Abstract
Changes in several regions within the brain have been associated with progression from healthy aging to Alzheimer's disease (AD), including the hippocampus, entorhinal cortex, and more recently, the inferior parietal lobule (IPL). In this study, the IPL was divided into three subregions: the gyrus, the banks of the sulcus, and the fundus to determine if these regions are independent of medial temporal regions in the progression of AD. Participants of the Alzheimer's Disease Neuroimaging Initiative (ADNI; n=54) underwent a structural MRI scan and neuropsychological exam, and were categorized as normal controls, mild cognitively impaired (MCI), or AD. FreeSurfer was initially used to identify the boundaries of the IPL. Each subregion was then manually traced based on FreeSurfer curvature intensities. Multivariate analyses of variance were used to compare groups. Results suggest that changes in thickness of the banks of the inferior parietal lobule are occurring early in the progression from normal to MCI, followed by changes in the gyrus and fundus, and these measures are related to neuropsychological performance.
Keywords: Alzheimer's disease, MCI, MRI, Inferior parietal lobule
1. Introduction
Alzheimer's disease (AD) is the most common form of dementia, and is the fifth leading cause of death in individuals over the age of 65. There are currently no definitive means for detecting who will develop AD. Magnetic resonance imaging (MRI) has provided a useful in vivo method for assessing changes that occur in the brain during the progression from healthy aging to AD, and offer potential means for identifying individuals in the presymptomatic stages, where treatment may delay or prevent the onset of this disease. MRI also offers the ability to track AD over time. As MRI post-processing techniques have been improved, the investigation of markers for development and progression to Alzheimer's disease has advanced. Several regions of interest have been intensely studied in subjects with both MCI and AD, such as the hippocampus and entorhinal cortex. More recently, regions beyond the medial temporal lobe such as the inferior parietal lobule (IPL) have begun to show promise as markers of AD.
The inferior parietal lobule is extending between the supramarginal gyrus rostrally, the lateral occipital cortex caudally, the superior parietal gyrus medially, and the middle temporal gyrus laterally (Desikan et al., 2006). Functionally, the IPL is involved with sensory and motor association. Both amyloid plaques and neurofibrillary tangles have been found at the time of autopsy in the IPL in MCI and AD subjects (Braak & Braak, 1991; Markesbery et al., 2006; Nelson et al., 2009). Studies in non-human primates have demonstrated connections between the IPL and both the entorhinal cortex (Ding et al., 2000; Van Hoesen & Pandya, 1975), and the hippocampus (Clower et al., 2001), suggesting that the IPL is a part of the memory circuitry. As such, pathology in this region may be contributing to the memory impairment that is a hallmark of AD.
Mild cognitive impairment (MCI) is thought to represent a preclinical stage of AD (Bennett et al., 2005; Morris et al., 2001; Petersen et al., 2006; Whitwell et al., 2007). Studies have found atrophy of parietal regions in the presymptomatic stages of AD (Desikan et al., 2009; Fan et al., 2008; Fox et al., 2001; Scahill et al., 2002; Whitwell et al., 2008), and that increased rates of atrophy in this region have been associated with conversion from MCI to AD (Desikan et al., 2008). Metabolic function in the IPL combined with genetic risk (carrying at least one copy of the APOE-4 allele) has been found to predict subsequent cognitive decline in normal subjects (Small et al., 2000). In addition, differences in blood flow to the IPL have been found in AD subjects both during rest (Scarmeas et al., 2004) and when performing memory tasks (Remy et al., 2005).
Because the IPL is a rather large structure, the purpose of this study was to determine if subregions within the IPL are differentially affected in the progression from normal to AD. In this study, the IPL was subdivided into the gyrus (G-IPL), the banks (B-IPL), and the fundus (F- IPL) based MRI measurements in normal (NC), MCI, and AD participants of the Alzheimer's Disease Neuroimaging Initiative.
2. Methods
2.1. Study population
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI). The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public-private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials.
The Principle Investigator of this initiative is Michael W. Weiner, M.D., VA Medical Center and University of California — San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada. The initial goal of ADNI was to recruit 800 adults, ages 55 to 90, to participate in the research -- approximately 200 cognitively normal older individuals to be followed for 3 years, 400 people with MCI to be followed for 3 years, and 200 people with early AD to be followed for 2 years.
The inclusion criteria for each group are as follows: NC: Mini Mental Status Exam (MMSE; Folstein et al., 1975) scores between 24-30, a Clinical Dementia Rating scale (CDR) (Morris, 1993) of 0, non-depressed, non-MCI, and non-demented; MCI (amnestic and multiple domain with memory impairment): subjective and objective memory complaint, MMSE scores between 24-30, objective memory loss measured by education adjusted scores on Wechsler Memory Scale Logical Memory II, a CDR of 0.5, absence of significant levels of impairment in other cognitive domains, essentially preserved activities of daily living, absence of dementia; AD (mild): MMSE scores between 20-26, CDR of 0.5 or 1.0, and meets NINCDS/ADRDA criteria (McKhann et al., 1984) for probable AD.
Participants underwent a neuropsychological battery at screening and MRI scanning at baseline, which are the data being evaluated in this study. Fifty-four participants (18 NC [55% female, average age 78.3], 18 MCI [50% female, average age 76.9], 18 AD [50% female, average age 76.6]) were pseudo-randomly selected (randomly selected from within each group by gender) from the larger pool of approximately 800 baseline scans in the ADNI to be included in the current study. The operator was blind to gender and group membership. Demographics, including age, education, and MMSE were available for all 54 participants included in this study (Table 1).
Table 1.
Demographics
| Normal | MCI All | MCI Stable | MCI Converters | AD | |
|---|---|---|---|---|---|
| Gender | 55% F | 50% F | 39% F | 80% F | 50% F |
| Age | 78.3 | 76.9 | 79 | 71.3 | 76.7 |
| Education | 16.3 | 15.9 | 15.2 | 17.8 | 15.1 |
| MMSE | 29.4 | 26.7 | 26.8 | 26.4 | 23.1 |
2.2. MRI acquisition and post-processing techniques
The imaging methods utilized by the ADNI have been described in detail previously (Jack et al., 2008). Scans were acquired from three manufacturers (General Electric Healthcare, Siemens Medical Solutions, and Philips Medical Systems) utilizing calibration techniques to maintain consistent protocols across scanners and sites. Raw dicom data of T1-weighted MP-RAGE scans acquired from 1.5 Tesla scanners at baseline visits from all 54 participants were obtained via the ADNI database (http://www.loni.ucla.edu/ADNI/). Images were processed through FreeSurfer version 4.0.3, a software program freely available at http://surfer.nmr.mgh.harvard.edu/, to obtain measurements of the IPL. The details of this post-processing program have been described elsewhere (Dale & Sereno, 1993; Dale et al., 1999; Fischl et al., 1999a; Fischl et al., 1999b; Fischl & Dale, 2000; Fischl et al., 2001; Fischl et al., 2002; Fischl et al., 2004a; Fischl et al., 2004b; Han et al., 2006; Jovicich et al., 2006; Segonne et al., 2004). Briefly, images were motion-corrected, intensity normalized using non-parametric, non-uniform (N3) correction (Sled et al., 1998), and stripped of non-brain tissue (Segonne et al., 2004). Images underwent a subcortical segmentation (Fischl et al., 2002; Fischl et al., 2004a), identification of the gray/white matter boundary, automated topography correction (Fischl et al., 2001; Segonne et al., 2007), and surface deformation (Dale & Sereno, 1993; Dale et al., 1999; Fischl & Dale, 2000). Surface representation was reviewed by an anatomically trained operator (SJG), and edits to ensure accurate surfaces were completed where necessary.
Once reviewed, further processing was completed, including surface inflation (Fischl, Sereno, & Dale, 1999), registration to a neuroanatomical atlas based on cortical folding patterns (Desikan et al., 2006; Fischl, Sereno, & Dale, 1999a; Fischl et al., 2004b), and maps of curvature and sulcal depth. The boundaries of each region of interest within the neuroanatomical atlas were visually inspected by the same neuroanatomically trained operator (SJG), and edits to these boundaries were completed where needed. This produced measurements of several neocortical and non-neocortical regions of interest, including the inferior parietal lobule, entorhinal cortex, hippocampus, and intracranial volume (ICV).
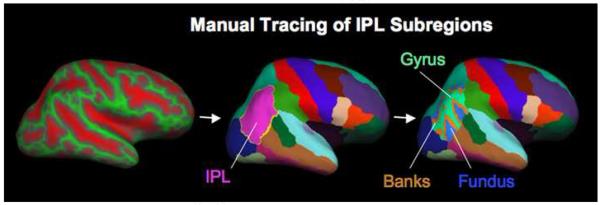
The boundaries of the IPL in this neuroanatomical atlas have been previously described (Desikan et al., 2006). This region of interest was manually subdivided into three regions by simultaneously viewing the neuroanatomical parcellation atlas and binary gray curvature maps on the inflated brain. The operator first manually traced the banks followed by the fundus of the right and left IPL by setting the curvature threshold to 0.0 and 0.55, respectively. The remainder of the IPL was then relabeled the gyrus of the IPL (Figure 1). Intrarater reliability was established by retracing these regions in a subset of participants (n=5), which demonstrated a correlation of 0.99 between tracings. This provided measurements of total surface area (TSA), average cortical thickness (ACT), and total gray matter volume (GMV) for each of the three subregions of the IPL: G-IPL, B-IPL, and F-IPL.
Figure 1.
Methods for manual tracing of each subregion of the inferior parietal lobule. First the sulcul-gyral pattern was loaded with the FreeSurfer parcellation atlas on the inflated brain, each subregion was then manually traced, and these subregions were then displayed with the parcellation atlas.
The GMV, TSA, and ACT of each IPL subregion, the entorhinal cortex, hippocampus, and neuropsychological (NP) performance were compared between groups using separate MANOVAs followed by Tukey pairwise comparisons when appropriate. Pearson correlations were used to determine the magnitude of the linear relationships between morphometric measures and neuropsychological performance. A principle components analysis was completed to determine which neuropsychological measures would be included in these correlations. Discriminant Function Analyses were used to determine the best predictors of group membership (NC, MCI, and AD) and converters versus non-converters in the MCI group.
2.3. Neuropsychological battery
The MMSE and Wechsler Memory Scale — Revised Logical Memory Immediate and Delayed (Wechsler, 1987) were administered at the screening visit. At the baseline visit, a battery of neuropsychological tests was given to all participants. This consisted of Alzheimer's Disease Assessment Scale Cognitive exam (Rosen, Mohs, & Davis, 1984), Clock Drawing Test, Auditory Verbal Learning test (AVLT)(Rey, 1964), Digit Span Forward and Backward, Category Fluency, Trail making A and B (Reitan, 1958), Wechsler Adult Intelligence Scale-Revised (WAIS-R) Symbol Digit Substitution, Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983), AVLT 30 minute Delay, and the American National Adult Reading Test.
3. Results
Kruskal-Wallis one-way analyses of variance were used to demonstrate that groups did not significantly differ in gender, age, or education. The MRI variables were compared using multivariate analyses of variance. It was found that except for the GMV of the fundus of the IPL in the right hemisphere, all GMV and ACT measures were significantly different between groups in both the right and left hemisphere (p<0.01). No TSA measures demonstrated a significant difference between groups.
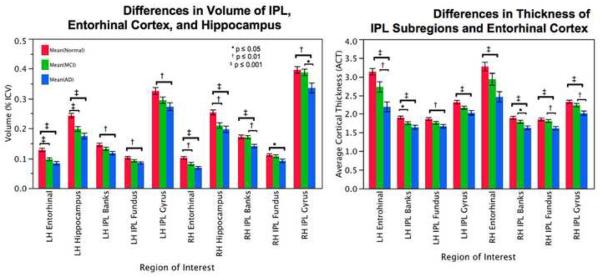
Results of the Tukey pairwise comparisons (Figure 2a) revealed more insight into these differences. When comparing volume in the right hemisphere, G-IPL was significantly different between NC and AD and MCI and AD; B-IPL was significantly different between NC and AD and MCI and AD; and F-IPL was significantly different between NC and AD. In the left hemisphere, GMV was significantly different for G-IPL between NC and AD; B-IPL was significantly different between NC and AD; and F-IPL was significantly different between NC and AD.
Figure 2.
a: Differences between NC, MCI, and AD in volume of the left and right subregions of the IPL, hippocampus, and entrohinal cortex. Volumes are expressed as percent intracranial volume (% ICV). b: Differences between NC, MCI, and AD in thickness of the left and right subregions of the IPL and entorhinal cortex.
When comparing thickness in regions of the right hemisphere (Figure 2b), G-IPL was significantly different between NC and AD and MCI and AD; B-IPL was significantly different between NC and AD and MCI and AD; and F-IPL was significantly different between NC and AD and MCI and AD. When comparing ACT in regions in the left hemisphere, G-IPL was significantly different between NC and AD; B-IPL was significantly different between NC and AD and NC and MCI; and F-IPL was significantly different between NC and AD. Since the main effect of group was not significant for the TSA measures, no follow up comparisons were made.
NP scores and p values demonstrating significant differences in NP performance are presented in Table 2. Traditionally NP tests have been portrayed as representing separate cognitive domains. Yet within each of the domain, sub-domains exist (i.e. cognitive flexibility versus set maintenance as subcomponents of executive function), NP tests have some crossover between domains and it is never fully clear how each sub-domain is affected by disease. In order to overcome these concerns, and to ensure that we understood how the various tests were inter-related in this dataset, a principle components analysis was used as a screening/data reduction tool to determine which of the NP tests to include in correlational analyses with the morphometric measurements. This broke the data down into three factors. The first factor consisted of Auditory Verbal Learning Immediate and Delay, the Boston Naming Test, and Categories; the second factor consisted of Trails A and Trails B, Digit Symbol, and Clock Command; and the third factor consisted of Trails B, Clock Command, Digit Span Forward, and Digit Span Backward. Scores from tests within each factor were selected for correlations in contrast to composite scores so that only known neuropsychological tests were evaluated. These included Clock Command, Auditory Verbal Learning Immediate and Delay, Digit Span Backward, Trails B, and Digit Symbol. Results of Pearson correlations were as follows (p<0.01): LH GMV of IPL Gyrus: Auditory Verbal Learning Delay; LH ACT of IPL Gyrus: Auditory Verbal Learning Delay; LH ACT of IPL Banks: Auditory Verbal Learning Delay; LH GMV of IPL Fundus: Auditory Verbal Learning Immediate, Trails B, and Digit Symbol; RH ACT of IPL Gyrus: Auditory Verbal Learning Delay and Digit Symbol; RH ACT of IPL Banks: Auditory Verbal Learning Delay and Digit Symbol; RH ACT of IPL Fundus: Auditory Verbal Learning Delay.
Table 2.
Neuropsychological Performance: Differences Between Groups
| Group Mean | p-values | |||||
|---|---|---|---|---|---|---|
| Test | Normal | MCI | AD | Normal vs MCI | MCI vs AD | Normal vs AD |
| Clock Command (Out of 5) | 4.67 | 4.33 | 3.33 | 0.483 | 0.003 | 0.000 |
| Clock Copy (Out of 5) | 4.78 | 4.72 | 4.44 | 0.964 | 0.405 | 0.276 |
| AV Immediate 1 (out of 15) | 4.94 | 4.28 | 3.11 | 0.424 | 0.080 | 0.003 |
| AV Immediate 2 | 7.39 | 5.17 | 4.22 | 0.001 | 0.246 | 0.000 |
| AV Immediate 3 | 9.50 | 6.44 | 4.50 | 0.001 | 0.040 | 0.000 |
| AV Immediate 4 | 10.61 | 7.06 | 4.89 | 0.000 | 0.018 | 0.000 |
| AV Immediate 5 | 11.39 | 7.06 | 5.06 | 0.000 | 0.056 | 0.000 |
| AV Interference | 4.72 | 4.00 | 3.00 | 0.226 | 0.063 | 0.001 |
| AV Immediate 6 | 8.61 | 2.83 | 1.67 | 0.000 | 0.401 | 0.003 |
| AV 30 Minute Delay | 12.72 | 8.61 | 6.17 | 0.004 | 0.123 | 0.000 |
| Digit Span Forward | 9.11 | 8.06 | 8.06 | 0.248 | 1.000 | 0.248 |
| Digit Span Backward | 7.39 | 6.28 | 5.33 | 0.224 | 0.336 | 0.009 |
| Categories-Animals | 19.56 | 16.50 | 12.56 | 0.193 | 0.069 | 0.001 |
| Categories-Vegetables | 15.44 | 10.61 | 8.22 | 0.001 | 0.157 | 0.000 |
| Trails A (Time to complete) | 32.06 | 39.61 | 52.50 | 0.302 | 0 036 | 0.001 |
| Trails B* (Time to complete) | 100.89 | 111.44 | 198.59 | 0.904 | 0.003 | 0.001 |
| Digit Symbol * | 48.11 | 38.67 | 29.12 | 0.019 | 0.020 | 0.000 |
| Boston Naming Test* | 27.94 | 24.50 | 20.82 | 0.094 | 0.074 | 0.000 |
One AD subject is missing from comparisons.
All regions of the IPL (RH and LH) and NP scores were entered into a discriminant function analysis to determine the best predictors of group membership, which revealed these were a combination of Auditory Verbal Learning Delay, Digit Symbol, and GMV of the banks of the right IPL, which were able to predict NC, MCI, and AD with 83.3%, 61.1% and 77.8% accuracy, respectively. The diagnoses of MCI subjects at future visits were evaluated, and five of the 18 MCI subjects in this study converted to AD between the 12-18 month visit. The same variables were entered into a second discriminant function analysis to determine the best predictors for future conversion from MCI to AD, which were the Boston Naming Test, Digit Span Backwards, and GMV of the fundus of the right IPL, which were able to predict stable MCI and converters with 92.3% and 100% accuracy, respectively.
When combined with measures of the hippocampus and entorhinal cortex, the measures that were most able to discriminate between NC, MCI, and AD were a combination of the GMV of the left entorhinal cortex, GMV of the right banks of the IPL, and ACT of the left entorhinal cortex, which were able to determine group membership with 66.7%, 50%, and 83.3%, respectively. When these measures were combined with total scores on the NP exam, group membership was best predicted for NC, MCI, and AD with Auditory Verbal Learning Delay, left hippocampus, GMV of the right banks of the IPL, and Digit Symbol, which were able to predict group membership with 88.9%, 72.2%, and 70.6% accuracy, respectively.
When assessing measures of the entorhinal cortex, hippocampus, and IPL subregions in MCI subjects, the measure that was able to predict stable versus converters was the TSA of the left entorhinal cortex, which was able to predict group membership with 84.6% and 80% accuracy, respectively.
4. Discussion
Little is known of how subregions of the IPL may be differentially affected in the progression to AD. In this study, three subregions of the IPL were compared among normal, MCI, and AD subjects. The first objective was to determine if these subregions of the IPL are differentially affected in the progression from normal to AD. Results suggest that not only are these regions differentially affected in the group of participants investigated, but also they are differentially affected in the right and the left hemisphere. Results also suggest that measures of these subregions of the IPL contribute information related to MCI and AD that is independent of entorhinal and hippocampal measures.
When comparing normal to MCI subjects, only one subregion of the IPL was found to differ between these groups, suggesting that the banks of the left IPL may be affected prior to the involvement of the gyrus and the fundus. In addition, while both the GMV and ACT demonstrated significant differences between groups, there were no significant differences between groups when assessing TSA. Since GMV is derived from the ACT and TSA measures this suggests that the differences in the GMV are driven by thickness changes rather that changes in surface area.
Few studies have addressed functional differences in the gyrus, banks, and fundus of the inferior parietal lobule. One study evaluated the contribution of regions of the parietal lobe in number processing (Chochon et al., 1999), and found that the banks of the intraparietal sulcus and deep portion of the postcentral sulcus are activated in number processing tasks, suggesting that these subregions may have unique functional roles in cognition. The present study identified differing relationships between the gyrus, banks, and fundus of the IPL in the right and left hemisphere and cognitive performance. It was found that while several subregions in both hemispheres were associated with performance on memory tasks, only a select few were associated with tasks involving numbers, including Digit Symbol (ACT of right gyrus and banks; GMV of left fundus) and Trails B (GMV of left fundus). This further suggests that these subregions may be functionally different, and therefore differentially affected in the progression to AD.
One further possibility is that these regions may be differentially affected due to perfusion differences in the gyrus, banks, and fundus of the IPL. Hypoperfusion of the inferior parietal region has been demonstrated in AD subjects when compared to those with MCI (Devanand et al., 2006; Grossman et al., 1997; Tranfaglia et al., 2009), and this may predict future progression from MCI to AD (Schroeter et al., 2009). It is possible that if this hypoperfusion is occurring differentially within the gyrus, banks, and sulcus of the IPL, this may contribute to differential effects in the progression from MCI to AD, contributing to the changes in the banks prior to the gyrus and fundus in the progression to AD.
The IPL has demonstrated laterality, both in correlations between volume and cognitive status (Keilp et al., 1996) and in atrophy related to progression to AD (Whitwell et al., 2008), with a predilection for the left IPL. This study was consistent with these findings, as the only significant difference between normal controls and MCI participants was in the average cortical thickness of the banks of the left IPL. However, significant differences between MCI and AD subjects occurred only in the right hemisphere in all measures of the subregions of the IPL (except for the GMV of the fundus), suggesting the right IPL may become more affected as subjects progress from MCI to AD. While the GMV of the fundus did not demonstrate significant differences between groups, it was found to be a predictor of future conversion from MCI to AD when combined with BNT, AV Delay, and Digit Span Backwards.
The second objective in this study was to investigate relationships between these subregions and neuropsychological performance. When considering NP performance, the only NP scores that were significantly different between the NC and MCI groups were auditory verbal learning immediate and delayed, Categories, Digit Symbol, and the Boston Naming Test. Significant correlations with the ACT of the banks of the left IPL (the only IPL subregion to demonstrate significant differences between NC and MCI) were found only with Auditory Verbal Learning Delay. There were no normal subjects included in this study who converted to MCI, and so it was not possible to determine predictors for conversion from normal to MCI. However, these data suggest that the above-mentioned test of memory may be sensitive to detecting the progression from normal to MCI.
When assessing MCI stable versus converters, the best predictors of future conversion were the Boston Naming Test, Digit Span Backwards, and GMV of the fundus of the right IPL. When the measure of the IPL were combined with those of the entorhinal cortex and hippocampus and entered into the discriminant function analysis, the IPL fell out of the results, leaving the TSA of the left entorhinal cortex to be the best predictor of conversion. However, the results of this second discriminant function analysis provided a less accurate prediction of future stable versus converters (here stable subjects were predicted with 84.6% accuracy compared with 92.3%, and converters were predicted with 80% as compared with 100% accuracy). This suggests that measures of the IPL are independent of entorhinal and hippocampal measures when comparing between groups and future conversion to AD.
To our knowledge no studies have investigated the differential effects of these subregions of the IPL in the progression to AD. This research suggests that detecting subtle changes within these subregions may identify individuals in the earliest stages of AD. These measures, combined with neuropsychological data, may provide useful biomarkers for future development of AD.
Limitations of this study included a small study population, due time intense tracing of each subregion of the IPL. It is possible that these initial tracings may be used to create an automated label for these subregions that can be incorporated into FreeSurfer for this specific data set and could be used to assess the entire ADNI study population in the future.
In addition, only the baseline visits of these participants were evaluated. While progression from MCI to AD was evaluated, the longitudinal change occurring in these subregions was not evaluated. Further investigation into the changes that may occur within each subregion of the IPL as participants are followed throughout the 36-month period of the ADNI project would likely provide further insight into the role of the IPL in the progression to AD.
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson and Johnson, Eli Lilly and Co., Medpace, Inc., Merck and Co., Inc., Novartis AG, Pfizer Inc, F. Hoffman-La Roche, Schering-Plough, Synarc, Inc., and Wyeth, as well as non-profit partners the Alzheimer's Association and Alzheimer's Drug Discovery Foundation, with participation from the U.S. Food and Drug Administration. Private sector contributions to ADNI are facilitated by the Foundation for the National Institutes of Health (www.fnih.org, http://www.fnih.org/, http://www.fnih.org, http://www.fnih.org/). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation. Funding for this specific project was supported by NIH grant AG000277.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Chochon F, Cohen L, van de Moortele PF, Dehaene S. Differential contributions of the left and right inferior parietal lobules to number processing. Journal of cognitive neuroscience. 1999;11:617–630. doi: 10.1162/089892999563689. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Cabral HJ, Fischl B, Guttmann CR, Blacker D, Hyman BT, Albert MS, Killiany RJ. Temporoparietal MR imaging measures of atrophy in subjects with mild cognitive impairment that predict subsequent diagnosis of alzheimer disease. AJNR.American journal of neuroradiology. 2009;30:532–538. doi: 10.3174/ajnr.A1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Fischl B, Cabral HJ, Kemper TL, Guttmann CR, Blacker D, Hyman BT, Albert MS, Killiany RJ. MRI measures of temporoparietal regions show differential rates of atrophy during prodromal AD. Neurology. 2008;71:819–825. doi: 10.1212/01.wnl.0000320055.57329.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Habeck CG, Tabert MH, Scarmeas N, Pelton GH, Moeller JR, Mensh BD, Tarabula T, Van Heertum RL, Stern Y. PET network abnormalities and cognitive decline in patients with mild cognitive impairment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:1327–1334. doi: 10.1038/sj.npp.1300942. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen G, Rockland KS. Inferior parietal lobule projections to the presubiculum and neighboring ventromedial temporal cortical areas. The Journal of comparative neurology. 2000;425:510–530. doi: 10.1002/1096-9861(20001002)425:4<510::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Fan Y, Resnick SM, Wu X, Davatzikos C. Structural and functional biomarkers of prodromal alzheimer's disease: A high-dimensional pattern classification study. NeuroImage. 2008;41:277–285. doi: 10.1016/j.neuroimage.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004a;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human brain mapping. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Cerebral cortex. Vol. 14. New York, N.Y.: 2004b. 1991. Automatically parcellating the human cerebral cortex; pp. 11–22. [DOI] [PubMed] [Google Scholar]
- Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of alzheimer's disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001;358:201–5. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- Grossman M, Payer F, Onishi K, White-Devine T, Morrison D, D'Esposito M, Robinson K, Alavi A. Constraints on the cerebral basis for semantic processing from neuroimaging studies of alzheimer's disease. Journal of neurology, neurosurgery, and psychiatry. 1997;63:152–158. doi: 10.1136/jnnp.63.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski B, Britson PJ, L Whitwell J, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW. The alzheimer's disease neuroimaging initiative (ADNI): MRI methods. Journal of magnetic resonance imaging : JMRI. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The boston naming test Lea and Febiger. Philadelphia: 1983. [Google Scholar]
- Keilp JG, Alexander GE, Stern Y, Prohovnik I. Inferior parietal perfusion, lateralization, and neuropsychological dysfunction in alzheimer's disease. Brain and cognition. 1996;32:365–383. doi: 10.1006/brcg.1996.0071. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Archives of Neurology. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer's disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Scheff SW, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Patel E, Markesbery WR. Alzheimer's-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment. Neuroscience letters. 2009;450:336–339. doi: 10.1016/j.neulet.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E. Neuropathologic features of amnestic mild cognitive impairment. Archives of Neurology. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the trail making tests as an indicator of organic brain damage. Perception & Motor Skills. 1958;8:253–266. [Google Scholar]
- Remy F, Mirrashed F, Campbell B, Richter W. Verbal episodic memory impairment in alzheimer's disease: A combined structural and functional MRI study. NeuroImage. 2005;25:253–266. doi: 10.1016/j.neuroimage.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen clinique en psychologie Presses Universitaires de France. Paris, France: 1964. [Google Scholar]
- Rosen WG, Mohs RC, Davis KL. A new rating scale for alzheimer's disease. The American Journal of Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in alzheimer's disease: Unbiased analysis of fluid-registered serial MRI. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N, Habeck CG, Zarahn E, Anderson KE, Park A, Hilton J, Pelton GH, Tabert MH, Honig LS, Moeller JR, Devanand DP, Stern Y. Covariance PET patterns in early alzheimer's disease and subjects with cognitive impairment but no dementia: Utility in group discrimination and correlations with functional performance. NeuroImage. 2004;23:35–45. doi: 10.1016/j.neuroimage.2004.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Stein T, Maslowski N, Neumann J. Neural correlates of alzheimer's disease and mild cognitive impairment: A systematic and quantitative meta-analysis involving 1351 patients. NeuroImage. 2009;47:1196–1206. doi: 10.1016/j.neuroimage.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME. Cerebral metabolic and cognitive decline in persons at genetic risk for alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:6037–42. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranfaglia C, Palumbo B, Siepi D, Sinzinger H, Parnetti L. Semi-quantitative analysis of perfusion of brodmann areas in the differential diagnosis of cognitive impairment in alzheimer's disease, fronto-temporal dementia and mild cognitive impairment. Hellenic journal of nuclear medicine. 2009;12:110–114. [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. temporal lobe afferents. Brain research. 1975;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale-revised Psychological Corporation. San Antonio: 1987. [Google Scholar]
- Whitwell JL, Petersen RC, Negash S, Weigand SD, Kantarci K, Ivnik RJ, Knopman DS, Boeve BF, Smith GE, Jack CR., Jr. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Archives of Neurology. 2007;64:1130–1138. doi: 10.1001/archneur.64.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr. MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]