It is an understatement to say that blood pressure regulation is complex, especially in terms of long-term control. Redundancy abounds between the intricate balance of vascular, neural and renal components. For the past 30 years, a major focus has been on elucidating how the endothelium contributes to vascular physiology and pathophysiology with many investigators suggesting a link between endothelial dysfunction and hypertension. After Yanagisawa et al. first described the endothelial-derived constricting factor, endothelin(ET)-1,1 many investigators assumed that the most potent vasoconstrictor thus far identified would play a critical role in maintaining systemic vascular resistance, and therefore, contribute to blood pressure regulation. However, 20 years later, we are still only beginning to understand its very complex, yet significant role in this realm. The difficulty in understanding the ET system is due to numerous factors including 1) the opposing roles of ETA and ETB receptors to produce vasoconstriction and vasodilation, respectively, along with the occasional ETB dependent vasoconstriction, 2) the irreversible nature of ligand binding to the ET receptors, 3) the localized nature of ET action such that plasma levels do not necessarily reflect synthesis, but rather, the balance of synthesis and clearance, 4) global knockout models for ET and its receptors result in lethal phenotypes, and 5) the relatively under-investigated function of ET receptors in the peripheral nervous system that can influence vascular tone and blood pressure.
Break-through evidence for the ET system in the control of blood pressure came from studies by Gariepy and collaborators who published several key papers revealing that genetic deficiency in ETB receptor function results in elevated blood pressure and salt-dependent hypertension.2,3 Mutation of the ETB receptor prevents development of the enteric nervous system and so animals homozygous for this trait develop megacolon and die at about the time of weaning. This certainly makes these animals a poor model for studying hypertension. However, these investigators came up with the clever idea of creating a transgenic rat that expresses a functional, non-mutated ETB receptor only in adrenergic tissues and so they do not develop intestinal agangliosis, but retain mutated, non-functional ETB receptors in the vasculature and kidney. As adults, these transgenic ETB receptor deficient rats have slightly elevated basal blood pressures when measured by telemetry, but more interestingly, a high salt diet produces a profound hypertension providing solid evidence for ETB control of sodium excretion.
Fortunately, we also have available a wide range of highly specific and selective pharmacological tools for studying the ET system by way of receptor antagonists.4 Long-term administration of ETB receptor specific antagonists produces hypertension that is salt-dependent similar to that observed with ETB receptor deficiency.5 The elevation in blood pressure due to ETB blockade can be reversed with an ETA receptor antagonist indicating that the ETB receptor may function primarily to protect from ETA dependent effects, through clearance of ET-1 as well as vasodilation. However, this does not explain the salt sensitivity of hypertension produced by ETB blockade or deficiency.
Renal collecting duct specific knockout mice developed by Kohan’s lab have clearly identified a role for the intrarenal ET-1/ETB receptor pathway in blood pressure regulation and facilitating sodium excretion.6,7 Similar to the global loss of ETB receptor function, the collecting duct specific ET-1 and ETB receptor knockout mice display elevated basal blood pressures that are exacerbated by a high salt diet. The latter studies provide definitive evidence for the renal collecting duct ET system as an important control system for blood pressure.
ET-1 and ETA receptor deficient mice, on the other hand, have developmental defects in the craniofacial region and do not survive beyond birth.8,9 Until now, our knowledge of how the ETA receptor may contribute to blood pressure regulation has been limited to pharmacological studies (even though there has yet to be an ETA selective agonist developed). ETA receptor blockade can lower blood pressure in both animals and humans with hypertension, particularly in those that are salt-dependent,10 but the physiological role of the ETA receptor has been more difficult to discern because there are mixed reports of whether ETA selective antagonists can or cannot lower blood pressure in normotensive animals or humans. Much of this uncertainty is likely due to variability in the methods, unknown in vivo selectivity of antagonists, and the genetic background of the model being tested.
In the current issue of Hypertension, Kisanuki et al. describe a newly developed mouse strain that represents a significant advance in our understanding of the physiological role of endothelial-derived ET-1.11 Using the Cre/loxP recombinase approach, these investigators created a mouse strain that does not express the preproET-1 gene in vascular endothelial cells (ET-1flox/flox;Tie2-Cre) in an effort to discern the physiological role of endothelial-derived ET-1 on blood pressure. These mice have lower blood pressures than intact genetic controls thus providing the best evidence to date that the balance of the physiological actions of endothelial ET-1 is to maintain a degree of elevated vascular tone. While the potency of ET-1 to cause vasoconstriction may have led some to believe that this would be a foregone conclusion, the opposing actions of ETA and ETB receptors have not allowed one to fully understand the net result of endogenous ET-1 activity. Furthermore, the effect of specific ETA versus ETB blockade, at least in a short-term setting, suggests that the predominant action of endothelial ET-1 would be via the ETB receptor. In other words, ETB blockade causes more dramatic increases in blood pressure compared to the blood pressure lowering effects of ETA antagonists. The difficulty in understanding this balance is because removing ETB actions not only results in loss of endothelial vasodilatory pathways, but also allows endogenous ET-1 to act on the ETA receptor unopposed.
Another important mouse model was developed by Bagnall and colleagues and involved endothelial-specific deletion of the ETB receptor.12 Similar to the ETB deficient rat, endothelial cell specific deletion of the ETB receptor increased plasma ET-1 concentrations consistent with a “clearance” function of the ETB receptor. Furthermore, these mice developed endothelial dysfunction as defined by an attenuated ability of isolated aorta to relax in response to acetylcholine and other stimuli. Importantly, these investigators observed that the loss of endothelial ETB receptors had no effect on blood pressure or the blood pressure response to changes in salt intake. The limiting feature of this study is that these animals were developed on a salt-sensitive background and so they need to be re-derived on a salt-resistant background in order to discern whether salt-sensitivity truly is unrelated to endothelial ETB receptors. Nonetheless, the findings of Bagnall et al. would indicate that the hypertension produced by global ETB blockade or genetic deficiency is most likely due to renal tubular ETB function and that collecting duct derived ET-1 targets renal tubular ETB receptors and not endothelial ETB receptors.
Amiri and colleagues have published a series of papers where ET-1 has been over-expressed in vascular endothelium.13–15 Over-expression of endothelial ET-1 in mice results in hypertrophic remodeling, oxidant stress, and vascular inflammation, but no effect on blood pressure. These effects are attenuated and exacerbated by ETA and ETB receptor blockade, respectively. Thus it appears as though the ETB receptor functions to protect the vasculature from the injurious effects of ETA receptor activity, but that elevated ET-1 production in and of itself is insufficient to raise blood pressure. Such findings are supported by reports demonstrating that chronic ET-1 infusion does not always produce hypertension, at least in rats on a normal salt diet.16,17
The study by Kisanuki et al. using their newly developed endothelial cell specific ET-1 knockout mice also addressed the interaction between ET-1 and other vasoactive systems.11 The endothelial cell ET-1 knockout mice had similar blood pressure responses to angiotensin II, norepinephrine, bradykinin, and the NOS inhibitor, L-NAME, as genetic controls. Therefore, it appears that endothelial cell ET-1 has little influence on these systems, at least in terms of the acute vascular responsiveness. Additional studies that produce chronic changes in these systems are still needed to help discern whether the balance of these factors is influenced by endothelial cell ET-1.
The lack of endothelial cell ET-1 clearly results in lower arterial pressure as demonstrated by both tail cuff and telemetry methods in the mouse. Acute administration of an ETA selective antagonist, FR139317, to a wild-type mouse also lowered blood pressure consistent with endogenous ET-1/ETA dependent tone. However, it is interesting to note that FR139317 lowered blood pressure to a similar degree in the control and endothelial cell ET-1 knockout mice even though the latter change was not statistically significant, most likely due to the low number of mice studied (n=5). In any event, the authors concluded that there remains some degree of ETA dependent vascular tone in endothelial cell ET-1 knockout mice. Since there is a wide range of cell types that synthesize ET-1, this may not be a surprising finding. Indeed, while endothelial cell ET-1 knockout mice have reduced ET-1 in heart, lung, kidney and brain, there remains measurable, albeit reduced, ET-1 in the plasma.
In conclusion, the development of endothelial cell ET-1 knockout mice represents another significant advance in our efforts to understand the physiological role of ET-1 in the control of blood pressure. On balance, we can be confident that endothelial cell derived ET-1 functions to maintain a higher level of vascular tone as a means of maintaining blood pressure, and therefore appears to contribute in a vasoconstrictor capacity to maintain blood pressure in its capacity as a major participant in the complex scheme of blood pressure control and fluid-volume balance. Furthermore, the endothelial ETB receptor appears to buffer ETA receptor activity both in terms of blood pressure and the mitogenic and pro-inflammatory actions of ET-1 as well as serving a permissive role in terms of endothelial-dependent relaxation. What is not clear, however, is how endothelial ET-1 actually produces the desired level of blood pressure since increases in peripheral resistance alone do not necessitate a long-term change in blood pressure without an influence on body-fluid homeostasis. What is the influence of endothelial ET-1 on kidney function? Is there a relationship between the vascular and renal tubular ET-1 systems? To what extent might the endothelial ET-1 system contribute to hypertension? What factors distinguish the physiological from the pathophysiological actions of ET-1? Does the neural ET-1 system operate independent of the vascular and renal systems? We can look forward to answers to these and many other questions in the coming years.
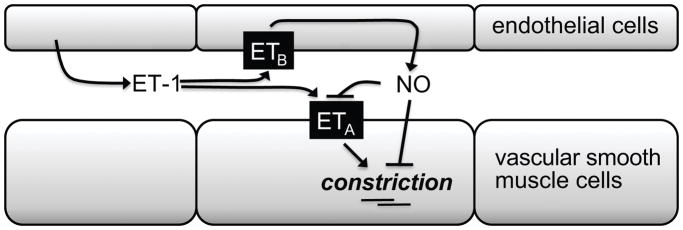
Figure 1.
Hypothetical scheme depicting the balance of ETA and ETB receptors in the vascular wall.
Acknowledgments
Sources of Funding
The author is supported by grants from the NIH (HL69999 and HL60653) and a grant from the Medical College of Georgia Cardiovascular Discovery Institute.
Footnotes
Disclosures
None.
References
- 1.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Gariepy CE, Williams SC, Richardson JA, Hammer RE, Yanagisawa H. Transgenic expression of the endothelin-B receptor prevents congenital intestinal aganglionosis in a rat model of Hirschsprung disease. J Clin Invest. 1998;102:1092–1101. doi: 10.1172/JCI3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gariepy CE, Cass DT, Yanagisawa M. Null mutation of endothelin receptor type B gene in spotting lethal rats causes aganglionic megacolon and white coat color. Proc Natl Acad Sci U S A. 1996;93:867–872. doi: 10.1073/pnas.93.2.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: great expectations or bleak house? Br J Pharmacol. 2008;153:1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol. 2001;281:F144–150. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 6.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 8.Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. doi: 10.1242/dev.125.5.813. [DOI] [PubMed] [Google Scholar]
- 9.Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, Oda H, Kuwaki T, Cao W, Kamada N, Hishage K, Ouchi Y, Azuma S, Toyada Y, Ishikawa T, Kumada M, Yazaki Y. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 10.Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ETA and ETB receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol. 2007;47:731–759. doi: 10.1146/annurev.pharmtox.47.120505.105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kisanuki YY, Emoto N, Ohuchi T, Kedzierski RM, Hammer RE, Yanagisawa H, Williams SC, Richardson JA, Suzuki T, Yanagisawa M. Low blood pressure in endothelial cell-specific endothelin-1 knockout mice. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.109.138701. in press. [DOI] [PubMed] [Google Scholar]
- 12.Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, Webb DJ, Kotelevtsev YV. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48:286–293. doi: 10.1161/01.HYP.0000229907.58470.4c. [DOI] [PubMed] [Google Scholar]
- 13.Amiri F, Paradis P, Reudelhuber TL, Schiffrin EL. Vascular inflammation in absence of blood pressure elevation in transgenic murine model overexpressing endothelin-1 in endothelial cells. J Hypertens. 2008;26:1102–1109. doi: 10.1097/HJH.0b013e3282fc2184. [DOI] [PubMed] [Google Scholar]
- 14.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 15.Amiri F, Ko EA, Javeshghani D, Reudelhuber TL, Schiffrin EL. Deleterious combined effects of salt-loading and endothelial cell restricted endothelin-1 overexpression on blood pressure and vascular function in mice. J Hypertens. doi: 10.1097/HJH.0b013e328338bb8b. [DOI] [PubMed] [Google Scholar]
- 16.Mortensen LH, Fink GD. Salt-dependency of endothelin-induced, chronic hypertension in conscious rats. Hypertension. 1992;19(6 Pt 1):549–554. doi: 10.1161/01.hyp.19.6.549. [DOI] [PubMed] [Google Scholar]
- 17.Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension. 2005;45:283–287. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]