Abstract
OBJECTIVE
To characterize the risks of CIN3 and cancer in women age 21–24 with human papillomavirus (HPV)-positive atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesion (LSIL) screening results in routine clinical practice.
MATERIALS AND METHODS
Quality assurance databases containing records of screening test and histology results from the Northern California Kaiser Permanente Medical Care Program Regional Lab were reviewed. Numbers of LSIL and HPV-positive ASC-US results and associated cancers and CIN3 in women age 21–24 during 2003–2007 were tabulated and the corresponding risks calculated overall and by year of age.
RESULTS
During the 5 year period 2003 to 2007, 1620 HPV-positive ASC-US and 2,175 LSIL were diagnosed in women age 21–24, for which corresponding histology is available. No invasive cancers were detected in association with LSIL and HPV-positive ASC-US screening results in this age group during this time period. The risk of cancer was therefore 0% (95%CI = 0.00%–0.10%). The risk of CIN3 associated with an HPV-positive ASC-US was 2.90% (95%CI = 2.14%–3.84%), with LSIL was 2.44% (95%CI = 1.83%–3.18%), and for the two combined the risk was 2.64% (95%CI = 2.15%–3.20%)
CONCLUSION
The risk of CIN3 and cancer is low enough that management of women age 21–24 with ASC-US and LSIL smears without immediate colposcopy should be considered, as is currently recommended for women age 20 and under.
Keywords: Pap, ASC-US, LSIL
Introduction
Improved understanding of the natural history of cervical intraepithelial neoplasia (CIN) has prompted recent changes in management guidelines based on age and HPV status (1, 2). Among these changes are the follow-up of atypical squamous cells of undetermined significance (ASC-US) and low-grade squamous intraepithelial lesion (LSIL) Paps without immediate colposcopy in women under age 21. The purpose of this study is to investigate the potential consequences of extending this policy to women under the age of 25.
A blueprint for defining management of cervical cancer screening abnormalities based on risk was recently published (3). As additional screening and triage modalities become available, rational management of the myriad different combinations of results requires consistent management for similar levels of risk. Defining the risks of CIN3 or cancer associated with LSIL and HPV-positive ASC-US screening test results in women ages 21 to 24 will inform management recommendations. Implementation of such a risk management model may better allocate resources towards those at greatest risk, and improve the well-being of those at lowest risk.
Materials and Methods
The Regional Laboratory of the Northern California Kaiser Permanente Medical Care Program provides cervical cytology services for approximately 65% of the Northern California Kaiser membership, a racially and ethnically diverse catchment of approximately 1 million women. Since the 1990s, the Pap and cervical biopsy correlation data generated as part of our quality assurance programs has been retained. In January of 2001, a Cervical Cancer Screening Guideline was approved for Kaiser Northern California similar to the 2001 American Society of Colposcopy and Cervical Pathology Consensus Guidelines (4) which included the introduction of oncogenic HPV testing for women of all ages with ASC-US cytology results, and the recommendation that women with ASC-US who tested positive for HPV should undergo colposcopy.
From 2003 to 2007, all cervical biopsy reports referable to abnormal screening tests in facilities served by the Regional Laboratory were manually reviewed and entered into the database by Regional Laboratory personnel under the direction of one of the authors (BF). A sample of 9.9% of all of the biopsy reports (for all cytologic diagnoses and ages) was subsequently re-reviewed by the investigators to assess the accuracy of the data entry. Using CIN2+ versus <CIN2+ as the cut point, classifications of abnormal histology in the database were found worthy of revision in 1.6% of cases, mostly in relation to “CIN1-2” which was reclassified as <CIN2 rather than CIN2+.
Abnormal Pap results for women age 21–24 were extracted from all Paps performed during January 1, 2003, through December 31, 2007, regardless of whether biopsy correlation was available. Paps occurring during the period January 1, 2003, through December 31, 2007 for which histologic correlation was available were mapped to the worst subsequent histologic diagnosis occurring from January 1, 2003 through June 30, 2008. The study cohort for the evaluation of the risk of CIN3 or cervical cancer of an LSIL or HPV-positive ASC-US screen was composed of those women aged 21 through 24 who experienced either of theses abnormal Pap results between January 1, 2003, and December 31, 2007, and had one or more subsequent histologic evaluations during the period from January 1, 2003 to June 30, 2008 in a facility served by the Regional Laboratory.
The risk of invasive cancer was evaluated in the cohort defined above, and for women 21–24 with an LSIL or HPV positive ASC-US Pap result between January 1, 2000 to December 31, 2007. The premise underlying the examination of this extended cohort is that clinical management, testing procedures and the nuances of cytologic and histologic diagnoses of CIN may have changed, but the diagnosis of the presence or absence of invasive cancer should have remained constant during this time period.
Results
From 2003 to 2007, 124,503 Paps were performed on women ages 21–24, of which 7,552 (6.07%) were ASC-US and 3,538 (2.84%) were LSIL. Adoption of reflex HPV testing for ASC-US triage was progressive during this time period, as the facility labs adapted to the accessioning of specimen transport media (STM) tubes, systems were designed and implemented to transport, track, store and sort these tubes, and Information Technology issues related to test ordering and results reporting were resolved. As a consequence 4,374/7,552 (57.92%) of the ASC-US Paps in this age group had an HPV test result during this time period. Of those tested, 2,905/4,374 (66.42%) of women age 21–24 with ASC-US cytology results were HPV positive.
In the 5 year period 2003–2007, 1,620 HPV-positive ASC-US and 2,175 LSIL in women age 21–24 had corresponding histology is available. No invasive cancers were detected in association with HPV-positive ASC-US (0.00%, 95%CI = 0.00%–0.22%) or LSIL cytology (0.00%, 95%CI = 0.00%–0.17%) in this age group within this time period. The results of HPV-positive ASC-US and LSIL were combined because it has been previously shown that these two screening outcomes were associated with equivalent risk of CIN2+. The risk of cancer for HPV-positive ASC-US and LSIL combined was 0.00% and the upper 95% confidence limit was 0.10%, or 10 cancers in 10,000 women. Thus, the negative predictive value (NPV) for cancer of an HPV-positive ASC-US or LSIL screening result in this population was 100% (95%CI = 99.90%–100%).
Extending the dataset from 2000–2007 for all women age 21–24 with HPV positive ASC-US and LSIL and available histologic evaluation increased the number of HPV-positive ASC-US to 2,536 and LSIL to 3,427 for a total of 5,963 HPV-positive ASC-US and LSIL Paps. No invasive cancers were diagnosed in this larger cohort. Thus, the risk of cancer in this population was 0% and the upper 95% confidence limit was 0.06%, or 6 per 10,000 women. The NPV for cancer in this expanded population with HPV-positive ASC-US and LSIL screening results was also 100% (95% CI = 99.94%–100%).
The risks of CIN3 by year of age following an HPV-positive ASCUS, LSIL, and the combination are shown in Table 1. The risk of CIN3 following a HPV-positive ASC-US result at age 21–24 was 2.90% (95%CI = 2.14%–3.84%). The risk of CIN3 following LSIL Pap at age 21–24 was 2.44% (95%CI = 1.83%–3.18%). The risk of CIN3 (n = 100) for a HPV-positive ASC-US or LSIL smear (n = 3,795 women age 21–24) was 2.63% (95%CI = 2.15%–3.20%).
Table 1.
Risk of CIN 3 Associated With ASCUS HPV-Positive and LSIL Screening Tests by Year of Age
| CIN 3 | ||||||
|---|---|---|---|---|---|---|
| Age, y | ASC HPV+ | LSIL | Total | |||
| 21 | 12/403 | 3.0% | 6/502 | 1.2% | 18/905 | 2.0% |
| 22 | 6/390 | 1.5% | 13/569 | 2.3% | 19/959 | 2.0% |
| 23 | 15/465 | 3.2% | 19/541 | 3.5% | 34/1006 | 3.4% |
| 24 | 14/362 | 3.9% | 15/563 | 2.7% | 29/925 | 3.1% |
| Total | 47/1620 | 2.9% | 53/2175 | 2.4% | 100/3795 | 2.6% |
Discussion
As the understanding of the natural history of cervical intraepithelial neoplasia and HPV infections in young women and the importance of age in the evaluation of risk of cervical cancer has evolved, the ASCCP Guidelines for the triage of women younger than 21 with ASC-US and LSIL cervical cytology have been revised to avoid immediate colposcopy (1). Based on the findings of the current analysis, we propose further revision of HPV-positive ASC-US and LSIL triage protocols in favor of more conservative management for women in the 21–24 year old age range, who could reasonably be followed with cytology as is currently recommended for women up through age 20. Such an approach would reflect the same understanding of HPV natural history currently underlying the recommendations for adolescents, and potentially reduce the untoward effects of continued screening of young women. Given our inability to demonstrate benefit from the practice of screening young women, attention to minimizing the adverse effects seems warranted. In addition to sparing young women unnecessary trauma, such a policy might also improve resource allocation in screening and prevention efforts.
Consideration of extending the age for conservative management of minor cytologic abnormalities is motivated by several factors. While screening in this age group detects CIN, it is difficult to demonstrate that treatment of CIN in this age group changes the incidence of cervical cancer. Second, regression of CIN is common in this age group, and the ability to separate lesions which will regress from those that will not is difficult. Third, the treatment of CIN in women who have not completed their childbearing is associated with adverse obstetrical outcomes. If the benefit of screening in this age group is difficult to document, and the risks of screening and treatment are clear, then consideration of more conservative management algorithms for mild cytologic abnormalities seems warranted if screening women under the age of 25 is to continue.
In a prospective study of 187 women with LSIL diagnosed between the ages of 13 and 22 conducted in our population, regression to a normal Pap smear without treatment occurred in 61% by 12 months and 91% by 36 months (5). Regression of ASC-US to normal by 24 months for women of all ages ranges from 68.19% to 97.3% and similar 24 month regression has been reported after LSIL, ranging from 47.4% to 92.5% (6, 7).
Between 2001 and 2003 the incidence and mortality from cervical cancer in women 20–24 in the SEER database was 1.5/100,000 and 0.1/100,000 women per year (8). In a review of 150,000 cervical screening results from the Northwest Permanente Medical Care Program, ASC and LSIL smears were most common in young women age 20–24 (9). In the SEER dataset, the risk for being diagnosed with cervical cancer in the next 10 years is 0.04% at age 20 (8). Note that for women of this age this risk of cervical cancer is less than the risk of breast cancer (0.05% in the next 10 years starting at age 20), for which screening is not recommended.
In Finland and the Netherlands, cervical screening begins at age thirty and is repeated every five years. The incidence of cervical carcinoma and associated mortality in these countries is significantly less than in the United States (4.0 and 0.9/100,000 women per year in 2003 in Finland and 4.9 and 1.4/100,000 in the Netherlands vs. 8.4/100,000 and 2.4 deaths/100,000 women per year in the U.S) (8, 10). While our populations and screening rates may differ, HPV infection is remarkably uniform worldwide save for minor differences in type distribution between ethnic groups (11). These results from Europe demonstrate that screening women under 30 is not required to achieve superior cervical cancer prevention. The absence of impact on cervical cancer rates as a result of screening of women under the age of 30 in the United States is documented by the data of Gustaffson et al. (Figure 1), who reported the effects of the introduction of cytologic screening in the state of Connecticut (12). The introduction of cytologic screening did not change the incidence of invasive cancer in women under the age of 30.
Figure 1.
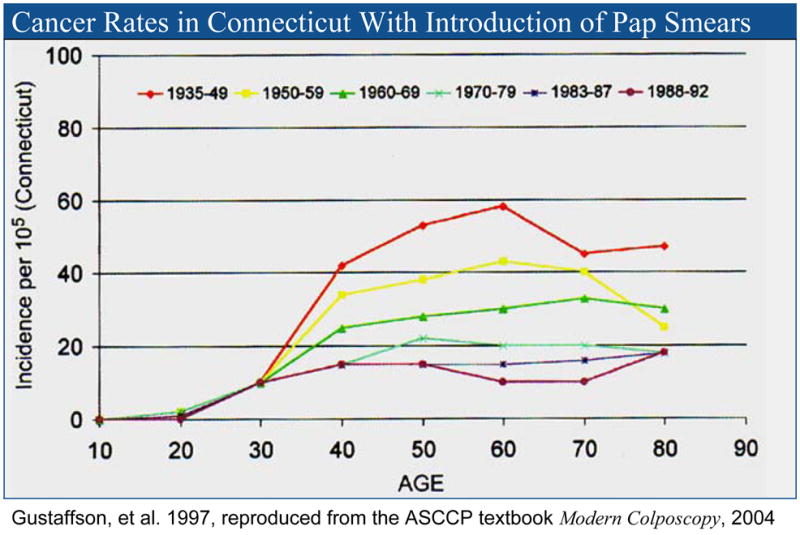
Effect of the Introduction of Cytologic Screening on Cervical Cancer Incidence in the State of Connecticut, by Age
One potential objection to the recommendation to make the management of women age 21–24 with ASC-US and LSIL smears consistent with that of women age 20 and under is that this would not be safe for high risk populations. Moscicki et al have addressed this concern in their recent publication reporting CIN3 rates of 6.5% for ASC-US and 5.73% for LSIL (13). There were no cancers associated with either diagnosis in the very high risk population of 13–24 year old women studied. High risk behaviors in the cohort included 38% who had been pregnant one or more times, a mean of 8 sexual partners, 88% with at least one new partner in the last 2 months, and 39% reported anal intercourse. Moscicki and colleagues concluded that “extending …conservative management to young women [with ASC-US and LSIL Pap smears] under the age of 25 years may be reasonable…” and recommended that guideline groups reexamine this issue.
Failing to demonstrate benefit from screening younger women means that the risks of screening must be stringently appraised (14). Following excisional procedures for the management of CIN (conization or LEEP), there is an increased risk for overall perinatal mortality, preterm delivery, low birth weight infants, and premature rupture of membranes (PROM) in subsequent pregnancies (14–16). When triaging young women with LSIL to colposcopy, the implication for future pregnancy must be considered, particularly in a population with a high likelihood of spontaneous resolution of disease (17) and at virtually no risk of cancer.
In ALTS the cumulative absolute risk of CIN3 at 2 years after an HPV-positive ASC-US Pap was 10.8%, and after an LSIL Pap was 10.7% (17). We believe that these differences are consequent to the older age (median of 25.0 years, mean of 27.5 years, range of 18–81 years) in the ALTS population, the two-year follow-up for more complete disease ascertainment and the inclusion of incident CIN3, and to the fact that LEEP at exit was undertaken for persistent minor abnormalities when colposcopy did not reveal CIN2+. This disease assessment at study exit was an important and informative component of the study design but not reflective of current (or contemplated) U.S. clinical practice recommendations. In addition, ALTS evaluated ASCUS by the definition then current, which included the cases that would be diagnosed as ASC-H under the present recommendations.
The grouping of ASC-US and LSIL together in this evaluation is warranted because of previous studies demonstrating that these screening results confer precisely the same risk of CIN2+ at initial evaluation and at two years (18). For this reason the relative numbers of ASC-US and LSIL evaluated are of minimal consequence, as both diagnoses portend similar risk, in this study and in ALTS, and they are therefore the same category for the purposes of clinical practice recommendations.
The strength of this study lies in the evaluation of clinical practice outside of a controlled prospective trial. The database has been developed and validated as part of a quality assurance program and provides the capability to capture data from a screened population that has not been selected by the exclusion criteria of a prospective study. Our membership is limited at the extremes of wealth, but it represents a diverse body of ethnic groups characterized elsewhere (19). Sample sizes that no study sponsor could afford are possible, and care for most women in the U.S. will be delivered outside of the clinical trial setting, hence we feel that evaluations of this kind complement the previously published experience.
Not all cases of HPV+ ASC-US and LSIL were included in this evaluation, as not all women with HPV positive ASC-US or LSIL underwent follow-up histological evaluation diagnosed by the Regional Laboratory. Women with HPV-positive ASC-US results underwent histologic evaluation in the clinics served by the Regional Laboratory in 1,620/2,905 (55.77%) of cases and LSIL Paps in 2,175/3,538 (61.48%) during this time period. Members with abnormal Paps may choose to have their colposcopy at facilities not served by the Regional Laboratory, or with gynecologic providers outside of the Kaiser system (survey data indicate that approximately 1% of our female members report Paps outside of the Kaiser system in the preceding 3 years despite Kaiser membership). Members or their providers may be non-compliant with recommendations for colposcopy, or members may change insurance or loose coverage following an abnormal Pap and prior to colposcopy. All of these factors decrease the number of cases available for evaluation but none of them are recognized to be associated with increased risk of adverse histologic diagnoses, and as a consequence those women for whom complete evaluation is available are felt to be a representative sample of the greater population.
In summary, given the low risk of an HPV-positive ASC-US or LSIL smear for CIN3 in women age 21 to 24 and safety from cancer, the likelihood of regression of abnormal cytology in this age group, the normally slow progression to invasive carcinoma, and risk for adverse pregnancy outcomes following treatment of CIN, we propose deferring immediate colposcopy in this population in favor of follow-up cytology at 12 months as currently recommended for women under the age of 21, thereby permitting the transient HR-HPV infections causing most of the HPV-positive ASC-US or LSIL screening results in young women to clear.
Acknowledgments
Sources of Financial Support: None
Dr. Castle was supported by the Intramural Research Program of the NIH, National Cancer Institute. The authors gratefully acknowledge the support of the Women’s Health Research Institute at Kaiser Permanente Northern California.
References
- 1.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 2.ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol. 2008;112:1419–44. doi: 10.1097/AOG.0b013e318192497c. [DOI] [PubMed] [Google Scholar]
- 3.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. Am J Obstet Gynecol. 2007;197:356, e1–6. doi: 10.1016/j.ajog.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. ASCCP Sponsored Consensus Conference. 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. JAMA. 2002;287:2120–9. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 5.Moscicki AB, Shiboski S, Hills NK, Powell KJ, Jay N, Hanson EN, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004;364:1678–83. doi: 10.1016/S0140-6736(04)17354-6. [DOI] [PubMed] [Google Scholar]
- 6.Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92:727–35. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 7.Schlecht NF, Platt RW, Duarte-Franco E, Costa MC, Sobrinho JP, Prado JC, et al. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J Natl Cancer Inst. 2003;95:1336–43. doi: 10.1093/jnci/djg037. [DOI] [PubMed] [Google Scholar]
- 8.SEER Cancer Statistics Review 1975–2005. National Cancer Institute; 2008. [Google Scholar]
- 9.Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: a population-based study. Am J Obstet Gynecol. 2004;191:105–13. doi: 10.1016/j.ajog.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Engholm G, Ferlay J, Christensen N, Bray F, Gjerstorff M, Klint A, et al. NORDCAN: Cancer Incidence, Mortality, and Prevalence in the Nordic Countries, Version 3.3. Association of Nordic Cancer Registries; Societies DC: 2008. [Google Scholar]
- 11.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson L, Ponten J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8:755–63. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- 13.Moscicki AB, Ma Y, Wibbelsman C, Powers A, Darragh TM, Farhat S, et al. Risks for cervical intraepithelial neoplasia 3 among adolescents and young women with abnormal cytology. Obstet Gynecol. 2008;112:1335–42. doi: 10.1097/AOG.0b013e31818c9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjoborg KD, Vistad I, Myhr SS, Svenningsen R, Herzog C, Kloster-Jensen A, et al. Pregnancy outcome after cervical cone excision: a case-control study. Acta Obstet Gynecol Scand. 2007;86:423–8. doi: 10.1080/11038120701208158. [DOI] [PubMed] [Google Scholar]
- 15.Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadler L, Saftlas A, Wang W, Exeter M, Whittaker J, McCowan L. Treatment for cervical intraepithelial neoplasia and risk of preterm delivery. JAMA. 2004;291:2100–6. doi: 10.1001/jama.291.17.2100. [DOI] [PubMed] [Google Scholar]
- 17.Walker JL, Wang SS, Schiffman M, Solomon D. Predicting absolute risk of CIN3 during post-colposcopic follow-up: results from the ASCUS-LSIL Triage Study (ALTS) Am J Obstet Gynecol. 2006;195:341–8. doi: 10.1016/j.ajog.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 18.Cox JT, Schiffman M, Solomon D. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188:1406–12. doi: 10.1067/mob.2003.461. [DOI] [PubMed] [Google Scholar]
- 19.Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595–600. doi: 10.1097/AOG.0b013e3181996ffa. [DOI] [PMC free article] [PubMed] [Google Scholar]


