Abstract
Objective
The purpose of this study was to demonstrate the utility of food-reinforced operant task performance in modeling binge-eating disorder (BED). We hypothesized that food reinforcement after a caloric preload would be related to BED status, but not hunger.
Methods and Procedures
We investigated the association between reports of hunger, binge tendency, and food reinforcement in a sample of 18 women (12 non-BED, 7 lean, 5 obese, and 6 obese BED). Participants completed two sessions of operant task performance after consuming 600 ml of flavored water or 600 ml of a 1 kcal/ml liquid meal.
Results
Under the water condition, food reinforcement did not differ between the non-BED and BED groups, and was positively correlated with hunger ratings across all participants (r = 0.55, P = 0.023). Under the liquid meal condition, food reinforcement was significantly decreased compared with the water condition in the non-BED group (t = −2.6, P = 0.026). There was also a significant difference between the non-BED and BED groups in the fed condition (41 ± 40, 117 ± 60, F = 10.3, P = 0.005, non-BED vs. BED, respectively, mean ± s.d.). The correlation between food reinforcement and hunger remained significant only in the non-BED group (r = 0.69, P = 0.011).
Discussion
Our results support the hypothesis that food reinforcement measured after a caloric preload is related to BED status but not hunger in those subjects with BED. The data also suggest that operant task performance can be useful in modeling BED criteria such as “eating when not physically hungry.”
INTRODUCTION
With the dramatic increase in the incidence of obesity among the US population, there is a renewed interest in the “reward” value of food as a contributing factor to its overconsumption (1–4). The “reward” or “reinforcing” value of a substance is measured most directly by using an operant task with responding contingent on the substance of interest. Many studies of animal ingestive behavior make use of operant tasks, which measure reinforcement by requiring expenditure of effort to acquire a substance, and provide a continuous, parametric measurement of excitatory drive and motivation associated with substance “seeking” (5–7). A common schedule of reinforcement, the progressive ratio, is widely used in studies with human participants to measure desire for substances of abuse under various physiological and psychological conditions (8,9) and has recently been used to measure the reinforcing efficacy of exercise in patients with anorexia nervosa (10). It has been used occasionally to measure the effect of food deprivation on motivation to eat (11–13), self-control in the presence of readily available food (14), to describe differences between smokers and nonsmokers (15), and between lean and obese individuals (16), and to measure the effect of mood on craving for sweet foods (17).
The progressive ratio task employed by Nasser et al. (18,19) used a computer to generate the progressive ratio schedule in which clicks on the computer mouse were credited toward receipt of $1 exchangeable for either food items or nonfood items. The break point (BP), the highest number of required responses completed, was determined for food items and nonfood items. The reinforcer with the highest BP was defined as the more “efficacious” reinforcer. Comparison of food BPs under varying conditions allows for the determination of the relative reinforcing value of food under those conditions in the groups being studied.
Epstein and Leddy (20) suggest that the reinforcing value of food may be a “more powerful determinant of food intake” than either hedonics or liking, food attributes that are related to laboratory food intake, a common method for measuring human eating behavior (21,22). An inherent problem with the test meal intake measurement is that it is dependent not only on excitatory appetitive factors but also on inhibitory postingestive factors (23), a situation that can be minimized with operant task paradigms. Previous work by Epstein et al. (24) demonstrated that food-reinforced operant task performance correlates with laboratory test meal intake in lean and obese subjects without a past or current history of an eating disorder, and work by Bulik and Brinded (25) demonstrated that food-reinforced operant task performance decreased after consumption of a laboratory meal in lean women without bulimia nervosa (BN), but not in lean women with BN. Because BN involves binge eating, the study by Bulik and Brinded (25) suggests the potential for using a food-reinforced operant task to also study binge-eating disorder (BED). Recent work by Wojnicki et al. (7) described an animal model of binge-eating behavior in nonfood-deprived lean rats using a progressive ratio operant task, and Boggiano et al. (26) have described a model of binge eating in lean and obese rats using actual intake of “palatable” food; however, we are not aware of any reports of studies using operant task performance to estimate binge-eating behavior in obese people with BED.
Tanofsky-Kraff and Yanovski (27) note that more information on the BED criteria of “eating when not physically hungry” is needed and may suggest additional options for obesity treatment interventions. To operationalize the difference in “eating when not physically hungry” between those with and without BED, we evaluated the relationship between self-reported binge-eating behavior and responding in an operant task measuring food reinforcement under a fasting and fed condition.
METHODS AND PROCEDURES
This study was approved by the Institutional Review Board of St Luke’s/Roosevelt Hospital Center, and all participants signed the approved consent form before taking part.
Participants
A total of 18 women participated in the study (non-BED group: n = 12, 7 lean and 5 obese; BED group: n = 6 obese). During a telephone screening procedure, participants were queried about their use of prescription drugs, smoking habits, presence of chronic metabolic illness, and actual intake patterns with respect to the food reinforcers used in the study. Participants were excluded if they reported regular use of prescription medication, except for oral contraceptives and hormone replacement therapy; smoking within the past 6 months; having a chronic metabolic illness such as diabetes, thyroid illness, renal disease; a diagnosis of an eating disorder or psychiatric disorder; or that they did not eat any of the food reinforcers used in the study. After passing the telephone screening, participants had a baseline visit where they signed the approved consent form, completed the beck depression inventory (BDI, 28) and the binge eating scale (BES, 29), were measured for weight and height, and were trained on the operant task. BED status was determined by clinical interview, using the eating disorder examination version 12, (30) updated to include items specific to the diagnosis of BED (31–33).
The mean BES score of the non-BED group was 8.4 ± 7.3, and the average BES score of the BED group was 22.5 ± 2.8 (mean ± s.d.), with all the scores within the binge range (34). None of the participants were clinically depressed as all BDI scores were <17.
Reinforcers
Food reinforcers included Hershey’s Kisses, plain M&M’s, miniature Hershey’s milk chocolate bars, Chips Ahoy chocolate chip cookies, Oreo cookies, Doritos brand tortilla chips and cheese nacho chips, Fritos corn chips, Cheetos, Lays potato chips, pretzel bites, popcorn (prepared and microwavable), Keebler peanut butter cheese crackers.
Nonfood reinforcers included word-puzzle books, Kleenex tissue, a bandana, Glad disposable s, “Jr” Bag Clips, “Subzero” Can Coolie, memo pads, Bic pens, Chapstick, sunblock, Curel Lotion, Dove and Yardley scented soaps, Ivory and Coast soaps, “Ranir” toothbrush travel kits, Scope mouthwash, Colgate toothpaste, travel-size flash light and batteries.
Operant behavioral task
The operant behavioral task used a computer to generate a progressive ratio schedule in which responses on the computer key were credited toward receipt of $1 amount of food or nonfood items, available immediately after completing the task. The participant was seated in front of a computer screen that had two interactive icons, a picture of a bowl of cereal, which represented all food items available, and a picture of a $1 bill, which represented the nonfood items. The two pictures remained the same throughout the entire task. Under each icon was a number and the participant was instructed to press the number on the computer keyboard corresponding to the icon of the reinforcer for which they wished to work.
The task was composed of 10 1-min trials, with the number of required responses increasing after each trial independently for each chosen reinforcer. The progressive ratio schedule began with 10 responses and progressed by 20 responses to a maximum of 190 responses for the tenth trial. (Note: the participant chose between the two reinforcers and made responses on the computer key to take part in the study. There was no option to not “work” for either reward and still receive compensation for participating in the study.) After all the 10 trials were completed, the “BP” (the highest number of required responses completed for a chosen reinforcer) was determined for food and nonfood items. The reinforcer with the highest BP was defined as the more “efficacious” reinforcer under the experimental conditions for a group of participants.
Preload meal
Our recent data on laboratory test meal intake (in obese women varying in BED status) (35,36) showed that when participants were asked to consume a liquid meal product (0.5 kcal/ml) until they were extremely full, their average intake was 544 ± 189 kcal (mean ± s.d.). On the basis of these data, we chose our preload conditions to deliver 600 ml of a commercially available liquid meal product equivalent to 600 kcal (Boost, 1 kcal/ml), or 600 ml of commercially available noncaloric fruit-flavored water (Fruit2 O or Dasani). These two preloads were matched for volume consumed and the presence of flavor. Consumed volume contributes to stomach distension, a variable signaling the absence of physical hunger.
Experimental design
We used a 2 × 2 (group and preload condition) design with each preload administered on a separate day to test our hypothesis. Test sessions were separated by a minimum of 2 days. Participants were instructed not to eat or drink anything but water for 2 h before the test sessions per Raynor and Epstein (14), and all participants’ data conformed to this requirement. To encourage consistency in meal intake behavior between the two test sessions, the test sessions were scheduled only between 10 AM and 12 PM, and between 2:30 and 4:30 PM. Participants chose their first test session time, and were required to complete the second test session within the same period on another day.
The preloads for the two test sessions were 600 ml of artificially sweetened fruit-flavored water (Fruit 2 O, or Dasani), or 600 ml of a 1 kcal/ml commercially available liquid meal (Boost). The order of preload presentation was counterbalanced across participants. On test days, participants were given 5 min to consume the preload, and rated their hunger before and 15 min after preload consumption. Hunger ratings were obtained using a 150-mm visual analogue scale anchored at 0 with “not at all” and at 100 with “extremely.” After rating their hunger for the second time, the participants viewed the available reinforcers for 2–3 min, and then performed the operant task. All of this occurred within 20 min of completing ingestion of the preload.
Data analysis
Primary outcome measures were food BP and hunger ratings by visual analog scale. (Data are presented as mean ± s.d.) We used a two-way ANOVA (group × preload) to evaluate between group differences in food BP between preloads. Regression analysis was used to demonstrate within group correlation between hunger ratings and food BP. Data were analyzed with the Statistical Package for the Social Sciences (SPSS version 14, 2006; SPSS, Chicago, IL). Two-tailed P < 0.05 was needed for significance.
Results
Table 1 shows a comparison of non-BED and BED groups with respect to demographics. Both groups were matched for age and preconsumption hunger rating (39.2 ± 24.4, 38.7 ± 24.4, F = 0.001, P = 0.97, non-BED and BED, respectively) but differed significantly in BMI, BDI, and BES. The postconsumption hunger rating for the caloric preload condition was significantly less than the hunger rating for the noncaloric preload condition (t = 3.4, P = 0.003) across both groups. However, there was no significant difference in postconsumption hunger rating between the groups for either preload (22.9 ± 15.7, 23.3 ± 28.7, non-BED vs. BED; 12.1 ± 13.7, 8.3 ± 16.0, non-BED vs. BED; water and Boost, respectively).
Table 1.
Comparison of Bed and non-Bed groups
| Variable | Non-BED (7 lean, 5 obese) | BED (n = 6 obese) | P value |
|---|---|---|---|
| mean ± s.d. | mean ± s.d. | ||
| Age | 37.5 ± 12.4 | 37.0 ± 15.0 | 0.94 |
| BMI | 27.5 ± 2.9 | 38.2 ± 4.8 | 0.008 |
| BDI | 3.8 ± 5.3 | 8.3 ± 5.3 | 0.02 |
| BES | 8.4 ± 7.3 | 22.5 ± 2.8 | 0.008 |
Data analyzed with ANOVA. P < 0.05 is considered significant.
BDI, beck depression inventory; BES, binge eating scale.
Relationship of hunger to food reinforcement
Under the noncaloric preload condition (water), the main effect of a correlation between food BP and hunger across all subjects (n = 17, one BED subject whose data point was clearly an outlier was excluded) was significant (r = 0.55, P = 0.023). Under the 600 kcal preload condition (Boost), the correlation between food BP and hunger, across all subjects, was not significant (r = 0.13, P = 0.59). When examined by group, there was a group × deprivation condition interaction observed, with a significant positive correlation found between food BP and hunger in the non-BED group (r = 0.69, P = 0.012) but not in the BED group (r = −0.23, P = 0.65).
Relationship of Bed classification to food reinforcement
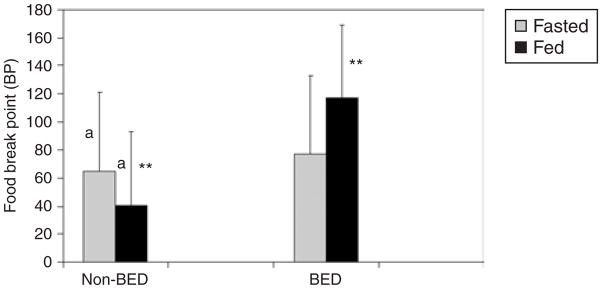
Figure 1 shows a comparison of food BP between fasted and fed conditions in subjects with and without BED. The two groups did not differ in the fasted condition (FBP = 65 ± 46 vs. 77 ± 65, F = 0.19, P = 0.66, non-BED vs. BED, respectively). However, there was a significant main effect within the non-BED group in the difference in FBP between the fasted and fed conditions (t = −2.6, P = 0.026). There was also a significant interaction effect (group × food deprivation condition) in the difference between the non-BED and BED groups in the fed condition (41 ± 40, 117 ± 60, F = 10.3, P = 0.005, non-BED vs. BED, respectively).
Figure 1.
Relationship of BED status and food deprivation state on food reinforcement. This figure shows a comparison of the differing effect of food deprivation and repletion on food reinforcement between subjects with and without BED. Error bars represent s.d. Significant differences are marked with similar letters or asterisks.
Post hoc analyses
Schebendach et al. (10) reported a positive correlation between exercise BP and BDI scores in those with anorexia nervosa. Consequently, we examined the relationship of BDI to food BP. There was a significant positive correlation between BDI score and food BP in the non-BED group after consumption of the water preload (r = 0.68, P = 0.022). There was a trend of a negative correlation between BDI and food BP in the BED group after the water preload (r = −0.79, P = 0.11). After consuming the caloric preload, there was no correlation between BDI and food BP in either group (r = −0.2, P = 0.56, non-BED group; r = −0.67, P = 0.21, BED group).
Because the non-BED group was composed of lean and obese individuals, and the BED group was composed only of obese individuals, we also performed a post hoc comparison between the obese individuals within each group to further demonstrate that our testing paradigm can model BED criteria as opposed to overeating in obese individuals. Table 2 shows the comparison of age, BMI, BDI, BES, and food BP for obese individuals with and without BED. There was no difference in age, BMI, BDI, or food BP after the noncaloric preload between these groups. There was a significant difference between groups in BES (13.4 ± 5.8, 22.5 ± 2.8, P = 0.008, non-BED vs. BED, respectively) and food BP after the caloric preload (38 ± 41, 117 ± 60, P = 0.035, non-BED vs. BED, respectively).
Table 2.
Comparison of obese individuals within the Bed and non-Bed groups
| Variable | non-BED (n = 5 obese) | BED (n = 6 obese) | P value |
|---|---|---|---|
| mean ± s.d. | mean ± s.d. | ||
| Age | 46.2 ± 11.2 | 37.0 ± 15.0 | 0.29 |
| BMI | 34.5 ± 7.7 | 38.2 ± 4.8 | 0.36 |
| BDI | 7.2 ± 6.8 | 8.3 ± 5.3 | 0.76 |
| BES | 13.4 ± 5.8 | 22.5 ± 2.8) | 0.008 |
| Food BP (noncaloric) | 74 ± 59 | 77 ± 65 | 0.95 |
| Food BP (caloric) | 38 ± 41 | 117 ± 60 | 0.035 |
Data analyzed with ANOVA. P < 0.05 is considered significant.
BDI, beck depression inventory; BES, binge eating scale; BP, break point.
DISCUSSION
We demonstrate that operant task performance is related (i) to the hunger rating after consuming the water preload across all subjects and (ii) to BED status after consuming a caloric preload, confirming our hypothesis that operant task responding for food after a caloric preload would be related to BED status but not hunger in the BED group. The relationship between food BP and hunger in both the non-BED and BED groups after the water preload is consistent with previous studies in humans (11–14) and animals (7,26), showing that food deprivation increases food-reinforced operant performance and food intake. The increased response for food after the caloric preload in the BED group agrees with results reported by Bulik and Brinded (25) in those with BN, and with results reported by Boggiano et al. (26) in binge-prone rats.
In contrast to the binge-eating behavior in response to intermittent access to palatable food in animal models (7,26,37), our results rely on a paradigm using continuous access to highly palatable food. Intermittent access to palatable food most closely resembles the binge eating associated with BN, a disorder characterized by periods of compensatory food restriction following a binge. However, in BED, the bingeing is not followed by compensatory behavior. Participants in our study were queried during screening about their habitual use of the food reinforcers, and were excluded from the study if they reported restricting use of these foods.
Although all of our participants had BDI scores below the clinical range for depression, a correlation was found between food BP and BDI under the noncaloric preload condition in both groups. The positive correlation observed in the non-BED group is opposite in direction to that reported by Willner et al. in rats (17) and Boggiano et al. (26) using stress paradigms. However, our results in the non-BED group agree with those of Willner et al. (17) in women, who showed an increase in responding for chocolate after laboratory induction of a depressive mood.
Study limitations
The main limitation of our study is the small within group “n.” Confirmation of these results with larger sample sizes is warranted. In addition, the association of BDI with food BP was observed only under the noncaloric preload condition, suggesting that the preloads themselves could possibly effect changes in mood across the test sessions. Monitoring of mood during test sessions should be included in future studies of food reinforcement when preload conditions are varied between test sessions, to control for differential effects of preloads on mood.
CONCLUSION
The demonstration of a significant relationship between food BP and BED status but not food BP and hunger, after consumption of a caloric preload, suggests that food-reinforced operant task performance can be used to model DSM-IV BED criteria such as “Eating when not physically hungry.” Previous work in the field of substance abuse has demonstrated that laboratory operant task performance can be used (i) to model seeking and use of dopamine agonist substances (i.e., cocaine, amphetamine), and (ii) to screen potential treatment paradigms for abuse of these substances (36,38–40). Given the recent reports on the association of genetic variations in dopamine receptors and transporter with binge eating (41–43), applying our laboratory model using food-reinforced operant task performance to obese individuals who meet the criteria for clinical BED may provide an additional tool for evaluating interventions for the treatment and prevention of BED.
Acknowledgments
This study was funded in part by P30DK26687. We thank Sami Hashim for critical review of the manuscript; Marci Gluck and Kochavi Galanti for performing the clinical interviews to establish BED status; Karen Tracey and Elizabeth Woods for technical assistance in the conduct of the study; Wendy Segal for assistance in data entry and management; and acknowledge the supply of Boost as an unrestricted gift from Mead Johnson and Company to A.G.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;24:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 2.Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- 4.Franken IH, Muris P. Individual differences in reward sensitivity are related to food craving and relative body weight in healthy women. Appetite. 2005;45:198–201. doi: 10.1016/j.appet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Evans SM, Nasser J, Comer SD, Foltin RW. Smoked heroin in rhesus monkeys: effects of heroin extinction and sweet fluid availability on measures of heroin seeking. Pharmacol Biochem Behav. 2003;74:723–737. doi: 10.1016/s0091-3057(02)01070-5. [DOI] [PubMed] [Google Scholar]
- 6.Foltin RW. Effects of dietary and pharmacological manipulations in appetitive and consummatory aspects of feeding in non-human primates. Appetite. 2005;45:110–120. doi: 10.1016/j.appet.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Wojnicki FHE, Roberts DCS, Corwin RLW. Effects of baclofen on operant performance for food pellets and vegetable shortening after a history of binge-type behavior in non-food deprived rats. Pharm Biochem Behav. 2006;84:197–206. doi: 10.1016/j.pbb.2006.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comer SD, Collins ED, Wilson ST, et al. Effects of an alternative reinforcer on intravenous heroin self-administration by humans. Eur J Pharmacol. 1998;345:13–26. doi: 10.1016/s0014-2999(97)01572-0. [DOI] [PubMed] [Google Scholar]
- 9.Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacology. 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- 10.Schebendach JE, Klein DA, Foltin RW, Devlin MJ, Walsh BT. Relative reinforcing value of exercise in inpatients with anorexia nervosa: model development and pilot data. Int J Eat Disord. 2007;40:446–453. doi: 10.1002/eat.20392. [DOI] [PubMed] [Google Scholar]
- 11.Lappalainen RI, Epstein LH. A behavioral economics analyses of food choice in humans. Appetite. 1990;14:81–93. doi: 10.1016/0195-6663(90)90002-p. [DOI] [PubMed] [Google Scholar]
- 12.Logue AW, King GR. Self-control and impulsiveness in adult humans when food is the reinforcer. Appetite. 1991;17:105–120. doi: 10.1016/0195-6663(91)90066-2. [DOI] [PubMed] [Google Scholar]
- 13.Kirk J, Logue A. Effects of deprivation level on humans’ self-control for food reinforcers. Appetite. 1997;28:215–226. doi: 10.1006/appe.1996.0071. [DOI] [PubMed] [Google Scholar]
- 14.Raynor HA, Epstein LH. The relative reinforcing value of food under differing levels of food deprivation and restriction. Appetite. 2003;40:15–24. doi: 10.1016/s0195-6663(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 15.Epstein LH, Wright SM, Paluch RA, et al. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. Am J Clin Nutr. 2004;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- 16.Saelens B, Epstein L. Reinforcing value of food in obese and non-obese women. Appetite. 1996;27:41–50. doi: 10.1006/appe.1996.0032. [DOI] [PubMed] [Google Scholar]
- 17.Willner P, Benton D, Brown E, et al. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharm. 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- 18.Nasser JA, Evans SM, Foltin RW. The relationship between self-reported eating behavior and performance in a behavioral task assessing food reinforcement. Appetite. 2002;39:93A. [Google Scholar]
- 19.Nasser JA, Geliebter A, Pi-Sunyer FX. Persistence of food reinforced operant task performance after a caloric preload in obese humans. Appetite. 2005;44:323A. [Google Scholar]
- 20.Epstein LH, Leddy JJ. Food Reinforcement. Appetite. 2006;46:22–25. doi: 10.1016/j.appet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JE, Crow S, Peterson CB, Wonderlich S, Crosby R. Feeding laboratory studies in patients with eating disorders. Int J Eating Disord. 1998;24:115–124. doi: 10.1002/(sici)1098-108x(199809)24:2<115::aid-eat1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Walsh BT, Boudreau G. Laboratory studies of binge eating disorder. Int J Eat Dis. 2003;34(Suppl):30–38. doi: 10.1002/eat.10203. [DOI] [PubMed] [Google Scholar]
- 23.Klein DA, Schebendach JS, Devlin MJ, Smith GP, Walsh BT. Intake, sweetness and liking during modified sham feeding of a sucrose solution. Physiol and Behav. 2006;87:602–606. doi: 10.1016/j.physbeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Epstein LH, Wright SM, Paluch RA, et al. Food hedonics and reinforcement as determinants of laboratory food intake in smokers. Physiol Behav. 2004;81:511–517. doi: 10.1016/j.physbeh.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Bulik CM, Brinded EC. The effect of food deprivation on the reinforcing value of food and smoking in bulimic and control women. Physiol Behav. 1994;55:665–672. doi: 10.1016/0031-9384(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 26.Boggiano MM, Artiga AI, Pritchett PC, et al. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs obese binge-eating and obesity with and without bingeeating. Int J Obes. 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- 27.Tanofsky-Kraff M, Yanovski SZ. Eating Disorder or disordered eating? Non-normative eating patterns in obese individuals. Obes Res. 2004;12:1361–1366. doi: 10.1038/oby.2004.171. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 29.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addict Behav. 1982;7:47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 30.Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment and Treatment. 12. Guilford Press; New York: 1993. pp. 317–331. [Google Scholar]
- 31.Celio AA, Wilfley DE, Crow SJ, Mitchell J, Walsh BT. A comparison of the binge eating scale, questionnaire for eating and weight patterns-revised, and the eating disorder examiniation questionnaire with instructions with the eating disorder examination in the assessment of binge eating disorder and its symptoms. Int J Eat Disord. 2004;36:434–444. doi: 10.1002/eat.20057. [DOI] [PubMed] [Google Scholar]
- 32.Allison KC, Crow SJ, Reeves RR, et al. Binge eating disorder and night eating syndrome in adults with type 2 diabetes. Obesity. 2007;15:1287–1293. doi: 10.1038/oby.2007.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galanti K, Gluck ME, Geliebter A. Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. Int J Eat Disord. 2007;40:727–732. doi: 10.1002/eat.20441. [DOI] [PubMed] [Google Scholar]
- 34.Marcus MD, Wing RR, Hopkins J. Obese binge eaters: affect, cognitions and response to behavioural weight control. J Consult Clin Psych. 1988;56:433–439. doi: 10.1037//0022-006x.56.3.433. [DOI] [PubMed] [Google Scholar]
- 35.Geliebter A, Hassid G, Hashim SA. Test meal intake in obese binge eaters in relation to mood and gender. Int J Eating Disord. 2001;29:488–494. doi: 10.1002/eat.1047. [DOI] [PubMed] [Google Scholar]
- 36.Nasser JA, Gluck ME, Geliebter A. Impulsivity and test meal intake in obese binge eaters. Appetite. 2004;43:303–307. doi: 10.1016/j.appet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Corwin RL. Bingeing rats: a model of intermittent excessive behavior? Appetite. 2006;46:11–15. doi: 10.1016/j.appet.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW. Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine selfadministration in humans. Psychopharmacology (Berl) 1999;143:102–110. doi: 10.1007/s002130050925. [DOI] [PubMed] [Google Scholar]
- 39.Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Gabapentin does not reduce smoked cocaine self-administration: employment of a novel self-administration procedure. Behav Pharmacol. 2007;18:71–75. doi: 10.1097/FBP.0b013e328014139d. [DOI] [PubMed] [Google Scholar]
- 40.Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- 41.Epstein LH, Wright SM, Paluch RA, et al. Relation between food reinforcement and dopamine genotypes and its effect on food intake in smokers. Am J Clin Nutr. 2004;80:82–88. doi: 10.1093/ajcn/80.1.82. [DOI] [PubMed] [Google Scholar]
- 42.Levitan RD, Masellis M, Basile VS, et al. The dopamine-4 receptor gene is associated with binge eating and weight gain in women with seasonal affective disorder. Biol Psychiatry. 2004;56:665–669. doi: 10.1016/j.biopsych.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Davis C, Levitan RD, Kaplan AS, et al. Dopamine transporter gene (DAT1) associated with appetite suppression to methylphenidate in a case-control study of binge eating disorder. Neuropsychopharmacology. 2007;32:2199–2206. doi: 10.1038/sj.npp.1301348. [DOI] [PubMed] [Google Scholar]