Abstract
A previous study from our laboratory demonstrated the presence within the rat striatum of dopaminergic terminals in different dynamical states, determined at least in part by the extent to which terminals are subject to autoinhibition. The present study is designed to test the hypothesis that heterogeneity in the basal tonic extracellular dopamine concentration contributes to the variable extent of autoinhibition. We probed basal extracellular dopamine concentrations using a previously demonstrated strategy that utilizes intrastriatal microinfusion of kynurenate, a substance that according to voltammetric measurements decreases extracellular dopamine from its basal concentration. In the striatum, however, we find that the response to kynurenate infusion is itself heterogeneous, allowing a broad classification of sites within the striatum as kynurenate-insensitive and kynurenate-sensitive, respectively. These newly identified kynurenate-insensitive and sensitive sites yield substantially and significantly different evoked DA release as measured by voltammetry during electrical stimulation of the medial forebrain bundle. Our findings confirm the hypothesis that heterogeneity in the local basal concentration of DA is responsible for the variable extent of autoinhibition within the striatum and support the conclusion the steady state and dynamical components of extracellular DA in this brain region are coupled.
Introduction
Central dopamine (DA) systems attract intense interest because they participate in multiple aspects of normal brain function and because DA dysfunctions are implicated in multiple CNS disorders, including Parkinson's disease (Booij et al. 1999; Hornykiewicz 2002; Dagher and Robbins 2009), schizophrenia (Stone et al. 2007), attention deficit hyperactivity disorder (Levy and Swanson 2001), and substance abuse (Koob and Bloom 1988). Current theories on the multiple functions of DA invoke the concept that extracellular DA operates on multiple timescales. In a recent review, Schultz pointed out that DA functions can be divided approximately between those involving rapid changes in extracellular DA concentrations (sub-seconds to seconds) and slow changes (minutes to tens-of-minutes) (Schultz 2007). Numerous studies have employed microdialysis (e.g. Hernandez et al 2007) and voltammetry (e.g Robinson and Wightman 2007) to elucidate DA events on different time scales.
Extracellular DA concentrations generally reflect a balance between the rate of DA release and clearance (Wightman and Zimmerman 1990; Smith and Justice 1994). Since DA release and clearance are each tightly regulated, extracellular DA concentrations appear to be precisely and intricately controlled (Wieczorek and Kruk 1995; Zahniser et al. 1999; Cowell et al. 2000; Chen et al. 2001; Rice and Cragg 2004; Zhang and Sulzer 2004). Voltammetry is effective for monitoring rapid DA dynamics (Wightman and Robinson 2002) whereas microdialysis is effective on the longer timescales (Watson et al. 2006; Nandi and Lunte 2009). However, the objective of the present study was to test the hypothesis that a coupling between the multiple DA timescales contributes to the control of extracellular DA concentrations. Thus, we used a single technique, voltammetry, to monitor DA on multiple timescales.
Fast-scan cyclic voltammetry in conjunction with carbon fiber microelectrodes facilitates the monitoring of extracellular DA dynamics in the rat striatum during electrical stimulation of the medial forebrain bundle (MFB) (Kuhr et al. 1987; Wightman et al. 1988). The dynamics of evoked release are both heterogeneous and sensitive to the manipulation of presynaptic D2 autoreceptors (Benoit-Marand et al. 2001; Kita et al 2007; Moquin and Michael 2009). The D2 antagonist, raclopride, increases the rate of evoked release whereas the D2 agonist, quinpirole, has the opposite effect. For this reason, herein we hypothesize that the inherent local heterogeneity of DA dynamics stems from different levels of occupation of D2 autoreceptors. Such a hypothesis implies local variations in the basal extracellular DA concentration, although direct observations of such a phenomenon have not been described to date because microdialysis, the preferred approach to assessing basal DA levels, averages the DA concentration over both distance (i.e. the length of the probe) and time (i.e. the sampling duration). Thus, in the present study, we employed voltammetry, which offers higher spatial resolution, to investigate local variations in basal DA.
Previously (Kulagina et al. 2001; Borland and Michael 2004; Mitala et al. 2008), we reported that voltammetry, under specific circumstances, provides a measure of long-timescale DA. Intrastriatal infusions of the glutamate antagonist, kynurenate (KYN), elicit a decrease in basal (i.e. non-evoked) DA as measured with a voltammetric electrode placed near the infusion site. We demonstrate herein that the striatum is heterogeneous in its response to KYN infusions. Thus, in regards to the basal DA concentration, the striatum exhibits both KYN-sensitive and KYN-insensitive sites. Moreover, sites where basal DA is KYN-sensitive and KYN-insensitive yield substantially and significantly different evoked DA responses during MFB stimulation. Collectively, our findings show that basal DA (i.e. long-timescale DA) contributes to the regulation of evoked DA (i.e. sub-seconds to seconds timescale DA), supporting the conclusion that a coupling exists between the multiple timescales of DA function.
Methods and Materials
Voltammetry
Carbon fiber microelectrodes were constructed by inserting a single carbon fiber (7 μm diameter, Thornell Carbon Fiber, T300, Amoco Performance Products, Inc., Greenville, SC) into a borosilicate glass capillary (0.75 mm I.D., 1 mm O.D., Sutter Instruments, Co., Novato, CA). The capillary was pulled to a fine tip around the fiber with a vertical micropipette puller (Narishige, Tokyo, Japan). The resulting tip was sealed with Spurr Epoxy (Polysciences, Inc., Warrington, PA) and the exposed fiber was trimmed to a length of 100 μm or 400 μm. Each electrode was pretreated with a triangular potential waveform (0–2 V vs. Ag/AgCl reference at 200 V/s for 1 s) in artificial cerebrospinal fluid (145 mM Na+, 1.2 mM Ca2+, 2.7 mM K+, 1.0 mM Mg2+, 152 mM Cl− and 2.0 mM phosphate, adjusted to pH 7.4). For fast scan cyclic voltammetry (FSCV) the resting potential was 0 V vs. Ag/AgCl and the voltage was swept linearly to 1.0 V, then to −0.5 V and back to 0 V at 300 V/s. During experiments involving MFB stimulation scans were performed at 10 Hz. During longer duration experiments involving KYN infusion scans were performed at 2.5 Hz. The DA voltammetric signal was obtained in the 500–700 mV potential range of the first potential sweep, which gives the maximum DA oxidation current. Voltammetric currents were converted to DA concentrations by post-calibration of the microelectrodes after they were extracted from the rat brain. Data collected during KYN infusions were corrected for signal drift by the method described by Borland and Michael (2004). Calibration was performed in artificial cerebrospinal fluid. DA identification was based on the appearance of background subtracted voltammograms (Borland and Michael 2004).
Animals and surgical procedures
All procedures involving animals were carried out with the approval of the Institutional Animal Care and Use Committee of the University of Pittsburgh. Male Sprague-Dawley rats (Hilltop, Scottdale, PA) (250–375g) were anesthetized with isoflurane (5% initially, 2.5% for maintenance) carried by oxygen throughout all experiments. While anesthetized, rats were wrapped in a homeothermic blanket at 37°C (EKEG Electronics, Vancouver, BC) and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) with the incisor bar 5 mm above the interaural line (Pellegrino et al. 1979). Holes were drilled through the skull and dura mater was dissected to permit electrode implantations. Electrical contact between brain tissue and a Ag/AgCl reference electrode was established with a salt bridge made with a plastic pipette tip plugged with a paper wick and filled with artificial cerebrospinal fluid.
Carbon fiber microelectrodes were placed in the striatum 2.5 mm anterior to bregma, 2.5 mm lateral from midline, and 4.5 mm below dura (Pellegrino et al. 1979). Stainless steel bipolar stimulating electrodes (MS303/a; Plastics One, Roanoke, VA, USA) were lowered towards the ipsilateral MFB (2.2 mm posterior to bregma, 1.6 mm from midline, and initially 7.5 mm below dura). From its initial position, the stimulating electrode was lowered slowly until evoked DA release was detected in the striatum: this is a well-established procedure for locating nigrostriatal DA fibers (Ewing et al. 1983; Kuhr et al. 1984; Heien et al. 2005). The optically isolated stimulus was a biphasic, constant-current square wave (2 ms pulse duration, 280 μA pulse height). Stimulation was delivered as either a single train (200 ms or 10 s) or four 1-s trains separated by 2-s intervals. Most of the experiments described in this study employed a stimulation frequency of 60 Hz: however, one study involved 45 Hz to confirm that slow-type responses occur at different frequencies (see Fig 3).
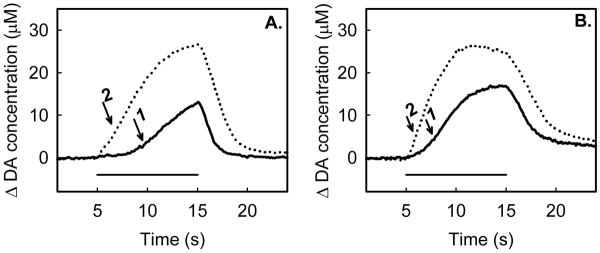
Figure 3.
Examples of very slow evoked DA release in striatum. At these sites, the pre-drug responses (solid lines) exhibit a prolonged onset in evoked release (arrow 1). Raclopride (2 mg/kg i.p.) eliminates the delay (arrow 2). Fig 3A and B are from two different animals with different stimulation frequencies (A: 45 Hz, B: 60Hz).
In some instances, as explained in the Results section, the placement of the recording electrode was optimized to facilitate the recording of fast-type or hybrid stimulus responses. This optimization involved lowering the electrode until a site yielding fast-type responses was located. In addition, in some of the experiments described below, we expressly wished to avoid fast-type and hybrid sites. In these instances, we employed the shorter (100 μm) electrodes as preliminary experiments showed that these shorter electrodes were more routinely located into slow-type sites without any procedure to optimize the placement.
Micropipets for the KYN infusions were constructed from fused silica capillary tubing (350 μm O.D., 25 μm I.D., Polymicro Technologies, Phoenix, AZ). The capillary was pulled to a fine tip with a horizontal laser-powered puller (Sutter instruments, Novato, CA) and trimmed to make the outer tip diameter ~30 μm. The inlet end of the capillary was attached to a 50 μL gastight syringe (Hamilton, Reno, NV) driven by a microprocessor-controlled driver (NA-1, Sutter Instruments, Novato, CA). The syringe and pipette were pre-filled with 1 mM KYN in artificial cerebrospinal fluid. The micropipette was mounted in a stereotaxic carrier at 10° from vertical and aligned with the microelectrode before either was lowered into the brain. Stereotaxic procedures were used to position the tip of the pipet 150 μm laterally from the striatal microelectrode in the same coronal plane. The infusion volume was 200 nL (2×10−10 mols KYN) and the infusion duration was 2 min.
Statistics
Three-way analysis of variance (ANOVA) was performed on the evoked DA concentration results in Figs 7 and 8. Two-way ANOVA was performed on the non-evoked DA concentration results in Fig 5, evoked DA concentration results in Fig 6, and normalized post-KYN evoked DA results in the inset of Fig 8. Post-hoc compare was performed using Tukey's criterion. Pre- and post-quinpirole response amplitudes (Fig 2) were analyzed by means of one-way ANOVA.
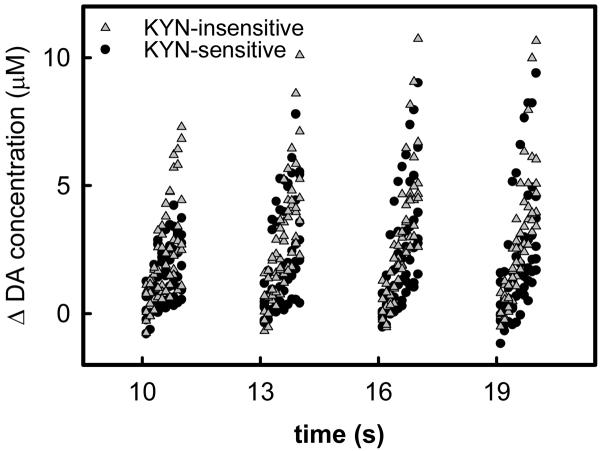
Figure 7.
The rising phase of evoked DA responses during 4 consecutive stimulus trains (1-s trains with 2-s intervals) at KYN-insensitive (grey solid triangles) and KYN-sensitive (black solid circles) sites are significantly different. The response to each train was re-zeroed in this plot. These responses were subjected to 3-way ANOVA: the responses at the KYN-insensitive and KYN-sensitive sites are significantly different (F(df=1, 400)=20.6, p<0.00001).
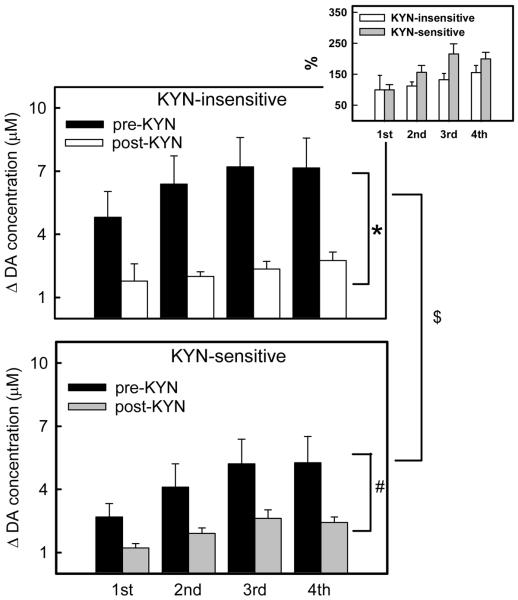
Figure 8.
Histograms of the peak amplitude of the response to 4 consecutive stimulus trains (1-s trains at 2-s intervals) at KYN-insensitive (top panel) and KYN-sensitive (bottom panel) striatal sites before (black bars) and after (white and gray bars) KYN-infusion. The inset panel shows the post-infusion amplitudes normalized with respect to the amplitude of the response to the first train. Data in the main panels were subjected to 3-way ANOVA: the KYN-insensitive and KYN-sensitive groups are significantly different (F(df=1, 80)=6.11, p<0.02, $). The Tukey test confirmed that the pre-infusion responses at the KYN-insensitive and KYN-sensitive sites are different (p<0.01) but not the post-infusion responses. The pre- and post-infusion groups are significantly different (F(df=1, 80)=50.5, p<0. 000000001). The effect of infusion was significant in both the KYN-insensitive (p<0. 0000001, *) and KYN-sensitive sites (p<0.005, #). Inset panel: these normalized responses were also subjected to 2-way ANOVA: The KYN-insensitive and KYN-sensitive sites are significantly different (F(df=1, 40)=5.19, p<0.03).
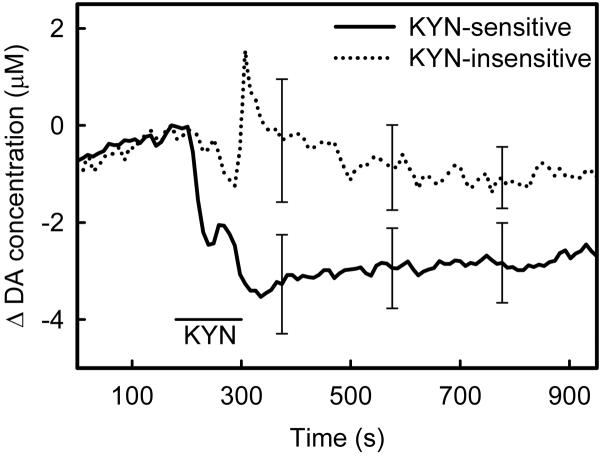
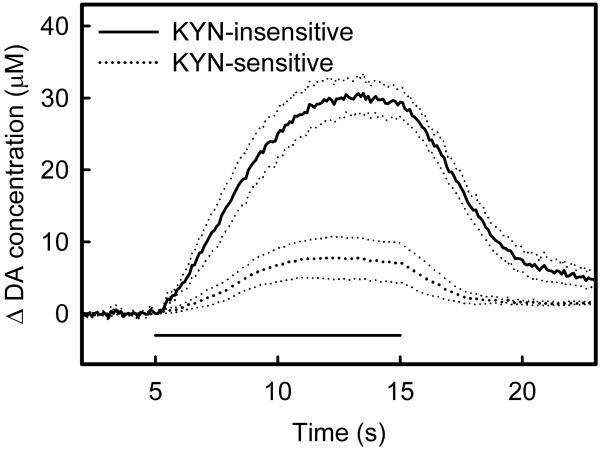
Figure 5.
The striatum is heterogeneous with respect to the response to KYN-infusion. The horizontal bar indicates the KYN infusion (2×10−10 mol in 200 nL aCSF). Some sites are KYN-insensitive (dotted line) while others are KYN-sensitive (solid line). Each response is the average (± SEM) of responses from 10 different animals. The data points indicated by error bars were subjected to two-way ANOVA: the KYN-insensitive and KYN-sensitive groups are significantly different (F(df=1,72)=8.93, p<0.004): time was not a significant factor.
Figure 6.
KYN-insensitive and KYN-sensitive recording sites yield substantially and significantly different evoked DA responses during a continuous 10 s stimulus train at 60 Hz (horizontal bar). Each trace is the mean ± the standard error of the responses recorded in 4 animals each. These data were subjected to 2-way ANOVA with group (KYN-insensitive and KYN-sensitive) and time as the classifications: evoked responses at the KYN-insensitive and KYN-sensitive sites are significantly different (F(df=1,606)=1984, p<0.000000000000001).
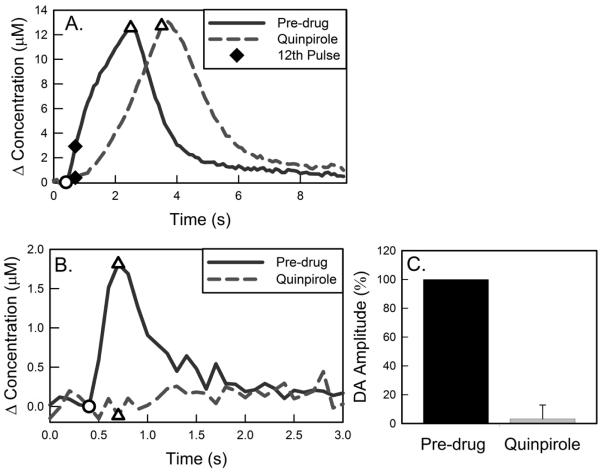
Figure 2.
Activation of dopamine D2-receptors with quinpirole slows evoked DA overflow and converts hybrid responses to slow-type responses. A) Before quinpirole, evoked release begins without delay after the onset of stimulation (60 Hz). Quinpirole delays the onset of evoked release and diminishes the amplitude of release evoked by the first 12 stim pulses (solid diamonds). B) Quinpirole abolishes 12-pulse stim responses. C) The decrease in DA release evoked by 12 stim pulses after quinpirole is significant (ANOVA: F(1,3) = 100.8; n = 3; p<0.001).
Chemicals and drugs
Kynurenic acid and dopamine hydrochloride were used as received from Sigma (St. Louis, MO, USA) and dissolved in artificial cerebrospinal fluid. Raclopride tartrate (2 mg/kg i.p.) and (−)quinpirole hydrochloride (1 mg/kg i.p) (Sigma Aldrich, St. Louis, MO) were dissolved in phosphate buffered saline (100 mM phosphate, pH 7.4). Isoflurane was obtained from Halocarbon (River Edge, NJ, USA). All solutions were prepared with ultrapure water (NANOPure; Barnstead, Dubuque, IA, USA).
Results
Evoked DA release in the striatum demonstrates heterogeneity of autoinhibition
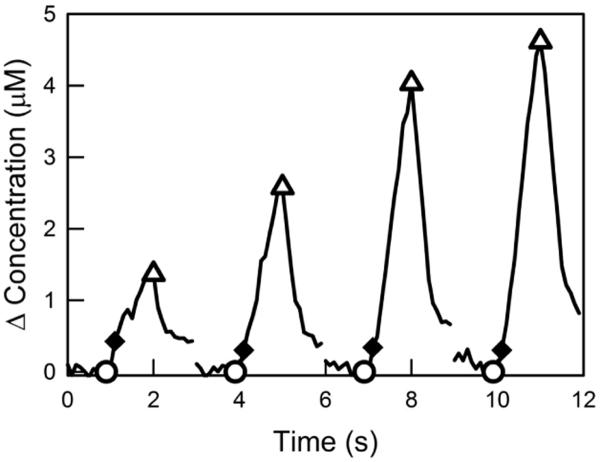
Responses evoked in the rat striatum during electrical stimulation of the medial forebrain bundle exhibit marked spatial heterogeneity (May and Wightman 1989a; May and Wightman 1989b; Moquin and Michael 2009). At some optimized recording locations, the evoked responses exhibit a hybrid behavior exhibiting a fast initial signal component that forms a leading shoulder on a slower subsequent signal component (Fig 1, evoked responses recorded with a 400-μm long electrode). Our previous report (Moquin and Michael 2009) established that the fast initial component is derived from non-autoinhibited DA terminals, whereas the slower subsequent component derives from autoinhibited DA terminals. The response amplitude after the first 12 pulses (200 ms) of each train is a measure of the fast initial component (Fig 1, solid diamonds), which exhibits its largest amplitude during the first stimulus train and depression during subsequent trains. The response amplitude after 60 pulses (1 s) of each train is a measure of the slower second component (Fig 1, open triangles), which exhibits its lowest amplitude during the first train and facilitation during subsequent trains. These hybrid responses demonstrate that the striatum exhibits subpopulations of DA terminals in distinct dynamical states (Moquin and Michael 2009).
Figure 1.
Kinetic hybrid responses during consecutive trains of MFB stimulation. During 1-s trains at 2-s intervals the fast-type signal component appears as a leading shoulder on the response. The signal recorded after 12 stimulus pulses (solid diamonds) was used to quantify the amplitude of the fast signal component. The signal recorded after 60 pulses (open triangles) was used to quantify the slow component.
Quinpirole (1 mg/kg i.p.) abolishes the fast component of hybrid responses (Fig 2a, responses recorded with 400-μm long electrodes). The fast component of hybrid responses was isolated by shortening the stimulus to 200 ms (12 pulses). Quinpirole (1 mg/kg i.p.) abolished all 12-pulse stimulus responses (Fig 2B and 2C). After quinpirole, even when the stimulus duration was extended, only slow-type evoked responses (slow initial responses rates and subsequent facilitation, Fig 2A) are observed. These results confirm that activation of D2 receptors abolishes the fast component of these responses.
Whereas Fig 2 illustrates evoked responses with fast-rising initial components, other striatal sites exhibit only slow-type release (Fig 3). The slow type responses are more typically observed in the striatum: slow type responses are the default response typically observed if the placement of the recording electrode is not optimized to locate fast-type or hybrid responses. The response in Fig 3A was evoked with a 45 Hz stimulus, yet an obvious increase in extracellular DA was observed only after a delay of about 3 s. Despite the delayed onset of evoked release, the signal returned towards baseline without delay at the end of the stimulus. The clearance of DA after the stimulus is catalyzed by the dopamine transporter (DAT), which is located on DA terminals. Thus, the rapid onset of clearance after the end of the stimulus implies the close proximity of the recording electrode to DA terminals. Consistent with this, raclopride (2 mg/kg i.p.) eliminated the delay in the onset of evoked release (Fig 3A), confirming the close proximity of the recording electrode to DA terminals. Hence, the delay in the onset of evoked release (pre-raclopride) is attributed to `silent' DA terminals, i.e. terminals sufficiently autoinhibited to be unable, at least initially, to release dopamine in response to the stimulus. Figure 3B is an example of the pronounced delay in evoked release but with a higher stimulus frequency (60 Hz), which confirms that the delayed response does not depend upon a specific stimulus frequency.
Kynurenate infusions affect evoked dopamine release
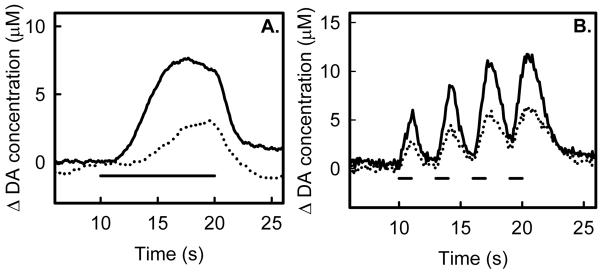
Intrastriatal infusion of KYN suppresses evoked DA release during both the continuous and multiple-train stimuli used in this study (Fig 4). Fig 4A is an example response to continuous stimulation with the onset of evoked release delayed for about 2 s after start of the stimulus. KYN infusion both extended the delay in the onset of release and decreased the overall response amplitude. Fig 4B is an example response to consecutive 1-s stimulus trains separated by 2-s intervals. KYN infusion suppressed the response to all four stimulus trains. Throughout this study, KYN infusions suppressed all evoked responses within the striatum, regardless of the style of the stimulus (continuous or multiple trains) and regardless of the dynamic feature of the pre-infusion response.
Figure 4.
Intrastriatal KYN infusion diminishes the amplitude of evoked DA release in response to continuous (A) and multiple-train (B) stimulation of the medial forebrain bundle (solid line: pre-KYN, dotted lines: post-KYN).
The effect of KYN infusion on the tonic basal DA pool
Rats were implanted with a stimulating electrode in the MFB and a voltammetric recording electrode in the ipsilateral striatum. No optimization of the placement of the recording electrode to locate fast-type or hybrid sites was performed; however sites exhibiting long onset delays in the response (e.g. Fig 3 and 4A) were excluded from the remainder of this study. Once the stimulating and recording electrodes were in position, a pre-infusion stimulus response was recorded. Then, the voltammetric signal (non-evoked) was recorded for 3 min before and 15 min after the start of KYN infusion. Finally, a post-infusion stimulus response was recorded: the post-infusion response was recorded 25 min after the pre-infusion response.
Intrastriatal KYN infusion decreased extracellular DA from its resting level at some striatal recording sites but not at others (Fig 5): these sites will henceforth be called KYN-sensitive and KYN-insensitive sites, respectively. KYN-sensitive sites are identified by a decrease in the basal voltammetric signal and by the appearance of characteristic DA oxidation and reduction peaks in the background subtracted voltammograms: this identification scheme was explained in detail by Borland and Michael (2004). In some cases, the infusion causes an artifact, which is responsible for the spike in the averaged response near 400 s in the KYN-insensitive group in Fig 5. Such spikes are infrequent (1 of 20 in this study) but have been observed before (Kulagina et al. 2001; Borland and Michael 2004). Inspection of the background-subtracted voltammograms associated with these spikes confirms that they are not DA-related. Hence, the striatum exhibits sites where the basal DA concentration is either KYN-sensitive or KYN-insensitive.
Heterogeneity of evoked DA responses
The remainder of this study examines the differences between evoked responses recorded at KYN-insensitive and KYN-sensitive sites (Note: all evoked responses in this study were affected by the KYN infusions: the terms “KYN-insensitive” and “KYN-sensitive” refer specifically to the basal DA response, as in Fig 5).
Electrical stimulation of the MFB with a continuous 10-s train (60 Hz) evokes substantially and significantly different responses at KYN-insensitive and KYN-sensitive sites (Fig 6). The responses in Fig 6 were recorded prior to KYN-infusion. The identification of the recording site as KYN-insensitive or KYN-sensitive was based on the voltammetric response to a subsequent KYN infusion. The infusion responses obtained at the sites in Fig 6 (n=4 each) are included in the data set used to construct Fig 5.
Pre-KYN infusion evoked responses were also recorded using four 1-s trains separated by 2-s intervals (Fig 7). The responses evoked by individual stimulus trains were re-zeroed to account for the fact that the voltammetric signal usually does not return to the pre-stimulus baseline during the 2-s interval between trains (Moquin and Michael 2009). As with Fig 6, the recording sites were identified as being KYN-insensitive or KYN-sensitive during a subsequent KYN-infusion. The infusion responses obtained at the sites in Fig 7 (n=6 each) are included in the data set used to construct Fig 5. The rising phase of the evoked responses from all 12 sites (Fig 7) were subjected to three-way ANOVA: the three factors were the experimental group (KYN-insensitive (triangles) and KYN-sensitive (circles)), the stimulus train (first, second, third, and fourth), and the recording time within each train (100 ms, 200 ms, 300 ms, etc.). The group factor is a highly significant contributor to the variance (F(df=1,400)=20.6, p<0.00001): thus, evoked responses at KYN-insensitive and KYN-sensitive sites are significantly different.
The maximum amplitudes of the responses to each stimulus train before and after KYN infusion (Fig 8) were also subjected to 3-way ANOVA (factors: experimental group, before and after KYN infusion, and the stimulus train number). All three factors are significant and there is a significant interaction between the experimental groups and the stimulus train. The experimental group is a significant factor (F(df =1,80)=6.1, p<0.02) and, according to Tukey pair-wise comparisons, the pre-infusion evoked responses in the KYN-insensitive and KYN-sensitive groups (Fig 8 top black bars v. Fig 8 bottom black bars) are significantly different (p<0.01). In both groups the pre-and post-infusion amplitudes (Fig 8 top black bars v. white bars; Fig 8 bottom black v. grey bars) are significantly different (Tukey pair-wise comparisons: KYN-insensitive p<0.0000001; KYN-sensitive p<0.005;). Interestingly, there is no significant difference between the absolute post-infusion amplitudes (Fig 8 top white bars v. bottom grey bars). However, when the post-KYN responses are normalized to the amplitude of the first stimulus train (Fig 8, inset), the KYN-sensitive and KYN-insensitive responses are significantly different (2-ANOVA, F(df=1,40)=5.19, p<0.03).
Discussion
Evoked DA responses in the rat striatum are heterogeneous in both their amplitude and dynamic profiles. Originally, this heterogeneity was attributed to an anatomical variation in the local density of DA terminals (May and Wightman 1989a; May and Wightman 1989b) and sites within the striatum were broadly classified as DAergic or non-DAergic. However, reexamination of this issue leads to the alternative conclusion that the heterogeneity of evoked release is instead caused by local variations in the degree to which DA terminals are autoinhibited (Moquin and Michael 2009). The present study is focused on the underlying source of the heterogeneity in autoinhibition. We examined the hypothesis that heterogeneity in the basal DA content of the striatal extracellular space is a contributing factor. Local heterogeneity in the basal DA concentration is not described in the existing literature because basal DA is typically measured by microdialysis, which is an averaging technique (Watson et al. 2006; Nandi and Lunte 2009).
In the present study, we find that the rat striatum, in addition to exhibiting heterogeneity in evoked responses, is also heterogeneous in manner in which basal (nonevoked) DA responds to intrastriatal KYN infusion (Fig 5). At some striatal sites (KYN-sensitive sites) the infusions cause the basal DA concentration to decrease from its resting level. At other sites (KYN-insensitive sites) there is no such effect. Furthermore, these distinct striatal sites yield substantially and significantly different evoked responses (Fig 6–8), supporting the overall conclusion that the heterogeneity in evoked release is coupled to the heterogeneity in the KYN sensitivity of the basal DA concentration. This coupling further supports the concept that DA terminals within the striatum are subdivided into fundamentally, and thus presumably functionally, distinct subpopulations in distinct dynamical states. It is interesting to note that the sites yielding the largest amplitude evoked responses (Fig 6–8) yielded the lowest amplitude infusion responses (Fig 5), and vice-versa, so the distinction between sites is not related in any simple manner to the density of local DA innervation (i.e. DA sites v. non-DA sites).
Thus, this study provides two findings that we regard as highly novel. First, that the striatum is spatially heterogeneous with respect to the steady-state concentration of extracellular DA. Second, that the heterogeneity of the steady-state extracellular DA contributes to the heterogeneity of the dynamical properties of DA terminals.
Autoreceptors contribute to the heterogeneity of evoked release
Evoked release in the rat striatum exhibits a range of dynamic behaviors, which we broadly classified as fast-type and slow-type (Moquin and Michael 2009). Hybrid responses (Moquin and Michael 2009 and Fig 1) indicate that DA terminals in the fast and slow states exist side-by-side within the striatum. The present study adds to the characterization of these sites by confirming that activation of D2 receptors with quinpirole abolishes the fast-type component of release (Fig 2). Quinpirole slows, but does not abolish, slow type release (Fig 2 and Moquin and Michael, 2009). These effects of quinpirole support the explanation that fast-type release is observed when the basal DA concentration is too low to activate D2 autoreceptors. On the other hand, slow-type release is observed when the basal DA concentration rises to a level sufficient to activate D2 autoreceptors.
The effects of the D2 antagonist, raclopride, on the dynamics of evoked release are complementary to those of quinpirole. Raclopride abolishes the delay in the onset of evoked release (Moquin and Michael 2009), even in cases where that delay is substantial (i.e. several seconds), as in Figs 3 and 4A. This is consistent with the explanation that the slow release behavior is a consequence of autoinhibition. These observations also show that sites yielding slow-type behavior cannot be considered non-DAergic sites (Venton et al. 2003) as DA's basal activity is clearly responsible for the on-going autoinhibition. Moreover, the non-DA sites defined anatomically by Venton et al (2003) are much smaller than the dimensions of the cylindrical (100 and 400 μm long) voltammetric electrodes used for this study. Finally, previous studies have established that D2 antagonists minimally affect fast-type release. When evoked release is sufficiently fast to be detected during so-called pseudo-1-pulse stimuli (1-to-4 pulses), D2 antagonists have little to no effect on the response (Garris et al. 1994; Garris and Wightman 1995; Benoit-Marand et al. 2001). This supports the conclusion that fast evoked responses occur when DA terminals are free of autoinhibition.
At first glance, it might appear counter-intuitive that the amplitude of fast-type release should be consistently smaller than the amplitude of slow-type release (e.g. Fig 1). However, it is necessary to consider the dynamics of release and the amplitude of release separately. This situation arises for two reasons. First, the fast component of release is short-lived because, although autoinhibition is absent prior to the onset of the stimulus, the stimulus itself raises the extracellular DA concentration, leading to a rapid onset of autoinhibition (Garris et al. 1994; Garris and Wightman 1995; Benoit-Marand et al. 2001) that results in the short-term depression of further release (Montague et al. 2004; Kita et al. 2007). This phenomenon is also observed in tissue slice preparations (Limberger et al. 1991; Kennedy et al. 1992; Phillips et al. 2002; Avshalumov and Rice 2003). On the other hand, slow-type release exhibits short-term facilitation rather than depression, so, whereas the initial rate of release is slow, the rate of release increases as the stimulation proceeds (Moquin and Michael 2009 and Figs 3 and 4). Thus, slow-type release eventually exceeds fast-type release in amplitude.
As discussed in our previous paper (Moquin and Michael 2009), we remain uncertain as to the mechanism underlying the short term facilitation of slow-type release.
Origin of the variations in autoinhibition
Voltammetric responses to intrastriatal infusion of KYN (Fig. 5) reveal the existence within the striatum of sites wherein the basal DA concentration is KYN-insensitive or KYN-sensitive. These distinct sites yield substantially and significantly different evoked responses (pre-infusion) (Fig 6–8). The amplitude of evoked release, regardless of the details of the stimulus, was significantly higher at sites where basal DA was KYN-insensitive, consistent with the interpretation that these sites contain lower basal DA concentration, making the basal response more challenging to detect but also causing less autoinhibition of evoked release. On the other hand, sites where basal DA appears KYN-sensitive exhibited significantly lower evoked release, consistent with the interpretation that these sites contain higher basal DA concentrations, making the basal response more detectable and resulting in greater autoinhibition of evoked release.
It is important to emphasize again that the sites yielding larger amplitude evoked responses yielded lower amplitude basal responses, and vice-versa. In our opinion, this eliminates the possibility that differences between the sites can be attributed to the properties of the recording or stimulating electrodes.
Overall, these findings support the conclusion that the presence within the striatum of subpopulations of DA terminals in different dynamical states is a direct consequence of variable autoinhibition stemming from local variations in the basal extracellular dopamine concentration. The ability of steady-state DA levels to affect the dynamical state of DA terminals may be described as a form of coupling between the various timescales of DA activity (Schultz 2007).
Autoinhibition is not the sole determinate of DA dynamics
However, our results also suggest that the tonic basal extracellular DA concentration is not the sole factor that determines the dynamics of evoked release. KYN infusion suppresses evoked release at both KYN-insensitive and KYN-sensitive locations (Fig 8), i.e. at all striatal locations in this study. This is not the expected results if the KYN infusion affects evoked release solely by adjusting the DAergic tone on autoreceptors. If that were the case, the effects of KYN infusions should more closely resemble those of a D2 antagonist and increase evoked release. In fact, the effects of KYN infusions (decreasing evoked release) more closely resemble those of an agonist, such as quinpirole. We attribute these findings to KYN's well-known DA-independent properties, which have been the subject of several prior studies of DA release (Lonart and Zigmond 1991; Kulagina et al. 2001; Borland and Michael 2004). These properties include KYN's ability to block both ionotropic glutamate receptors and the α7 nicotinic receptor (Hilmas et al. 2001; Rassoulpour et al. 2005; Poeggeler et al. 2007; Mitala et al. 2008; Amori et al. 2009). KYN's DA-independent actions, however, do not appear to call into question the conclusion that the heterogeneity in evoked release prior to any KYN infusion is largely driven by local variations in autoinhibition.
It is interesting to note, however, that KYN infusions eliminated the significant difference in the absolute amplitude of evoked responses at KYN-insensitive and KYN-sensitive locations (Fig 8 top white bars v. bottom grey bars), although a slight difference remains between the normalized responses (Fig 8 inset). This observation shows that KYN almost abolishes the heterogeneity of evoked release. Thus, KYN's combined DA-dependent and DA-independent actions homogenize evoked release, supporting the conclusion that subpopulations of DA terminals within the striatum exist in different dynamical states as a consequence of the presynaptic inputs they receive, which include but are not necessarily limited to the autoinhibition caused by the basal extracellular DA concentration. Although we show that the basal DA concentration is not the only source of the well-known heterogeneity in evoked DA release, it clearly plays a major role. For this reason, we conclude that a coupling exists between the slow-acting basal DA and the fast-acting DA release associated with impulse flow in nigrostriatal DA fibers.
Kynurenic acid
In this study, we employed KYN as a pharmacological tool to manipulate both the basal DA concentration and evoked DA release. KYN is an endogenous compound in the brain formed as a result of tryptophan metabolism by glia (Amori et al 2009). Disruptions in endogenous KYN levels are associated with schizophrenic symptoms in human patients (Schwarcz et al 2001), so there is intense interest in KYN itself. At micromolar concentrations, KYN acts as a broad spectrum antagonist of the ionotropic glutamate receptors. However, at endogenous nanomolar concentrations, KYN also blocks the α7 nicotinic receptor (Hilmas et al 2001), which has led to the suggestion that KYN's actions on glutamate may be an indirect consequence of its anticholinergic actions. Delivery of exogenous DA to the striatum by reverse dialysis decreases dialysate DA levels (Rassoulpour et al 2005) and inhibition of endogenous KYN synthesis enhances DA levels (Amori et al 2009). Our study was not specifically designed to address the actions of endogenous KYN as we used exogenous KYN at high concentration. Nevertheless, our observations that intrastriatal KYN infusions decrease DA, both basal and evoked, are consistent with the actions ascribed to endogenous KYN (Rassoulpour et al 2005; Amori et al 2009).
Possible functional implications
DA depletion studies provide evidence of the functional significance of steady-state extracellular DA concentrations (Abercrombie et al. 1990; Kirchhoff et al. 2009). The deficits associated with experimental or clinical DA depletions are alleviated by DA agonists (Maneuf et al. 1997; Schwarz 2003; Antonini and Barone 2008) and DA replacement with L-DOPA (Abercrombie et al. 1990; Birkmayer and Hornykiewicz 1998; Nagatsua and Sawadab 2009), therapies that in the absence of functional DA terminals are presumed to restore steady-state rather than dynamical DAergic transmission. Thus, our direct observation of steady-state basal DA functionally impacting the dynamics of DA release complements the insights derived from the effectiveness of these pharmacotherapeutic strategies for at addressing the symptoms associated with DA depletion. Moreover, other studies have identified pathological consequences of higher-than-normal steady-state DA concentrations (Murphy et al. 1996; Miyazaki and Asanuma 2008), giving rise to the concept that steady-state DA concentrations must be carefully regulated, i.e. that normal brain function depends upon steady-state DA concentrations that are neither too low nor too high. However, our study substantially extends this concept by suggesting that point-to-point variations in steady-state DA concentrations are functionally significant as well, i.e. our findings lead us to suggest that there should also be control over the size of DA terminal subpopulations exhibiting fast (non-autoinhibited) and slow (autoinhibited) dynamical behaviors.
Acknowledgement
This research was supported by the National Institutes of Health (grant no. MH 075989)
Abbreviations
- DA
dopamine
- DAT
dopamine transporter
- KYN
kynurenate
- MFB
medial forebrain bundle
Literature Cited
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525:36–44. doi: 10.1016/0006-8993(90)91318-b. [DOI] [PubMed] [Google Scholar]
- Amori L, Wu HQ, Marinozzi M, Pellicciari R, Guidetti P, Schwarcz R. Specific inhibition of kynurenate synthesis enhances extracellular dopamine levels in the rodent striatum. Neuroscience. 2009;159:196–203. doi: 10.1016/j.neuroscience.2008.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonini A, Barone P. Dopamine agonist-based strategies in the treatment of Parkinson's disease. Neurol Sci. 2008;29(Suppl 5):S371–4. doi: 10.1007/s10072-008-1049-4. [DOI] [PubMed] [Google Scholar]
- Avshalumov MV, Rice ME. Activation of ATP-sensitive K+(KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc. Natl. Acad. Sci. USA. 2003;100:11729–11734. doi: 10.1073/pnas.1834314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O. The effect of l-3,4-dihydroxyphenylalanine (=DOPA) on akinesia in parkinsonism. Parkinsonism Relat Disord. 1998;4:59–60. doi: 10.1016/s1353-8020(98)00013-3. [DOI] [PubMed] [Google Scholar]
- Booij J, Tissingh G, Winogrodzka A, van Royen EA. Imaging of the dopaminergic neurotransmission system using single-photon emission tomography and positron emission tomography in patients with parkinsonism. European Journal of Nuclear Medicine. 1999;26:171–182. doi: 10.1007/s002590050374. [DOI] [PubMed] [Google Scholar]
- Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91:220–9. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV, Rice ME. H2O2 is a novel, endogenous modulator of synaptic dopamine release. Journal of Neurophysiology. 2001;85:2468–2476. doi: 10.1152/jn.2001.85.6.2468. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, Gnegy ME. Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol. 2000;389:59–65. doi: 10.1016/s0014-2999(99)00828-6. [DOI] [PubMed] [Google Scholar]
- Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson's disease. Neuron. 2009;61:502–10. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Ewing AG, Bigelow JC, Wightman RM. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science. 1983;221:169–171. doi: 10.1126/science.6857277. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J. Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Regional differences in dopamine release, uptake, and diffusion measured by fast-scan cyclic voltammetry. In: Boulton A, Baker G, Adams RN, editors. Neuromethods Vol 27: voltammertic methods in brain systems. Humana Press; Totowa: 1995. pp. 179–220. [Google Scholar]
- Heien MLAV, Khan AS, Ariansen JL, Cheer JF, Phillips PEM, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc. Natl. Acad. Sci. USA. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Rossell S, Tucci S, Paredes D, Rada P. Improvement of the temporal resolution of brain microdialysis: sampling in seconds. In: Westerink BHC, Cremers TIFH, editors. Handbook of Behavioral Neuroscience, Vol 16: Handbook of Microdialysis Methods, Applications and Clinical Aspects. Academic Press; Amsterdam: 2007. pp. 267–277. [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent. Amino Acids. 2002;23:65–70. doi: 10.1007/s00726-001-0111-9. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem. 1992;59:449–55. doi: 10.1111/j.1471-4159.1992.tb09391.x. [DOI] [PubMed] [Google Scholar]
- Kirchhoff J, Mork A, Brennum LT, Sager TN. Striatal extracellular dopamine levels and behavioural reversal in MPTP-lesioned mice. Neuroreport. 2009;20:482–6. doi: 10.1097/WNR.0b013e32832984d6. [DOI] [PubMed] [Google Scholar]
- Kita JM, Parker LE, Phillips PE, Garris PA, Wightman RM. Paradoxical modulation of short-term facilitation of dopamine release by dopamine autoreceptors. J Neurochem. 2007;102:1115–24. doi: 10.1111/j.1471-4159.2007.04621.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–23. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kuhr WG, Ewing AG, Caudill WL, Wightman RM. Monitoring the stimulated release of dopamine with in vivo voltammetry. I: characterization of the response observed in the caudate nucleus of the rat. J. Neurochem. 1984;43:560–569. doi: 10.1111/j.1471-4159.1984.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Kuhr WG, Wightman RM, Rebec GV. Dopaminergic neurons: simultaneous measurements of dopamine release and single-unit activity during stimulation of the medial forebrain bundle. Brain Res. 1987;418:122–8. doi: 10.1016/0006-8993(87)90968-1. [DOI] [PubMed] [Google Scholar]
- Kulagina NV, Zigmond MJ, Michael AC. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 2001;102:121–8. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Levy F, Swanson JM. Timing, space and ADHD: the dopamine theory revisited. Aust N Z J Psychiatry. 2001;35:504–11. doi: 10.1046/j.1440-1614.2001.00923.x. [DOI] [PubMed] [Google Scholar]
- Limberger N, Trout SJ, Kruk ZL, Starke K. “Real time” measurement of endogenous dopamine release during short trains of pulses in slices of rat neostriatum and nucleus accumbens: role of autoinhibition. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:623–9. doi: 10.1007/BF00174745. [DOI] [PubMed] [Google Scholar]
- Lonart G, Zigmond MJ. High glutamate concentrations evoke Ca(++)-independent dopamine release from striatal slices: a possible role of reverse dopamine transport. J Pharmacol Exp Ther. 1991;256:1132–8. [PubMed] [Google Scholar]
- Maneuf YP, Crossman AR, Brotchie JM. The cannabinoid receptor agonist WIN 55,212-2 reduces D2, but not D1, dopamine receptor-mediated alleviation of akinesia in the reserpine-treated rat model of Parkinson's disease. Exp Neurol. 1997;148:265–70. doi: 10.1006/exnr.1997.6645. [DOI] [PubMed] [Google Scholar]
- May LJ, Wightman RM. Effects of D-2 antagonists on frequency-dependent stimulated dopamine overflow in nucleus accumbens and caudate-putamen. J Neurochem. 1989a;53:898–906. doi: 10.1111/j.1471-4159.1989.tb11789.x. [DOI] [PubMed] [Google Scholar]
- May LJ, Wightman RM. Heterogeneity of stimulated dopamine overflow within rat striatum as observed with in vivo voltammetry. Brain Res. 1989b;487:311–20. doi: 10.1016/0006-8993(89)90835-4. [DOI] [PubMed] [Google Scholar]
- Mitala CM, Wang Y, Borland LM, Jung M, Shand S, Watkins S, Weber SG, Michael AC. Impact of microdialysis probes on vasculature and dopamine in the rat striatum: A combined fluorescence and voltammetric study. J. Neurosci. Methods. 2008;174:177–185. doi: 10.1016/j.jneumeth.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M. Dopaminergic neuron-specific oxidative stress caused by dopamine itself. Acta Med Okayama. 2008;62:141–50. doi: 10.18926/AMO/30942. [DOI] [PubMed] [Google Scholar]
- Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, Kilpatrick MR, Wightman RM. Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci. 2004;24:1754–9. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moquin KF, Michael AC. Tonic autoinhibition contributes to the heterogeneity of evoked dopamine release in the rat striatum. J. Neurochem. 2009;110:1491–1501. doi: 10.1111/j.1471-4159.2009.06254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH. Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA. 1996;93:1325–9. doi: 10.1073/pnas.93.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsua T, Sawadab M. L-dopa therapy for Parkinson's disease: past, present, and future. Parkinsonism Relat Disord. 2009;15(Suppl 1):S3–8. doi: 10.1016/S1353-8020(09)70004-5. [DOI] [PubMed] [Google Scholar]
- Nandi P, Lunte SM. Recent trends in microdialysis sampling integrated with conventional and microanalytical systems for monitoring biological events: a review. Anal Chim Acta. 2009;651:1–14. doi: 10.1016/j.aca.2009.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Plenum Press; New York: 1979. [Google Scholar]
- Phillips PEM, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Poeggeler B, Rassoulpour A, Wu HQ, Guidetti P, Roberts RC, Schwarcz R. Dopamine receptor activation reveals a novel, kynurenate-sensitive component of striatal N-methyl-D-aspartate neurotoxicity. Neuroscience. 2007;148:188–97. doi: 10.1016/j.neuroscience.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulpour A, Wu HQ, Ferre S, Schwarcz R. Nanomolar concentrations of kynurenic acid reduce extracellular dopamine levels in the striatum. J Neurochem. 2005;93:762–5. doi: 10.1111/j.1471-4159.2005.03134.x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–4. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. Frontiers in Neuroengineering: Electrochemical Methods for Neuroscience. CRC Press Taylor Francis Group; Boca Raton, Fl: 2007. Rapid dopamine release in freely moving rats; pp. 17–34. [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annual Review of Neuroscience. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol. Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- Schwarz J. Rationale for dopamine agonist use as monotherapy in Parkinson's disease. Curr Opin Neurol. 2003;16(Suppl 1):S27–33. doi: 10.1097/00019052-200312001-00006. [DOI] [PubMed] [Google Scholar]
- Smith AD, Justice JB. The effect of inhibition of synthesis, release, metabolism and uptake on the microdialysis extraction fraction of dopamine. J Neurosci Methods. 1994;54:75–82. doi: 10.1016/0165-0270(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol. 2007;21:440–52. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. J Neurochem. 2003;87:1284–95. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Anal Chem. 2006;78:1391–9. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- Wieczorek W, Kruk ZL. Influences of neuronal uptake and D2 autoreceptors on regulation of extracellular dopamine in the core, shell and rostral pole of the rat nucleus accumbens. Brain Res. 1995;699:171–82. doi: 10.1016/0006-8993(95)00894-v. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–23. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Robinson DL. Transient changes in mesolimbic dopamine and their association with `reward'. J Neurochem. 2002;82:721–35. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–44. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther. 1999;289:266–77. [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7:581–2. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]