Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) agonists are commonly used to treat cardiovascular diseases, and are reported to have several effects on cardiovascular function that may be due to PPARγ-independent signaling events. Select angiotensin receptor blockers (ARBs) interact with and modulate PPARγ activity, thus we hypothesized that a PPARγ agonist may exert physiologic effects via the angiotensin II type 1A receptor (AT1AR). In AT1AR-overexpressing HEK 293 cells, both angiotensin II (Ang II) and the PPARγ agonist troglitazone (Trog) enhanced AT1AR internalization and recruitment of endogenous β-arrestin1/2 (βarr1/2) to the AT1AR. A fluorescence assay to measure diacylglycerol (DAG) accumulation showed that although Ang II induced AT1AR-Gq protein-mediated DAG accumulation, Trog had no impact on DAG generation. Trog-mediated recruitment of βarr1/2 was selective to AT1AR as the response was prevented by an ARB and Trog-mediated βarr1/2 recruitment to β1-adrenergic receptor (β1AR) was not observed. In isolated mouse cardiomyocytes, Trog increased both % and rate of cell shortening to a similar extent as Ang II, effects which were blocked with an ARB. Additionally, these effects were found to be βarr2-dependent, as cardiomyocytes isolated from βarr2-KO mice showed blunted contractile responses to Trog. These findings show for the first time that the PPARγ agonist Trog acts at the AT1AR to simultaneously block Gq protein activation and induce the recruitment of βarr1/2, which leads to an increase in cardiomyocyte contractility.
Keywords: PPARγ, troglitazone, AT1AR, β-arrestin, cardiomyocyte, contractility
Introduction
Angiotensin II (Ang II)-mediated activation of the angiotensin type 1A receptor (AT1AR), a member of the seven-transmembrane receptor (7TMR) family, leads to the initiation of both Gq protein-dependent and -independent signaling cascades. Gq protein-dependent signaling involves 2nd messenger generation (e.g. diacylglycerol (DAG) accumulation), ultimately leading to a hypertrophic response that can be maladaptive over time, but that can be attenuated via the use of angiotensin receptor blockers (ARBs) [1; 2]. G protein-independent signaling involves recruitment of β-arrestins 1 and/or 2 (βarr1/2) [3], multifunctional scaffold proteins that are involved in numerous cell signaling events including initiation of receptor internalization, activation of protein kinase and anti-apoptotic cascades and transactivation of epidermal growth factor receptor [3; 4; 5]. β-arrestin-dependent signaling has been shown to be both physiologically relevant and beneficial in the cardiovascular system. In cardiomyocytes, β-arrestin signaling mediates protection against chronic sympathetic stimulation [6] and increases contractility in response to AT1AR stimulation [7]. Additionally, β-arrestin signaling has been shown to activate anti-apoptotic pathways downstream of AT1AR in vascular smooth muscle cells [5]. Thus, an agent that acts selectively at the AT1AR to induce beneficial β-arrestin signaling without increasing detrimental Gq protein activity could effective in the treatment of cardiovascular disorders.
Recent studies have shown that synthetic agonists for the transcription factor peroxisome proliferator-activated receptor γ (PPARγ) oppose Ang II-mediated effects, reducing the hypertrophic response of cardiomyocytes to administration of Ang II as well as decreasing Ang II-mediated signaling in blood vessels and subsequent development of hypertension [8; 9; 10; 11]. Synthetic agonists for PPARγ are typically used in the treatment of Type II diabetes to increase insulin sensitivity [12]. These agents have been shown experimentally and clinically to improve cardiovascular function via increased vascular reactivity and cardiac function, decreased inflammation and reduced visceral fat storage (as reviewed in [13]). The mechanisms by which PPARγ agonists oppose Ang II-mediated signaling are not fully understood, but may be independent of PPARγ itself. In fact, PPARγ agonists such as troglitazone (Trog) have been shown to induce acute intracellular signaling responses independent of the effects of PPARγ on transcriptional processes, though a role for 7TMRs in mediating these responses has not been explored [14; 15].
While PPARγ agonists have been shown to oppose Ang II signaling, a subset of angiotensin receptor blockers (ARBs) have been shown to be selective PPARγ modulators (SPARRMs), increasing gene transcription and modulating metabolic pathways to reduce glucose and triglyceride levels and increase insulin sensitivity [16; 17; 18]. Since PPARγ agonists can induce PPARγ-independent signaling, and select ARBs can activate PPARγ, we tested the hypothesis that PPARγ agonists activate AT1AR signaling. Here, we show that Trog stimulates the AT1AR to induce endogenous βarr1/2 recruitment and AT1AR internalization without Gq protein activation. Moreover, cardiomyocyte contractility is increased in response to Trog, an effect that is sensitive to both an ARB and βarr2 expression, indicating that activation of AT1AR-β-arrestin-mediated signaling is a mechanism by which PPARγ agonists can induce PPARγ-independent physiologic effects.
Materials and Methods
Materials
HEK 293 cells stably expressing hemagglutinin-tagged AT1AR (HA-AT1AR cells), the plasmid construct for monomeric cyan fluorescent protein (mCFP)/mYFP-tagged diacylglycerol reporter (DAGR) and βarr2 knockout mice were kindly provided by Dr. RJ Lefkowitz (Duke University Medical Center). Troglitazone (Trog) was obtained from Calbiochem (San Diego, CA). Ang II, isoproterenol and losartan were purchased from Sigma-Aldrich (St. Louis, MO).
Cell culture and pharmacological treatment
HEK 293 cells stably expressing FLAG-tagged β1-adrenergic receptor (FLAG-β1AR cells) and HA-AT1AR cells were maintained in MEM supplemented with 10% FBS and 1% pen/strep at 37°C. Cells were serum-starved for 1hr prior to drug treatments outlined in figure legends.
Fluorescent microscopy
HA-AT1AR cells were seeded into 35mm glass-bottom confocal dishes (MatTek Corporation, MA) coated with 10 μg/mL collagen (Sigma-Aldrich). After 5 min stimulation, cells were fixed with 4% paraformaldehyde/PBS for 20 min and permeabolized for 5 min with ice-cold 0.2% Triton-X 100 in PBS. Cells were blocked for 1hr and all antibodies were diluted in 0.1% BSA in PBS. HA-AT1AR was visualized using monoclonal anti-HA clone HA-7 (Sigma-Aldrich) at 1:1,000 overnight, 4°C, followed by goat anti-mouse IgG, Dylight594 (Thermo Scientific, Rockford IL) at 1:1000 for 1hr. Samples were imaged using a Leica DMI6000B inverted microscope with the Leica DFC360 FX 1.4-megapixel monochrome digital camera. This system was controlled by and deconvolution carried out using Leica AF6000 software. Each condition was performed independently 3 times.
Chemical crosslinking and immunoprecipitation
Crosslinking was performed as previously described [19]. Immunoprecipitation (IP) of samples was performed using 200 to 500 μg protein and overnight incubation at 4°C with 25 μL of monoclonal anti-HA agarose or anti-FLAG M2 agarose (Sigma-Alrich). βarr1/2 recruitment was normalized to total βarr1/2 levels and amount of immunoprecipitated HA-AT1AR or FLAG-β1AR.
Immunoblotting
Following drug treatment, cells were rinsed in ice-cold PBS then collected and lysed in buffer containing 1% NP40, 20mM Tris, pH 7.4, 137mM NaCl, 100μg/mL PMSF, 10% Glycerol, 10mM NaF, 0.36mg/mL Na3VO4 and EDTA-free HALT protease inhibitor cocktail (Thermo Scientific, Rockford, IL). Protein estimation was carried using Pierce 660nm Protein Assay Reagent (Thermo Scientific), and immunoblotting of samples performed as previously described [19]. The LI-COR biosciences Odyssey system was used for detection of immunoblots using anti-β-arrestin1/2 (D24H9) at 1:5,000 (Cell Signaling Technology, Danvers, MA) and anti-FLAG M2 at 1:10,000 (Sigma-Aldrich). IRDye800 conjugated anti-HA epitope tag was used at 1:20,000 (Rockland, Gilbertsville, PA). Non-conjugated primary antibodies were detected with IRDye680 Donkey anti-rabbit IgG (H+L) at 1:5,000 in Odyssey Blocking Buffer (LI-COR Biosciences, Lincoln, NE).
DAGR assay
HA-AT1AR cells transfected with DAGR were seeded into 35mm glass-bottom confocal dishes (MatTek Corporation, MA) coated with 10 μg/mL fibronectin. Prior to assay, cells were rinsed and incubated in buffer containing 125mM NaCl, 5mM KCl, 1.5mM MgCl2, 1.5mM CaCl2, 10mM glucose, 0.2% BSA, 10mM HEPES, pH 7.4. Cells were stimulated with drugs and underwent fluorescence resonance energy transfer (FRET) analysis as described previously [20], with quantification of the DAGR ratio over time calculated as FRET intensity/mCFP intensity, normalized to baseline.
Cardiomyocyte Contractility
Mice used in these studies were C57BL/6 mice and βarr2 knockout mice (described previously, [7; 21]), 3–6 months old and 25–40g. 10–15 cardiomyocytes were used per treatment per heart. Animals were handled according to approved protocols and animal welfare regulations of the Institutional Review Board at Duke University Medical Center. Myocyte isolation, visualization and analysis were carried out as previously described [22; 23].
Statistical Analysis
Data are presented as mean±s.e.m. All statistical analyses were performed using One-way ANOVA with Tukey's multiple comparisons test via Prism 5.0 software.
Results
Troglitazone induces AT1AR internalization and β-arrestin recruitment without activation of Gq protein
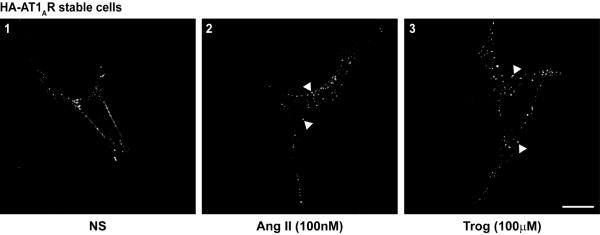
To determine if a PPARγ agonist can stimulate the AT1AR, we performed several assays to explore different facets of receptor activation. A common response to 7TMR stimulation is internalization, thus we initially aimed to determine if the PPARγ agonist Trog induces AT1AR internalization. HA-AT1AR cells were treated with Trog or Ang II, as a positive control for internalization, and HA-AT1AR puncta formation was assessed via immunofluorescence analysis. HA-AT1AR puncta formation was increased in response to both Ang II and Trog (Fig. 1, panels 2 and 3 arrowheads), indicating that the PPARγ agonist stimulates AT1AR internalization.
Figure 1. The PPARγ agonist troglitazone increases AT1AR internalization.
HA-AT1AR cells stimulated with Ang II (100nM, panel 2) or Trog (100μM, panel 3) for 5 min underwent immunofluorescent analysis for HA-AT1AR localization. Ang II and Trog stimulation each enhanced AT1AR internalization as indicated by the increased formation of HA-AT1AR-containing puncta (arrowheads), as compared to non-stimulated cells (NS, panel 1). Representative images shown, n = 3 of each condition, scale bar = 10μm.
Downstream effects of AT1AR activation are mediated by both Gq protein-dependent and βarr1/2-dependent signaling [24]. Gq protein activity can be assessed using DAGR, a fluorescent biosensor containing a diacylglycerol (DAG) binding domain flanked by mCFP and mYFP that produces an increase in intermolecular FRET in response to DAG generation at the membrane [20]. HA-AT1AR cells expressing DAGR underwent stimulation with Trog, or Ang II as a positive control for Gq protein activation. In response to Ang II, the FRET ratio increased sharply, indicating a rapid generation of DAG at the membrane (Fig. 2A) however stimulation with the same concentration of Trog (100μM) that induced AT1AR internalization did not increase DAG production, as shown by an unaltered FRET ratio. These results indicate that, while Trog induces AT1AR internalization, it does not stimulate AT1AR-mediated Gq protein activity.
Figure 2. The PPARγ agonist troglitazone induces βarr1/2 recruitment to the AT1AR in a Gq protein-independent manner.
(A) FRET analysis was used to assess the Gq protein-mediated response to agonist stimulation by detecting changes in the FRET ratio of transiently-transfected DAGR in HA-AT1AR cells. Ang II (100nM) stimulation induced a rapid increase in DAGR ratio which peaked at 90 sec, while Trog (100μM) stimulation produced no response in DAGR ratio, n = 3 independent experiments. (B) HA-AT1AR cells were stimulated for 5 min with Ang II (1–10nM) or Trog (10–100μM) and underwent crosslink/IP with HA-agarose gel. βarr1/2 was recruited to HA-AT1AR in a concentration-dependent manner in response to both Ang II and Trog, as summarized in histogram. *P<0.05, †P<0.01 and ‡ P<0.001 versus non-stimulated cells (NS), n = 6 each.
Since βarr1/2 recruitment to activated AT1ARs is required for receptor internalization [24], we tested whether Trog acts to recruit endogenous βarr1/2 to the AT1AR. HA-AT1AR cells were stimulated with increasing concentrations of Trog, or Ang II as a positive control for βarr1/2 recruitment, and underwent chemical crosslinking, immunoprecipitation of HA-AT1AR and immunoblotting analysis. Trog and Ang II each significantly increased βarr1/2 recruitment to HA-AT1AR in a concentration-dependent manner (Fig. 2B). Concentrations of Ang II in the low nanomolar range (5–10nM) produced equivalent βarr1/2 recruitment elicited by concentrations of Trog in the high micromolar range (50–100μM), concentrations shown to induce AT1AR internalization (Fig. 1) and previously shown by others to mediate PPARγ-independent effects [14; 25]. These data show that while Trog has no effect on AT1AR-coupled Gq protein activity, it does induce βarr1/2 recruitment to the AT1AR and promote receptor internalization.
Troglitazone-mediated β-arrestin recruitment is selective for the AT1AR
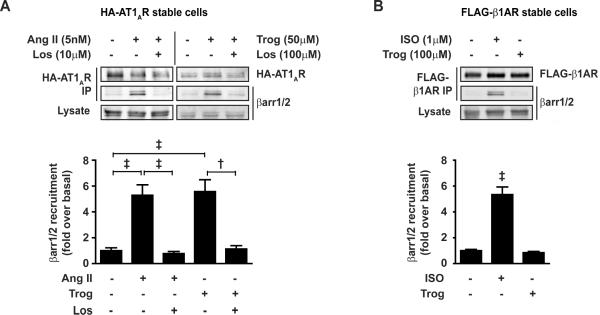
Trog-mediated βarr1/2 recruitment to the AT1AR suggests that Trog acts at the AT1AR itself to stimulate the response, thus we tested whether β-arrestin recruitment could be blocked with an ARB. Using matched submaximal concentrations of Trog (50μM) and Ang II (5nM) for βarr1/2 recruitment, the ability of the ARB, losartan (Los), to block Trog-mediated βarr1/2 recruitment to AT1AR was assessed via immunoprecipitation. As a positive control for Los-mediated inhibition of the AT1AR, Ang II-mediated βarr1/2 recruitment to HA-AT1AR was prevented by Los pretreatment (Fig. 3A). Similarly, Trog-mediated βarr1/2 recruitment to HA-AT1AR was ablated by pretreatment with Los. These results indicate that Trog acts directly at the AT1AR to induce βarr1/2 recruitment, an effect that can be surmounted with an ARB.
Figure 3. The PPARγ agonist troglitazone recruits β-arrestin to the AT1AR selectively.
(A) HA-AT1AR cells were stimulated with Ang II (5nM) ± Losartan (Los, 10μM) or Trog (50μM) ± Los (100μM) for 5 min and underwent crosslink/IP as described in Figure 2B. βarr1/2 recruitment to AT1AR was significantly increased following stimulation with either Ang II or Trog, an effect blocked by 5 min Los pretreatment, as summarized in histogram. †P<0.01 and ‡ P<0.001, n ≥ 3 each. (B) FLAG-β1AR cells were stimulated with ISO (1μM) or Trog (100μM) for 5 min and underwent crosslink/IP with FLAG-M2 agarose. βarr1/2 recruitment to β1AR was significantly increased following stimulation with ISO, but not Trog, as summarized in histogram. ‡ P<0.001 versus cells with no pharmacological treatment, n = 3 each.
To investigate whether βarr1/2 recruitment in response to Trog is selective to the AT1AR or is a nonselective response to high concentrations of Trog at 7TMRs in general, we tested the ability of Trog to induce recruitment of βarr1/2 to the β1-adrenergic receptor (β1AR). FLAG-β1AR cells were stimulated with the highest concentration of Trog (100μM) shown to induce βarr1/2 recruitment to the AT1AR, or isoproterenol (ISO, 1μM) as a positive control for βarr1/2 recruitment to the β1AR. While ISO stimulation induced a significant increase in βarr1/2 recruitment to the β1AR, Trog was unable to promote β-arrestin recruitment to the β1AR (Fig. 3B), indicating that Trog-induced βarr1/2 recruitment is selective for the AT1AR.
Troglitazone increases cardiomyocyte contractility via the AT1AR in a β-arrestin-dependent manner
To determine if a PPARγ agonist induces a physiologic effect mediated by AT1AR signaling, we measured the effect of Trog on the contractility of freshly isolated murine cardiomyocytes. Treatment of wild-type cardiomyocytes with concentrations of Trog that were shown to induce βarr1/2 recruitment to AT1AR (50–100μM, Fig. 2B) resulted in a significant increase in both the percentage and rate of cell shortening (Fig. 4A, black bars). These Trog-mediated effects on contractility were statistically indistinguishable from those of Ang II. Importantly, the Trog-mediated increase in cardiomyocyte contractility was blocked by pretreatment with an ARB (valsartan, Val). These results confirm our observation that Trog acts via the AT1AR and indicate that this interaction is capable of mediating a physiologic increase in cardiomyocyte contractility.
Figure 4. The PPARγ agonist troglitazone induces AT1AR-β-arrestin-dependent cardiomyocyte contractility.
(A) Isolated wild-type or βarr2-KO cardiomyocytes were field-stimulated at 0.5 Hz basally or following treatment with Ang II (10μM), Trog (50 or 100μM), Val (10μM) or ISO (1μM). Histograms summarize % (upper) and rate (lower) of cardiomyocyte shortening. †P<0.001 versus cells with no pharmacological treatment, n ≥ 3 individual hearts. (B) Comparison of the % change in contractility, relative to cells with no pharmacological treatment, in response to Ang II (10μM) or Trog (50 or 100μM) in wild-type versus βarr2-KO cardiomyocytes. *P<0.05 versus wild-type within corresponding treatment group.
Since βarr2 plays a role in AT1AR-mediated myocyte contractility [7], we tested whether βarr2 signaling is required for Trog-mediated effects on myocyte contractility. Cardiomyocytes isolated from βarr2 knockout mice (βarr2-KO) exhibited blunted cell shortening responses to both Ang II and Trog stimulation that were not statistically different from untreated cells (Fig. 4A, grey bars). To ensure that βarr2-KO cardiomyocytes were responsive to a contractile stimulus, they were treated with ISO, which produced a significant increase in contractile response. Comparison of the contractility increases achieved in wild-type versus βarr2-KO cardiomyocytes revealed a significantly diminished response to both Ang II and Trog in the absence of β-arr2 (Fig. 4B). Thus, βarr2 is a key mediator of Trog-induced cardiomyocyte contractility.
Discussion
In this study, we show that the PPARγ agonist Trog acts at the AT1AR to induce AT1AR internalization and ARB-sensitive recruitment of βarr1/2, in the absence of Gq protein activation. Moreover, we show that Trog increases cardiomyocyte contractility in an ARB-sensitive manner and is, at least partially, β-arrestin2-dependent. Although PPARγ agonists classically activate PPARγ to mediate gene transcription, it has also been shown that these agents can exert PPARγ-independent cell signaling processes [14; 15; 26]. Our observation that a PPARγ agonist acts at the AT1AR to rapidly recruit βarr1/2 suggests a lack of role for PPARγ-mediated transcriptional events in this process. With regard to cardiomyocyte function, our data highlights the ability of Trog to induce an acute physiologic response via AT1AR signaling, increasing cardiomyocyte contractility. These data, coupled with the ARB-sensitive nature of Trog-mediated AT1AR-β-arrestin signaling, suggest that Trog-mediated effects at the level of AT1AR are independent of PPARγ activation.
PPARγ agonists, including Trog and ciglitazone, have been previously reported to exert off-target effects, influencing intracellular processes independent of PPARγ activation in different cell lines and tissue [14; 15; 26]. One of these processes involved alterations in intracellular and/or extracellular Ca2+-handling, dependent upon the PPARγ agonist, though a role for AT1AR in this process was not explored [14]. Such effects on Ca2+-handling may explain our observation that a portion of the Trog-induced cardiomyocyte contractility was βarr2-independent, as approximately half the contractile response remained in the absence of βarr2 (Fig. 4C). In our study, we show Trog-mediated AT1AR effects are independent of Gq protein activation but dependent on β-arrestin recruitment. The ability of a 7TMR to utilize β-arrestin to exert downstream signaling is now being recognized as a mechanism to stimulate physiologic effects and accumulating data suggests that β-arrestin-mediated signaling opposes detrimental G protein-mediated activity to confer beneficial cellular effects [3; 6; 27].
The ability of ligands to stabilize receptors in certain conformations that activate particular signaling pathways but not others has been increasingly explored [28; 29]. [Sar1, Ile4, Ile8]-Ang (SII) was shown to act at the AT1AR to preferentially enhance β-arrestin-mediated signaling with no effect on Gq protein activity and has been suggested to be more cardioprotective than conventional ARBs that prevent both G protein- and β-arrestin-dependent signaling [7; 19; 30]. Our study shows that the PPARγ agonist Trog acts similarly to SII by preferentially enhancing β-arrestin recruitment to AT1AR and subsequent cardiomyocyte contractility. We postulate that PPARγ-mediated activation of AT1AR-β-arrestin signaling may in part provide an explanation for the cardioprotective effects of PPARγ agonists reported in the literature [8; 9; 10]. An additional benefit of PPARγ agonist action at the AT1AR may be the lack of potentially detrimental Gq protein-mediated signaling, especially under conditions of heightened Ang II stimulation, such as the development of hypertension. Others have suggested that the use of SPARRMs to simultaneously block AT1AR activation and induce PPARγ activity would be clinically more beneficial than conventional PPARγ agonists by exerting both antihypertensive and antidiabetic effects [17; 18]. Alternatively, using Trog as an example, we suggest PPARγ agonists may show enhanced clinical usefulness by simultaneously inducing beneficial PPARγ- and AT1AR-βarrestin-mediated effects without a concomitant increase in potentially detrimental AT1AR-Gq protein-mediated signaling.
Conclusions
Our study shows that the PPARγ agonist Trog stimulates β-arrestin recruitment to the AT1AR and induces receptor internalization independent of Gq protein activity. Effects of PPARγ agonists on acute cardiomyocyte function have not previously been reported, and here we show Trog increases cardiomyocyte contractility in a βarr2-dependent manner. We propose that a PPARγ agonist with enhanced efficiency at inducing β-arrestin recruitment to the AT1AR, in the absence of Gq protein-mediated effects, could provide a means of potentiating the beneficial effects of both PPARγ agonists and ARBs in the treatment of cardiovascular ailments.
Acknowledgements
We thank Rhonda Carter and Kristine Porter for excellent technical assistance. This research was funded by the Jefferson School of Pharmacy (Start-up Funds, D.G.T.) and the National Institutes of Health (HL-56687, H.A.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–37. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hoogwerf BJ. Renin-angiotensin system blockade and cardiovascular and renal protection. Am J Cardiol. 105:30A–5A. doi: 10.1016/j.amjcard.2009.10.009. [DOI] [PubMed] [Google Scholar]
- [3].DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- [4].Tilley DG, Kim IM, Patel PA, Violin JD, Rockman HA. beta-Arrestin mediates beta1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling. J Biol Chem. 2009;284:20375–86. doi: 10.1074/jbc.M109.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. {beta}-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem. 2009;284:8855–65. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–58. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rajagopal K, Whalen EJ, Violin JD, Stiber JA, Rosenberg PB, Premont RT, Coffman TM, Rockman HA, Lefkowitz RJ. Beta-arrestin2-mediated inotropic effects of the angiotensin II type 1A receptor in isolated cardiac myocytes. Proc Natl Acad Sci U S A. 2006;103:16284–9. doi: 10.1073/pnas.0607583103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].De Ciuceis C, Amiri F, Iglarz M, Cohn JS, Touyz RM, Schiffrin EL. Synergistic vascular protective effects of combined low doses of PPARalpha and PPARgamma activators in angiotensin II-induced hypertension in rats. Br J Pharmacol. 2007;151:45–53. doi: 10.1038/sj.bjp.0707215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Asakawa M, Takano H, Nagai T, Uozumi H, Hasegawa H, Kubota N, Saito T, Masuda Y, Kadowaki T, Komuro I. Peroxisome proliferator-activated receptor gamma plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation. 2002;105:1240–6. doi: 10.1161/hc1002.105225. [DOI] [PubMed] [Google Scholar]
- [10].Benkirane K, Viel EC, Amiri F, Schiffrin EL. Peroxisome proliferator-activated receptor gamma regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen-activated protein kinase in blood vessels in vivo. Hypertension. 2006;47:102–8. doi: 10.1161/01.HYP.0000196728.05488.c3. [DOI] [PubMed] [Google Scholar]
- [11].Wei-Guo Z, Hui Y, Shan L, Yun Z, Wen-Cheng N, Fu-Lin Y, Fang-Yan F, Jun-Hua G, Jian-Hua Z. PPAR-gamma agonist inhibits Ang II-induced activation of dendritic cells via the MAPK and NF-kappaB pathways. Immunol Cell Biol. 2009 doi: 10.1038/icb.2009.100. [DOI] [PubMed] [Google Scholar]
- [12].Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65:772–81. doi: 10.1016/j.cardiores.2004.12.008. [DOI] [PubMed] [Google Scholar]
- [13].Patel CB, De Lemos JA, Wyne KL, McGuire DK. Thiazolidinediones and risk for atherosclerosis: pleiotropic effects of PPar gamma agonism. Diab Vasc Dis Res. 2006;3:65–71. doi: 10.3132/dvdr.2006.016. [DOI] [PubMed] [Google Scholar]
- [14].Dewar BJ, Gardner OS, Chen CS, Earp HS, Samet JM, Graves LM. Capacitative calcium entry contributes to the differential transactivation of the epidermal growth factor receptor in response to thiazolidinediones. Mol Pharmacol. 2007;72:1146–56. doi: 10.1124/mol.107.037549. [DOI] [PubMed] [Google Scholar]
- [15].Gardner OS, Shiau CW, Chen CS, Graves LM. Peroxisome proliferator-activated receptor gamma-independent activation of p38 MAPK by thiazolidinediones involves calcium/calmodulin-dependent protein kinase II and protein kinase R: correlation with endoplasmic reticulum stress. J Biol Chem. 2005;280:10109–18. doi: 10.1074/jbc.M410445200. [DOI] [PubMed] [Google Scholar]
- [16].Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- [17].Schupp M, Clemenz M, Gineste R, Witt H, Janke J, Helleboid S, Hennuyer N, Ruiz P, Unger T, Staels B, Kintscher U. Molecular characterization of new selective peroxisome proliferator-activated receptor gamma modulators with angiotensin receptor blocking activity. Diabetes. 2005;54:3442–52. doi: 10.2337/diabetes.54.12.3442. [DOI] [PubMed] [Google Scholar]
- [18].Marshall TG, Lee RE, Marshall FE. Common angiotensin receptor blockers may directly modulate the immune system via VDR, PPAR and CCR2b. Theor Biol Med Model. 2006;3:1. doi: 10.1186/1742-4682-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. Beta-blockers alprenolol and carvedilol stimulate beta-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci U S A. 2008;105:14555–60. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Violin JD, Dewire SM, Barnes WG, Lefkowitz RJ. G protein-coupled receptor kinase and beta-arrestin-mediated desensitization of the angiotensin II type 1A receptor elucidated by diacylglycerol dynamics. J Biol Chem. 2006;281:36411–9. doi: 10.1074/jbc.M607956200. [DOI] [PubMed] [Google Scholar]
- [21].Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–8. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- [22].Rockman HA, Choi DJ, Akhter SA, Jaber M, Giros B, Lefkowitz RJ, Caron MG, Koch WJ. Control of myocardial contractile function by the level of beta-adrenergic receptor kinase 1 in gene-targeted mice. J Biol Chem. 1998;273:18180–4. doi: 10.1074/jbc.273.29.18180. [DOI] [PubMed] [Google Scholar]
- [23].Barki-Harrington L, Luttrell LM, Rockman HA. Dual inhibition of beta-adrenergic and angiotensin II receptors by a single antagonist: a functional role for receptor-receptor interaction in vivo. Circulation. 2003;108:1611–8. doi: 10.1161/01.CIR.0000092166.30360.78. [DOI] [PubMed] [Google Scholar]
- [24].Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–25. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- [25].Gardner OS, Dewar BJ, Earp HS, Samet JM, Graves LM. Dependence of peroxisome proliferator-activated receptor ligand-induced mitogen-activated protein kinase signaling on epidermal growth factor receptor transactivation. J Biol Chem. 2003;278:46261–9. doi: 10.1074/jbc.M307827200. [DOI] [PubMed] [Google Scholar]
- [26].Rosa AO, Egea J, Martinez A, Garcia AG, Lopez MG. Neuroprotective effect of the new thiadiazolidinone NP00111 against oxygen-glucose deprivation in rat hippocampal slices: implication of ERK1/2 and PPARgamma receptors. Exp Neurol. 2008;212:93–9. doi: 10.1016/j.expneurol.2008.03.008. [DOI] [PubMed] [Google Scholar]
- [27].Lee MH, El-Shewy HM, Luttrell DK, Luttrell LM. Role of beta-arrestin-mediated desensitization and signaling in the control of angiotensin AT1a receptor-stimulated transcription. J Biol Chem. 2008;283:2088–97. doi: 10.1074/jbc.M706892200. [DOI] [PubMed] [Google Scholar]
- [28].Galandrin S, Oligny-Longpre G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–30. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- [29].Galandrin S, Bouvier M. Distinct signaling profiles of beta1 and beta2 adrenergic receptor ligands toward adenylyl cyclase and mitogen-activated protein kinase reveals the pluridimensionality of efficacy. Mol Pharmacol. 2006;70:1575–84. doi: 10.1124/mol.106.026716. [DOI] [PubMed] [Google Scholar]
- [30].Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A. 2007;104:16657–62. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]