedic Formulations Central Council for Research in Ayurveda and Siddha, Ministry of Health and Family Welfare, Government of India. 1987:41–60. [Google Scholar]
- 17.Basu V, Gangadevi T, Subramaniam A. Antihypoglycemic activity of cassia kleini leaf extract in glucose fed normal rats and alloxan induced diabetic rats. Indian J pharmacol. 2002;34:209–15. [Google Scholar]
- 18.Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol. 1969;22:246. doi: 10.1136/jcp.22.2.246-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker KM, England JD, Da Costa J, Hess RL, Goldstein DE. Improved colorimetric assay for glycosylated hemoglobin. Clin Chem. 1981;27:669–72. [PubMed] [Google Scholar]
- 20.Seifter S, Dayton S, Novice B, Muntwyler E. The estimation of glycogen with anthrone reagent. Arch Biochem. 1950;25:191–200. [PubMed] [Google Scholar]
- 21.Atta-Ur-Rahman, Zaman K. Medicinal plants with hypoglycemic activity. J Ethnopharmacol. 1989;26:1–55. doi: 10.1016/0378-8741(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 22.Chakkvarthy BK, Gupta S, Gambir SS, Gode KD. Pancreatic Beta cell Regeneration.A novel antidiabetic mechanism of Pterocarpus marsupium Roxb. Indian J Pharma. 1980;12:123–7. [Google Scholar]
- 23.Malaisse WJ. Alloxan toxicity to the pancreatic B-cell: A new hypothesis. Biochem Pharmacol. 1982;31:3527–34. doi: 10.1016/0006-2952(82)90571-8. [DOI] [PubMed] [Google Scholar]
- 24.Dagogo-Jack S, Santiago JV. Pathophysiology of type 2 diabetes and modes of action of therapeutic interventions. Arch Intern Med. 1997;157:1802–17. [PubMed] [Google Scholar]
- 25.Yki-Järvinen H, Taskinen MR. Interrelationships among insulin's antilipolytic and glucoregulatory effects and plasma triglycerides in nondiabetic and diabetic patients with endogenous hypertriglyceridemia. Diabetes. 1988;37:1271–8. doi: 10.2337/diab.37.9.1271. [DOI] [PubMed] [Google Scholar]
- 26.Danze PM, Tarjoman A, Rousseaux J, Fossati P, Dautrevaux M. Evidence for an increased glycation of IgG in diabetic patients. Clin Chim Acta. 1987;166:143–53. doi: 10.1016/0009-8981(87)90416-5. [DOI] [PubMed] [Google Scholar]
- 27.Defronzo RA, Bonadonna RC, Ferrannini I. Pathogenesis of type 2 (non-insulin dependent) diabetes mellitus: A balanced overview. Diabetologia. 1992;35:389–97. doi: 10.1007/BF00401208. [DOI] [PubMed] [Google Scholar]


