Abstract
Many studies indicate calcitriol has potent anti-tumor activity in different types of cancers. However, high levels of vitamin D can produce hypercalcemia in some patients. Glucocorticoids are used to ameliorate hypercalcemia and to enhance calcitriol anti-tumor activity. Calcitriol in combination with the glucocorticoid dexamethasone (Dex) increased vitamin D receptor (VDR) protein levels and ligand binding in squamous cell carcinoma VII (SCC). In this study we found that both calcitriol and Dex induce VDR- and glucocorticoid receptor (GR)-mediated transcription respectively, indicating both hormone receptors are active in SCC. Pre-treatment with Dex increases VDR-mediated transcription at the human CYP24A1 promoter. Whereas, pre-treatment with other steroid hormones, including dihydrotestosterone and R1881, has no effect on VDR-mediated transcription. Real-time PCR indicates treatment with Dex increases Vdr transcripts in a time-dependent manner, suggesting Dex may directly regulate expression of Vdr. Numerous putative glucocorticoid response elements (GREs) were found in the Vdr gene. Chromatin immunoprecipitation (ChIP) assay demonstrated GR binding at several putative GREs located within the mouse Vdr gene. However, none of the putative GREs studied increase GR-mediated transcription in luciferase reporter assays. In an attempt to identify the response element responsible for Vdr transcript regulation, future studies will continue to analyze newly identified GREs more distal from the Vdr gene promoter.
1. Introduction
Calcitriol (1α,25(OH)2D3), the active form of vitamin D has an essential role in calcium homeostasis [1]. Calcitriol promotes cell differentiation and cell cycle arrest, while inhibiting cell growth in a number of cancer cell types. Numerous studies demonstrate calcitriol has significant anti-tumor activity both in vivo and in vitro in a number of tumor models including squamous cell carcinoma, breast, colon and prostate [2]. In addition to a direct anti-proliferative effect on tumor cells, our laboratory recently demonstrated that calcitriol inhibits proliferation of tumor derived endothelial cells and tumor angiogenesis [3–5]. The action of calcitriol is exerted through binding to the vitamin D receptor (VDR), a member of the nuclear receptor superfamily. In the presence of ligand, VDR binds to vitamin D response elements (VDREs) to either increase or repress transcription of target genes [2,6,7]. VDR levels are known to be regulated at the level of transcription by a number of transcription factors including VDR itself. In addition, treatment with calcitriol stabilizes VDR and increases its own activity by phosphorylation at several residues [8–10]. Recent findings also indicate micro-RNAs may target VDR mRNAs in human cells. For example micro-RNA-125b decreases the amount of VDR protein [11].
Hypercalcemia, the dose-limiting toxicity of vitamin D, could limit use as an anti-cancer agent. Glucocorticoids, such as dexamethasone (Dex), have been shown to inhibit calcium absorption by intestine and increase calcium excretion in kidney. Thus, glucocorticoids are utilized to overcome hypercalcemic effects of calcitriol [12,13]. Previously, our laboratory examined effects of glucocorticoids, such as Dex, and demonstrated Dex increases VDR protein levels and anti-proliferative effects of calcitriol in vitro and in vivo by using the SCC model [14,15]. We present here data exploring regulation of VDR by glucocorticoids.
2. Expression and activity of VDR
Effects of vitamin D are mediated by the active metabolite calcitriol, a secosteroid hormone, which binds to intracellular VDR. VDR, a ligand dependent transcription factor, forms a heterodimer with retinoid X receptor (RXR). VDR-RXR heterodimers in turn bind to VDREs on the promoter region to either increase or repress transcription of target genes [16]. Calcitriol and VDR are critical for calcium homeostasis and normal mineralization of bone [1,2]. Effects of calcitriol and VDR activity have been explored in vivo using VDR knockout (VDR KO) mice models. VDR KO mice are hypocalcemic and exhibit a Type II rickets phenotype. In VDR KO mice, impaired calcium absorption has been proposed as the primary defect responsible for the phenotype [1,17]. Primary target organs of calcitriol include the small intestine to regulate calcium absorption, bone where it regulates calcium deposition and kidney where calcitriol regulates its metabolism [1,2,18]. In addition to calcium homeostasis, a number of observations indicate calcitriol plays a role in regulation of cellular differentiation, cell cycle arrest, proliferation and apoptosis in different tissues and cell types where VDR is expressed.
3. Glucocorticoids regulate VDR expression
Anti-proliferative effects of calcitriol in combination with glucocorticoids have been studied in vivo and in vitro. The glucocorticoid Dex sensitizes SCC cells to anti-tumor effects of calcitriol, increases VDR-ligand binding and increases VDR protein levels. Further studies indicate treatment with calcitriol or Dex alone induces cell cycle arrest in SCC, which is further enhanced upon combination treatment. Activation of apoptosis was also studied in SCC cells where Dex dramatically increases calcitriol-induced apoptosis [14,15]. To further investigate how glucocorticoids increase VDR and calcitriol effects, SCC cells were treated with Dex. Dex increased de novo Vdr transcription as seen by real-time PCR in the SCC model. Induction with 500 nM Dex increased Vdr transcript after 6, 12 or 24h of treatment with an induction of 4–6 times compared to ethanol control (Figure 1). These results are consistent with a recent finding showing that calcitriol and glucocorticoids regulate mouse VDR at the level of mRNA in adipocytes [19]. Mice harboring subcutaneously implanted SCC tumors were used to study effects of Dex on calcitriol anti-tumor activity. In addition to increased anti-proliferative effects of calcitriol in mice treated with a combination of calcitriol-Dex, there was an increase in VDR measured by hormone binding in SCC cells from implanted tumors. Interestingly, hormone binding was increased in the kidney but reduced in intestinal mucosa. Indicating differential regulation of VDR by Dex is tissue specific. Tissue specific VDR regulation may result in both an anti-proliferative effect and reduction of calcitriol induced hypercalcemia within mice as Dex reduces calcium absorption in the intestine [12,15,20–22]. This preclinical data and the need to develop new anti-cancer therapies led to evaluate the combination treatment in clinical trials and to extend the study to different models [5,13]. Increased anti-proliferative effects of calcitriol and VDR by Dex, has also been observed in tumor associated endothelial cells [5]. Contrarily, effects of Dex on calcitriol-mediated actions in prostate cells indicate Dex does not increase calcitriol anti-proliferative effects (Figure 2) or VDR-mediated transcription (data not shown). This observation is further supported by data showing decreased GR expression in prostate as prostate cancer progresses [23]. Calcitriol plus Dex treatment of androgen-independent prostate cancer patients was demonstrated to be safe, feasible and has anti-tumor activity. Although we believe a better prognosis can be predicted in light of GR status in each individual. In the SCC model it has been shown that RU486, an anti-glucocorticoid that binds GR, inhibits the ability of Dex to enhance anti-proliferative effects, cell cycle arrest, and apoptosis produced by calcitriol [14]. Results from studies using RU486 indicate a direct effect of glucocorticoids/GR in modulating actions of calcitriol. We examined the mouse Vdr gene for presence of glucocorticoids response elements (GREs) using the online suite Nubiscan [24]. Twenty putative GREs with a score of 0.6 or higher were identified between the upstream sequence (5 kb) and the first 4 introns of the Vdr gene. Chromatin immuno-precipitation (ChIP) assays demonstrate GR occupancy at several putative GREs after 2 or 3h of treatment with 100 nM Dex (data not shown). However, none of the putative GREs were able to significantly increase GR-mediated transcription in the presence of 100 nM Dex by using luciferase reporter assays (Figure 3). Previous studies indicate the sequence immediately upstream of exon 1c of the human VDR gene may function as a hormone-responsive TATA-containing promoter that responds to Dex [6]. However, this promoter region is not present in the mouse Vdr gene [25]. Our studies and the studies of others consistently suggest a direct regulation of VDR by glucocorticoids via GR in mouse and human cells. However, the mechanism involved remains unknown. It is possible that one or more GREs located farther from the mouse Vdr transcription start site may act as enhancers. Other possible mechanisms include changes in Vdr mRNA/protein stability and changes in expression/activity of transcription cofactors that may modulate Vdr expression.
Figure 1. Dexamethasone induces VDR transcripts.
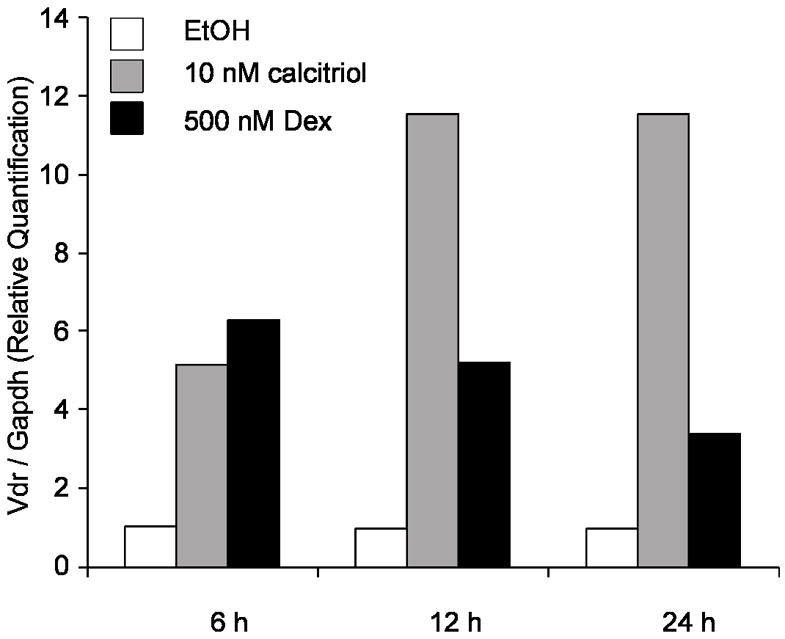
SCC cells were treated for 6, 12 and 24h with 500 nM Dex and 10 nM calcitriol. mRNA was measured using TaqMan® real-time PCR. Both 500 nM Dex and 10 nM calcitriol increased Vdr mRNA expression. Vdr mRNA transcripts levels were normalized to Gapdh expression. The results are expressed as relative quantitation compared to the ethanol control. Results of one representative experiment.
Figure 2. Dexamethasone does not increase the anti-proliferative effect of calcitriol in C4-2 cells.
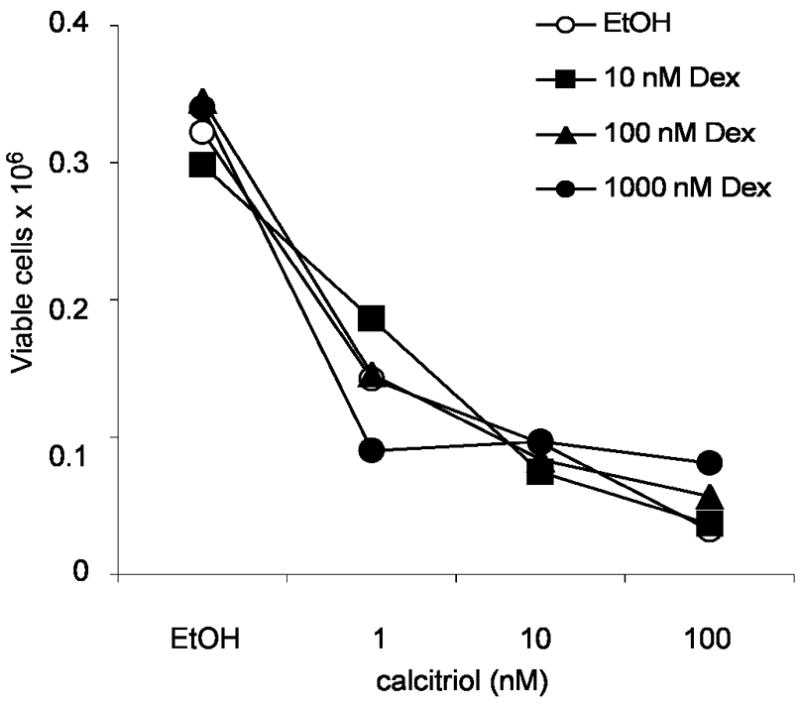
2×105 cells were seeded in 12-well plates using RPMI-10%FBS. Cells were allowed to attach for 24h and were treated with 10, 100, or 1000 nM Dex plus increasing concentrations of calcitriol or ethanol (control). After 6 days of treatments cells were trypsinized and cell viability was assessed by trypan blue dye exclusion assay. Results of one representative experiment.
Figure 3. Analysis of putative glucocorticoids response elements present in the mouse Vdr gene.
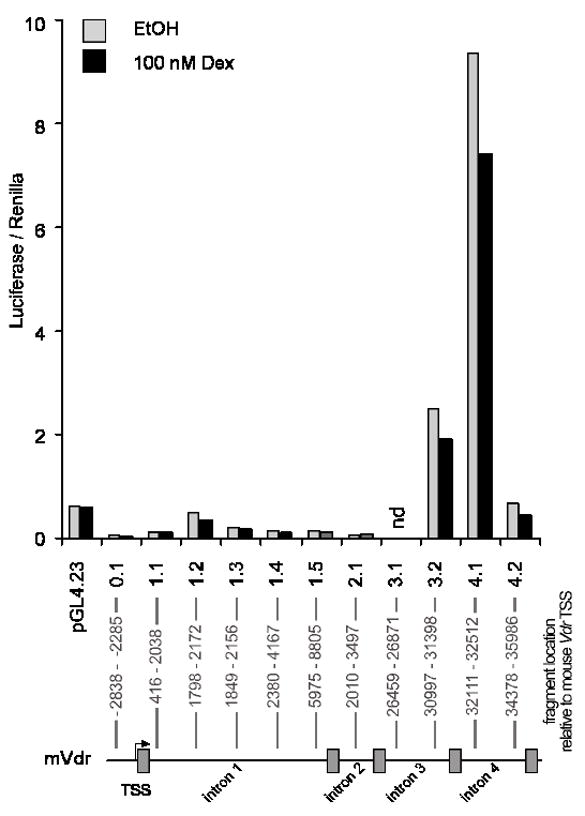
GREs found between the first 5kb upstream of mouse Vdr transcription start site and in between the first 4 introns were cloned into the minimal promoter vector pGL4.23 (Promega). The constructs were named by the intron where the putative GREs were found, follow by the region cloned between the same intron (e.g. pGL4.23-1.1 contains the GRE present in the intron 1 located at most 5′ position and pGL4.23-0.1 contains the 2 GREs found in the upstream region). SCC cells were transfected and recovered overnight in RPMI-10% charcol stripped FBS medium. After 24h of treatment with 100 nM Dex, cells were lysed and assess for luciferase activity to test the ability of GREs to enhance transcription. The results of one representative experiment are shown as units of luciferase normalized by the renilla reporter control.
4. VDR targets and calcitriol effects are modulated by dexamethasone
Glucocorticoids sensitize SCC cells to anti-tumor effects of calcitriol along with increasing VDR protein levels. Consequent increases in VDR by Dex also increase VDR-mediated transcription. Pre-treatment with Dex significantly increased VDR-mediated transcription at the human CYP24A1 promoter. Pre-treatment with other steroid hormones including dihydrotestosterone and R1881 has no effect on VDR-mediated transcription, indicating effects of Dex on VDR-mediated transcription are specific to glucocorticoids. Also in vivo results show glucocorticoids increase VDR and its target CYP24A1. Mice receiving 2 mg/kg per day for 5 days increased both Vdr mRNA and markedly increase Cyp24a1 at the level of mRNA and enzymatic activity [26]. Previous studies indicate treatment with calcitriol or Dex produces a decrease of p-Erk and p-Akt survival signals in SCC which is further potentiated by combination treatment. Activation of apoptosis was also examined by measuring cleavage of the effector caspase-3. Dex increased calcitriol-induced cleavage of caspase-3 and combination of calcitriol with Dex led to cleavage of full length PARP.
5. Conclusions
Our recent results indicate Dex potentates calcitriol effects by increasing VDR. Increases in VDR are proposed to occur at the level of transcription. Treatment of SCC cells with Dex produces an important increase of Vdr transcripts. Similar effects have been observed in mouse adipocytes and human breast cancer cell lines. The Vdr gene contains a number of putative GREs. Rapid increase in Vdr transcript levels may indicate glucocorticoids directly induce Vdr de novo transcription. Several putative GREs shows GR occupancy by ChIP assay, however none of the putative GREs show enhanced transcription by using luciferase reporter assays. Future directions include studying the direct role of GR in dexamethasone effect by using siRNAs targeting GR, potential distal enhancers, and whether glucocorticoids modulate VDR stability at either protein or mRNA levels.
6. Addendum in proof
The regulation of Vdr expression in mouse by glucocorticoids/GR signaling axis was recently addressed by the goup of Dr. Pike (Lee A. Zella, Mark B. Meyer, Robert D. Nerenz, Seong Min Lee, Melissa L. Martowicz and J. Wesley Pike, Molecular Endocrinology 24 (1): 128–147, 2010). In this work ChIP on Chip was used to identify GR binding sites that may regulate Vdr expression in mouse cells. A fragment of 2.2 kb, that binds GR, located 5.1 kb upstream of Vdr Transcription start site was found to be transcriptionally active in a luciferase reporter assay [27].
Acknowledgments
We thank Jason Kirk for reviewing the manuscript. This work was supported by NIH/NCI CA67267, CA85142 and CA95045. AAH was supported by pre-doctoral fellowship from DOD PCRP050202 and CONICYT-CHILE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, Bieglmayer C, Kato S, Cross HS. Characterization of a vitamin d receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–1435. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 2.Deeb KK, Trump DL, Johnson CS. Vitamin d signalling pathways in cancer: Potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 3.Chung I, Han G, Seshadri M, Gillard BM, Yu WD, Foster BA, Trump DL, Johnson CS. Role of vitamin d receptor in the antiproliferative effects of calcitriol in tumor-derived endothelial cells and tumor angiogenesis in vivo. Cancer Res. 2009;69:967–975. doi: 10.1158/0008-5472.CAN-08-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung I, Yu WD, Karpf AR, Flynn G, Bernardi RJ, Modzelewski RA, Johnson CS, Trump DL. Anti-proliferative effects of calcitriol on endothelial cells derived from two different microenvironments. J Steroid Biochem Mol Biol. 2007;103:768–770. doi: 10.1016/j.jsbmb.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 5.Flynn G, Chung I, Yu WD, Romano M, Modzelewski RA, Johnson CS, Trump DL. Calcitriol (1,25-dihydroxycholecalciferol) selectively inhibits proliferation of freshly isolated tumor-derived endothelial cells and induces apoptosis. Oncology. 2006;70:447–457. doi: 10.1159/000098872. [DOI] [PubMed] [Google Scholar]
- 6.Byrne IM, Flanagan L, Tenniswood MP, Welsh J. Identification of a hormone-responsive promoter immediately upstream of exon 1c in the human vitamin d receptor gene. Endocrinology. 2000;141:2829–2836. doi: 10.1210/endo.141.8.7618. [DOI] [PubMed] [Google Scholar]
- 7.Jehan F, DeLuca HF. The mouse vitamin d receptor is mainly expressed through an sp1-driven promoter in vivo. Arch Biochem Biophys. 2000;377:273–283. doi: 10.1006/abbi.2000.1788. [DOI] [PubMed] [Google Scholar]
- 8.Arriagada G, Paredes R, Olate J, van Wijnen A, Lian JB, Stein GS, Stein JL, Onate S, Montecino M. Phosphorylation at serine 208 of the 1alpha,25-dihydroxy vitamin d3 receptor modulates the interaction with transcriptional coactivators. J Steroid Biochem Mol Biol. 2007;103:425–429. doi: 10.1016/j.jsbmb.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsieh JC, Jurutka PW, Galligan MA, Terpening CM, Haussler CA, Samuels DS, Shimizu Y, Shimizu N, Haussler MR. Human vitamin d receptor is selectively phosphorylated by protein kinase c on serine 51, a residue crucial to its trans-activation function. Proc Natl Acad Sci U S A. 1991;88:9315–9319. doi: 10.1073/pnas.88.20.9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh JC, Jurutka PW, Nakajima S, Galligan MA, Haussler CA, Shimizu Y, Shimizu N, Whitfield GK, Haussler MR. Phosphorylation of the human vitamin d receptor by protein kinase c. Biochemical and functional evaluation of the serine 51 recognition site. J Biol Chem. 1993;268:15118–15126. [PubMed] [Google Scholar]
- 11.Mohri T, Nakajima M, Takagi S, Komagata S, Yokoi T. Microrna regulates human vitamin d receptor. Int J Cancer. 2009;125:1328–1333. doi: 10.1002/ijc.24459. [DOI] [PubMed] [Google Scholar]
- 12.Lee GS, Choi KC, Jeung EB. Glucocorticoids differentially regulate expression of duodenal and renal calbindin-d9k through glucocorticoid receptor-mediated pathway in mouse model. Am J Physiol Endocrinol Metab. 2006;290:E299–307. doi: 10.1152/ajpendo.00232.2005. [DOI] [PubMed] [Google Scholar]
- 13.Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin d3) and dexamethasone in androgen-independent prostate cancer. Cancer. 2006;106:2136–2142. doi: 10.1002/cncr.21890. [DOI] [PubMed] [Google Scholar]
- 14.Bernardi RJ, Trump DL, Yu WD, McGuire TF, Hershberger PA, Johnson CS. Combination of 1alpha,25-dihydroxyvitamin d(3) with dexamethasone enhances cell cycle arrest and apoptosis: Role of nuclear receptor cross-talk and erk/akt signaling. Clin Cancer Res. 2001;7:4164–4173. [PubMed] [Google Scholar]
- 15.Yu WD, McElwain MC, Modzelewski RA, Russell DM, Smith DC, Trump DL, Johnson CS. Enhancement of 1,25-dihydroxyvitamin d3-mediated antitumor activity with dexamethasone. J Natl Cancer Inst. 1998;90:134–141. doi: 10.1093/jnci/90.2.134. [DOI] [PubMed] [Google Scholar]
- 16.Carlberg C. Rxr-independent action of the receptors for thyroid hormone, retinoid acid and vitamin d on inverted palindromes. Biochem Biophys Res Commun. 1993;195:1345–1353. doi: 10.1006/bbrc.1993.2191. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin d receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF. Vitamin d and bone health. J Nutr. 1996;126:1159S–1164S. doi: 10.1093/jn/126.suppl_4.1159S. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Zemel MB. 1alpha, 25-dihydroxyvitamin d and corticosteroid regulate adipocyte nuclear vitamin d receptor. Int J Obes (Lond) 2008;32:1305–1311. doi: 10.1038/ijo.2008.59. [DOI] [PubMed] [Google Scholar]
- 20.Hirst M, Feldman D. Glucocorticoids down-regulate the number of 1, 25-dihydroxyvitamin d3 receptors in mouse intestine. Biochem Biophys Res Commun. 1982;105:1590–1596. doi: 10.1016/0006-291x(82)90970-6. [DOI] [PubMed] [Google Scholar]
- 21.Kim MH, Lee GS, Jung EM, Choi KC, Jeung EB. The negative effect of dexamethasone on calcium-processing gene expressions is associated with a glucocorticoid-induced calcium-absorbing disorder. Life Sci. 2009;85:146–152. doi: 10.1016/j.lfs.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Kim MH, Lee GS, Jung EM, Choi KC, Oh GT, Jeung EB. Dexamethasone differentially regulates renal and duodenal calcium-processing genes in calbindin-d9k and -d28k knockout mice. Exp Physiol. 2009;94:138–151. doi: 10.1113/expphysiol.2008.044339. [DOI] [PubMed] [Google Scholar]
- 23.Yemelyanov A, Czwornog J, Chebotaev D, Karseladze A, Kulevitch E, Yang X, Budunova I. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene. 2007;26:1885–1896. doi: 10.1038/sj.onc.1209991. [DOI] [PubMed] [Google Scholar]
- 24.Podvinec M, Kaufmann MR, Handschin C, Meyer UA. Nubiscan, an in silico approach for prediction of nuclear receptor response elements. Mol Endocrinol. 2002;16:1269–1279. doi: 10.1210/mend.16.6.0851. [DOI] [PubMed] [Google Scholar]
- 25.Halsall JA, Osborne JE, Hutchinson PE, Pringle JH. In silico analysis of the 5′ region of the vitamin d receptor gene: Functional implications of evolutionary conservation. J Steroid Biochem Mol Biol. 2007;103:352–356. doi: 10.1016/j.jsbmb.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 26.Akeno N, Matsunuma A, Maeda T, Kawane T, Horiuchi N. Regulation of vitamin d-1alpha-hydroxylase and -24-hydroxylase expression by dexamethasone in mouse kidney. J Endocrinol. 2000;164:339–348. doi: 10.1677/joe.0.1640339. [DOI] [PubMed] [Google Scholar]
- 27.Zella LA, Meyer MB, Nerenz RD, Lee SM, Martowicz ML, Pike JW. Multifunctional enhancers regulate mouse and human vitamin d receptor gene transcription. Mol Endocrinol. 24:128–147. doi: 10.1210/me.2009-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]


