Abstract
Objective
The impact of glucocorticoids and mineralocorticoids on chronic otitis media (COM) in toll-like receptor 4-deficient C3H/HeJ mice was investigated.
Study Design
To evaluate control of COM by steroids with differences in their anti-inflammatory (prednisolone, dexamethasone), and fluid absorption functions (fludrocortisone, aldosterone). A minimum sample size of 5 animals for each group was required based on power analysis calculations. Sample sizes ranged from 7-17 mice per treatment group.
Subjects and Methods
ABR thresholds were performed at baseline, 2 weeks and 4 weeks. Histopathology was evaluated on all mice ears at the end of the study.
Results
ANOVA of ABR threshold change showed significant treatment effects (p <0.05) by both steroid types at all time intervals and ABR frequencies except 4 weeks/8 kHz. Histologic assessment showed prednisolone-treated mice had a higher rate of clearance of middle and inner ear inflammation (62%) than control mice (4%).
Conclusion
It was concluded that steroid treatments can improve the physiology of chronic middle and inner ear disease seen with COM.
Introduction
Chronic otitis media (COM) not only causes significant middle ear pathology, but can also impact the inner ear. Movement of cytokines, inflammatory cells, and bacterial products through the round window membrane places the cochlea at significant risk for sensorineural hearing loss, tissue remodeling, and other permanent changes. Although inner ear pathology in COM has been described in clinical reports and temporal bone studies,1,2 we have little insight into the underlying pathologic mechanisms due to lack of an appropriate animal model for chronic middle ear disease. We have recently reported that approximately 50% of C3H/HeJ mice develop spontaneous persistent middle ear disease with significant inner ear inflammation as well.3 This mouse strain has a gene defect in its toll-like receptor 4 (TLR4) which is the normal binding site for the endotoxin (lipopolysaccharide) of gram-negative bacteria. This defect is a single amino acid substitution in TLR4 that reduces its ability to respond to gram-negative infections.4 Their TLR4 defect makes them hyporesponsive to gram-negative bacteria, which they cannot clear easily upon colonization or infection. As a result, spontaneous COM develops due to colonization by a common mouse gram-negative bacterium Klebsiella oxytoca.5
Because not only the middle ear, but also the inner ear is at risk in COM, clinical intervention strives to suppress inflammation and protect the inner ear from permanent damage. The corticosteroids have differential effects on the glucocorticoid receptor (anti-inflammation and immunosuppression) and mineralocorticoid receptor (sodium transport and fluid reabsorption).6 Although glucocorticoids are given for their immune suppression functions, some (prednisone, methylprednisolone) also have equal binding affinity for the mineralocorticoid receptor. An exception is dexamethasone, which has little or no mineralocorticoid receptor binding affinity because of its chemical configuration. Mineralocorticoids, such as the natural hormone aldosterone and synthetic fludrocortisone, have little or no binding affinity for the glucocorticoid receptor. It has been reported that steroids may have an impact on Na+ and fluid transport through the middle ear mucosal epithelium.7,8 This fluid homeostasis function of the middle ear epithelium is likely to have a role in removing fluid that accumulates during inflammation in acute and chronic OM.
Therefore, these C3H/HeJ mice with COM were treated with various therapeutic steroids to determine if such treatments could control their middle and inner ear pathology. The goal of the study was to differentiate which steroid role, fluid homeostasis (mineralocorticoids), immune suppression (glucocorticoids) or both (steroid with both actions), would perform best in controlling middle ear fluid and inflammatory cell infiltration in the C3H/HeJ mouse model of COM. Evaluation of inner ear inflammation also would provide some insight into which steroids best control the cochlear sequelae of COM.
Methods
Mice
C3H/HeJ mice were obtained at two months of age from Jackson Laboratories and housed for 3-6 months. C3H/HeJ mice were screened for OM by anesthetizing them with ketamine (100 mg/ml; 0.067 mg/gm) and xylazine (20 mg/ml; 0.013 mg/gm). A Zeiss® otomicroscope was used to examine their ears at 25X power for signs of OM: middle ear effusion, bulging TM, erythema, or pus in the ear canal. C3H/HeJ mice with bilateral COM were selected for the study. Sample size was calculated using a power of 0.8 and p < 0.05. Using these parameters, an n of 5 animals (10 ears) was planned as a minimum number for each study group. Group sizes varied due to numbers of mice with bilateral otitis media from shipment to shipment of mice as the study progressed.
Steroid Treatments
Steroids were selected for their relative functions in immune suppression and fluid reabsorption (Na+ transport). Prednisolone is predominantly immunosuppressive but also has strong mineralocorticoid receptor binding, so it has both functions (Table 1). On the other hand, dexamethasone has little or no mineralocorticoid effect (Table 1). Aldosterone has no glucocorticoid receptor binding, hence no immune suppression, whereas fludrocortisone has a strong mineralocorticoid effect and only a minor glucocorticoid function (Table 1). Mice with bilateral COM were treated for 4 weeks with these various steroids: prednisolone (n=17), dexamethasone (n=16), aldosterone (n=7) and fludrocortisone (n=7). Untreated mice (n=12) served as controls and were given tap water without any steroid. Prednisolone, fludrocortisone and aldosterone were given in the drinking water at the following doses: prednisolone 5 mg/kg/day; fludrocortisone 10 μg/kg/day; aldosterone 15 μg/kg/day.6 Dexamethasone was given as a subcutaneous injection daily at 0.75 mg/kg/day. The study drugs used were given in equivalent therapeutic doses.6
Table 1.
Steroids to Control Inflammation and Fluid
| Glucocorticoid Effect (Immunosuppression) | |||
|---|---|---|---|
| + | - | ||
| Mineralocorticoid Effect (Fluid Homeostasis) | + | Prednisolone | Fludrocortisone Aldosterone |
| - | Dexamethasone | Water | |
Auditory Brainstem Response Electrophysiology
ABR thresholds were measured before treatment and at 2 and 4 weeks after treatment was initiated. ABR thresholds were measured at 4, 8, 16 and 32 kHz according to our standard protocol on all ears to determine levels of cochlear function and middle ear status.9 Comparison of baseline thresholds with those at 2 and 4 weeks of treatment would demonstrate if any of the steroids were altering the progression of hearing loss seen in the untreated controls. An analysis of variance (ANOVA, SPSS®) of the ABR thresholds shifts was performed to determine if the treatment groups were different from the untreated controls. If overall group effects were seen, post-hoc Tukey tests were conducted to differentiate which steroid groups were significantly different from controls. In all statistical tests, a probability less than 0.05 was chosen as significant. Paired T tests were also performed of the four average ABR thresholds for the prednisolone-treated animals and controls at baseline and 4 weeks. Data used for the paired T tests was pooled ear ABR data.
Middle Ear Histopathology
Histologic examination was made of middle ear and inner ear pathology at the termination of the study. After the 4 week ABR measurement, animals were euthanized by an overdose of anesthetic (ketamine and xylazine), intracardially perfused with fixative (1.5% glutaraldehyde - 3% paraformaldehyde in 0.1M Phosphate buffer), and the dissected skulls immersed in fixative overnight. The middle and inner ears were left intact and connected to each other by the skull base so both ears were processed together for histology and sectioning. Tissues were microwave decalcified in EDTA, embedded in glycol methacrylate plastic, sectioned in the horizontal plane at 5 μm, and every tenth section serially mounted on glass slides, stained, and coverslipped. Slides were examined with the Leica® DMLB microscope. Middle ear inflammation was qualitatively evaluated for presence of fluid, inflammatory cell infiltration, and edematous changes in the middle ear mucosa and round window (RW) membrane. The inner ear was assessed for pathology. Of particular interest was the presence or absence of bacteria, inflammatory cell infiltrates, and fibrillar deposition.
Role of the funding source
The funding agency for this study had no involvement in study design, collection, analysis or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
All animal procedures were approved by OHSU IACUC.
Results
Steroid Treatments
All mice survived the steroid treatments with no attrition or adverse effects. None avoided drinking the water containing the drugs or became dehydrated. The injections of dexamethasone were well tolerated. Therefore, changes in thresholds are presumed to be the result of progression of middle ear and inner ear disease and not due to additional systemic illness or adverse drug effects.
ABR Electrophysiology
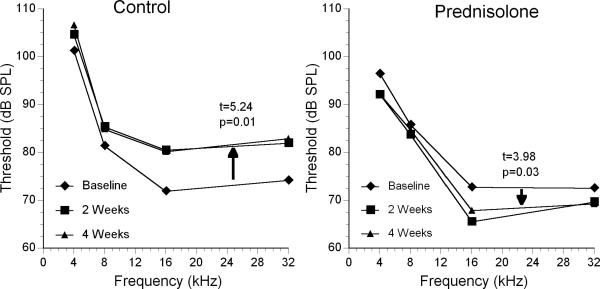
The group receiving no treatment showed significant worsening of the ABR thresholds at both 2 and 4 weeks compared to baseline (Fig 1). A statistical comparison of thresholds at baseline and 4 weeks showed this elevation was significant (paired t = 5.24; p = 0.01). On the other hand, prednisolone-treated mice showed a decrease in thresholds (Fig 1) that also was statistically significant (paired t = 3.98; p = 0.03). The other steroids did not show as dramatic a change in threshold during the treatment period. Because the extent of middle and inner ear disease, and therefore threshold, varies considerably between individual mice, group baselines and absolute thresholds were difficult to compare statistically due to different treatment starting points. Therefore, an ANOVA was conducted of the change in thresholds at 2 weeks and 4 weeks to provide a better comparison of treatment effects.
Figure 1.
Average ABR threshold change from baseline after 2 and 4 weeks of treatment with prednisolone compared to untreated controls. Paired t values and probability performed for the averaged ABR thresholds at four frequencies for the control and prednisolone-treated animals at baseline and 4 weeks.
ANOVA comparing ABR threshold change by time of treatment showed a significant treatment effect. The treatment groups at 2 weeks were statistically different at all 4 frequencies (Table 2). This also was the case for 4 week treatment threshold changes except that no statistically significant group differences were seen at 8 kHz (p = 0.098). Threshold shifts at the different frequencies in untreated mice ranged from 4 to 10 dB from baseline, demonstrating the continuing decline in hearing sensitivity over the 4 week period (Fig. 1). To differentiate which groups were statistically different from controls, post-hoc comparisons were carried out (Table 2). It appeared that all of the steroid treatment groups had less hearing decline at some frequencies than the untreated COM mice.
Table 2.
ANOVA and Tukey Post-hoc Analysis of Treatment Effects on ABR Threshold Change
| Differences at 2 Weeks | Differences at 4 Weeks | |||||||
|---|---|---|---|---|---|---|---|---|
| Group ANOVA | 4 kHz | 8 kHz | 16 Khz | 32 Khz | 4 kHz | 8 kHz | 16 Khz | 32 Khz |
| F ratio probability |
3.42 0.011 |
3.18 0.016 |
17.65 0.001 |
4.56 0.002 |
4.38 0.003 |
2.01 0.098 |
8.05 0.001 |
4.37 0.003 |
| Post-hoc vs. Controls: |
||||||||
| Prednisolone |
MD = -7.73
SE=2.68 (-15.17,-0.28) p=0.038 |
MD=-6.05
SE=2.17 (-12.09,-.018) p=0.049 |
MD=-15.79
SE=1.91 (-21.09,-10.48) p=0.001 |
MD=-10.67
SE=2.90 (-18.45,-2.88) p=0.002 |
MD=-9.67
SE=2.55 (-16.7,-2.57) p=0.002 |
MD=-4.53 SE=2.24 (-10.76,1.70) p=0.266 |
MD=-13.10
SE=2.34 (-19.60,-6.59) p=0.001 |
MD=-11.97
SE=2.90 (-20.02,-3.92) p=0.001 |
| Dexamethasone | MD=-5.25 SE=2.72 (-12.79,2.29) p=0.308 |
MD=-5.81 SE=2.21 (-11.93,.305) p=0.071 |
MD=-8.27
SE=1.93 (-13.64,-2.89) p=0.001 |
MD=-3.26 SE=2.94 (-11.1,4.62) p=0.781 |
MD=-7.03 SE=2.59 (-14.21,149) p=0.058 |
MD=-4.69 SE=2.27 (-11.00,1.62) p=0.246 |
MD=-6.78
SE=2.37 (-13.36,-.194) p=0.04 |
MD=-6.08 SE=2.94 (-14.23,2.06) p=0.241 |
| Fludrocortisone | MD=-1.23 SE=3.38 (-10.62,8.16) p=0.996 |
MD=-4.35 SE=2.74 (-11.97,3.26) p=0.510 |
MD=-6.51 SE=2.41 (-13.20,.178) p=0.06 |
MD=-1.14 SE=3.66 (-10.96,8.67) p=0.998 |
MD=-7.09 SE=3.22 (-16.03,1.85) p=0.188 |
MD=-4.21 SE=2.83 (-12.08,3.65) p=0.574 |
MD=-8.61
SE=2.95 (-16.81,-.40) p=0.035 |
MD=-5.13 SE=3.66 (-15.29,5.01) p=0.627 |
| Aldosterone | MD=1.26 SE=3.38 (-8.12,10.66) p=0.996 |
MD=-0.07 SE=2.74 (-7.69,7.54) p=1.000 |
MD=-6.79
SE=2.41 (-13.48,-.106) p=0.045 |
MD=-6.43 SE=3.66 (-16.25,3.38) p=0.369 |
MD=-1.80 SE=3.22 (-10.74,7.13) p=0.981 |
MD=0.43 SE=2.83 (-7.43,8.29) p=1.000 |
MD=-5.68 SE=2.95 (-13.88,2.52) p=0.313 |
MD=-5.49 SE=3.66 (-15.64,4.65) p=0.565 |
MD = mean difference
SE = standard error
95% confidence intervals (listed under each SE)
Significant p values (< 0.05) noted in bold
Glucocorticoids
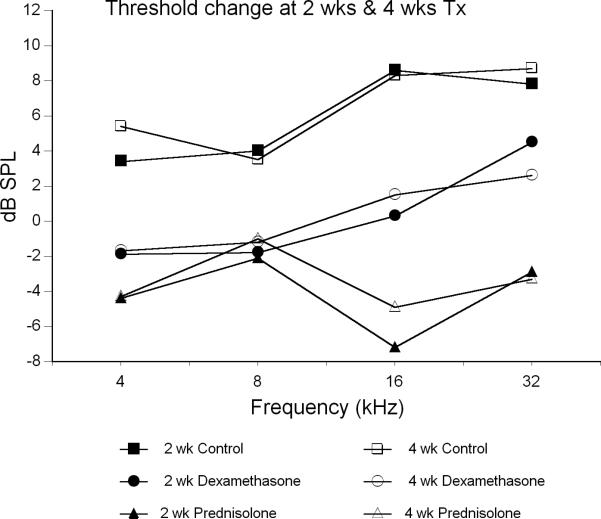
Significant differences were found in the glucocorticoid treatment groups compared to controls (Fig 2). Prednisolone had the strongest treatment effect (Table 2), as suggested by the threshold data in Figure 1. Prednisolone-treated animals were significantly better than controls at all frequencies after 2 weeks and at all frequencies except 8 kHz at 4 weeks (Table 2). Average improvement in thresholds from baseline for this group ranged from 2 to 7 dB over the treatment period (Fig. 2). Thus, the prednisolone treatment prevented the hearing decline seen in untreated controls and actually improved thresholds.
Figure 2.
Average ABR threshold change from baseline after 2 and 4 weeks of treatment with glucocorticoids compared to untreated controls. Probability values from Tukey post-hoc comparisons (Table 2).
Dexamethasone treatment was only moderately effective, showing a slight difference from controls at 16 kHz at 2 weeks and at 4 weeks (Table 2). It showed a trend for being different at 4 kHz at 4 weeks (p = 0.058), but this did not quite reach the statistical probability cutoff of 0.05. After 4 weeks of treatment, the dexamethasone mice showed +/- 2 dB changes from baseline (Fig 2), suggesting the drug may have slowed the decline of hearing, but did not dramatically improve hearing levels like prednisolone.
Mineralocorticoids
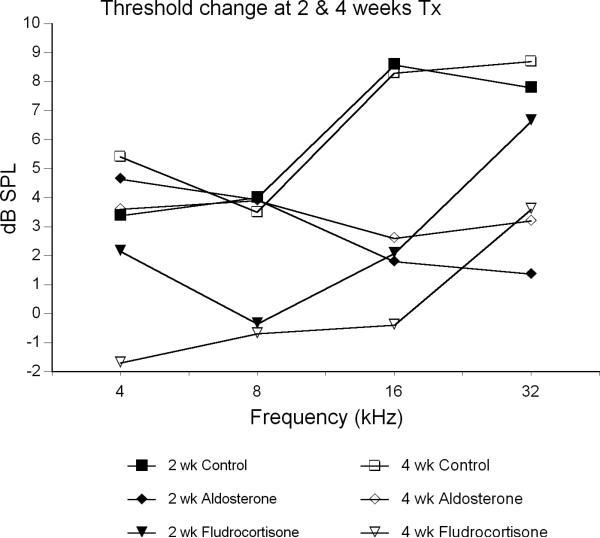
Mineralocorticoids overall were much less effective than the glucocorticoids in reversing the decline in hearing (Fig 3; Table 2). At 4 weeks, mice receiving fludrocortisone showed a +/- 2 dB threshold change from baseline. However, their difference from controls only reached statistical significance at 16 kHz (p = 0.035). Furthermore, the treatment effect from aldosterone was significant (p = 0.045) only at 2 weeks (16 kHz). By 4 weeks, aldosterone mice showed increased thresholds of 3-4 dB, a decline in hearing similar to controls.
Figure 3.
Average ABR threshold change from baseline after 2 and 4 weeks of treatment with mineralocorticoids compared to untreated controls. Probability values from Tukey post-hoc comparisons (Table 2).
Thus, while overall group differences were seen by ANOVA, the most consistent differences from control mice were limited to the prednisolone treatment. The other treatments, while showing less of hearing decline than controls, were only sporadically different from controls by post-hoc tests.
Histology
Untreated mice
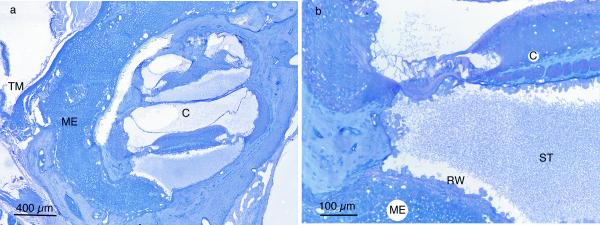
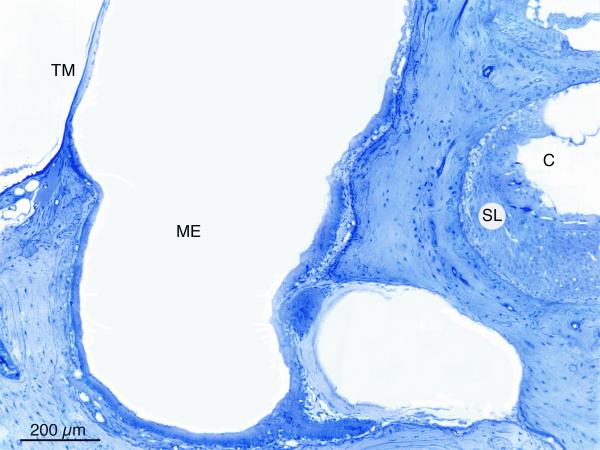
Histologic examination of the middle and inner ears revealed the amount and character of cellular infiltrates in both untreated and treated animals. The cellular infiltrate in the infected middle ears was dense, highly cellular (macrophages, neutrophils), and often filled the entire middle ear space (Fig 4). Many animals with infected middle ears also had a significant amount of inner ear inflammation. This was characterized by bacteria and/or inflammatory cells in the scala vestibuli and/or scala tympani (Fig 4). This middle and inner ear disease was also frequently seen in cochleae of untreated COM ears, indicating the significant impact of middle ear disease on the inner ear.
Figure 4.
(A) Middle ear (ME) and inner ear (C) inflammation in dexamethasone-non-responder C3H/HeJ ear. The middle ear inflammatory cells fill the entire space medial to the tympanic membrane. (B) Higher magnification shows inflammatory cells and fibrillar debris in scala tympani (ST). External to the round window membrane (RW) are densely packed inflammatory cells in middle ear (ME). (TM – tympanic membrane)
No untreated mice cleared their middle ear inflammation during the study period (Table 3). All 24 middle ears of the 12 untreated mice had persistent OM and 23 (96%) of the inner ears showed some degree of inflammation. This continued inflammation in the untreated mice over the treatment period implied that once they develop middle ear disease it does not clear on its own. This observation suggested that any histologic absence of inflammation in the steroid treated mice was probably due to the treatment and not spontaneous recovery.
Table 3.
Clearance or Persistence of Inflammation within the Middle and Inner Ear
| Middle ear | Inner ear | Controls | Pred | Dex | Fludro | Aldo |
|---|---|---|---|---|---|---|
| Clear | Clear | 0 | 8 (26%) | 0 | 0 | 0 |
| Clear | Inflamed | 0 | 3 (9%) | 0 | 0 | 0 |
| Inflamed | Clear | 1 (4%) | 10 (29%) | 0 | 0 | 3 (21%) |
| Inflamed | Inflamed | 23 (96%) | 13 (38%) | 32 (100%) | 14 (100%) | 11 (79%) |
| Total Ears | 24 | 34 | 32 | 14 | 14 | |
Steroid treatments
A total of 17 bilaterally inflamed mice (34 ears) received the prednisolone treatment. Of these, 11 (35%) middle ears were clear at the end of the treatment period (Table 3), although 3 of their ipsilateral inner ears had residual inflammation. These clear middle ears had no inflammatory cells or fluid remaining, suggesting they had cleared as a result of the treatment (Fig 5). Also, 29% of prednisolone-treated ears had middle ear inflammation only (without inner ear manifestations) compared to only 4% of controls (Table 3). Only 38% of prednisolone ears had both middle and inner ear inflammation, which was in contrast to 96% of controls. Thus it appeared that prednisolone treatment had a significant effect of reducing inflammation in both the middle and inner ears, paralleling the improvement in ABR thresholds in this group.
Figure 5.
Prednisolone treated ear showing absence of all middle ear (ME) and inner ear (C) inflammation. (TM – tympanic membrane)
As suggested by the ABR data, dexamethasone had little impact on the progression of hearing loss and middle and inner ear disease. All 16 mice still had bilateral middle and inner ear inflammation at the end of the treatment period (Table 3; Fig 4). This also was the case with the mineralocorticoids (Table 3). Although 3 aldosterone-treated middle ears (21%) did not show inner ear inflammation, the remaining 79% did, as well as all fludrocortisone ears. Histologically these were similar in appearance to the untreated controls.
Thus, the histologic assessments paralleled the findings of the ABR electrophysiology. The most significant improvement in histologic manifestation of inflammation was with the prednisolone treatments that cleared middle ear disease in more than one-third of mice. Also, the incidence of inner ear inflammation was significantly decreased. The other steroids did not appreciably reduce the inflammation in either the middle or inner ears.
Discussion
The untreated mice in the present study show the significant progression of inflammation and threshold elevation that occurs with chronic otitis media. Even during the 4 week study period, thresholds increased by several dB and all but one mouse had some degree of inflammation in the inner ear.
Treated mice showed the glucocorticoid prednisolone was the most effective of the steroids in controlling the hearing loss associated with chronic otitis media. Significant hearing improvement over controls was seen at both 2 and 4 weeks in nearly all frequencies. This was also reflected in the reduction of histologic manifestations of inflammation in both the middle ear and inner ear. The other glucocorticoid dexamethasone was not as effective, in spite of the fact it is a commonly prescribed glucocorticoid for suppurative OM and labyrinthitis. The efficacy of prednisolone may relate to the fact it has a significant fluid absorption function as well, mediated by its strong binding affinity for the mineralocorticoid receptor. Thus, prednisolone might be better at improving middle ear disease because it also is clearing the effusion that accompanies the chronic inflammation.7,8 Herman et al10 provided evidence from middle ear epithelium tissue culture that dexamethasone was more effective than aldosterone in generating the short circuit current, which is dependent on Na+ transport. Those results are at variance with the current findings, but the reason isn't immediately clear. They also showed aldosterone, a natural mineralocorticoid, had no effect on Na+ transport, which is quite unexpected given its role in Na+ transport by increasing Na+ channels and producing Na+, K+-ATPase. Portier et al11 showed that sodium absorption was enhanced fourfold in cultured middle ear epithelial cells via increase in expression of the α-subunit of the epithelial sodium channel (α-ENaC). Various reports suggest the mixed action glucocorticoids and mineralocorticoids could enhance clearing of middle ear fluid in the setting of a middle ear effusion.
The mineralocorticoids, aldosterone and fludrocortisone, were not very effective in our study in clearing middle ear inflammation, presumably because of they have little influence in immune suppression. Although they appeared to prevent further progression of middle ear disease, they were not consistently better than no treatment. This suggests fluid absorption without suppression of inflammation is simply not sufficient to control ongoing disease. Certainly more studies are necessary to clarify this hormonal control of middle ear fluid clearance, but these studies offer preliminary evidence of the respective roles of corticosteroid groups.
Studies of the efficacy of steroids for treatment of middle ear inflammation in both animals and humans exist. A rat model of tympanic membrane healing showed decreased sequelae (myringosclerosis) to the eardrum from myringotomy if rats were treated with intramuscular dexamethasone compared to saline or negative controls (RU486).12
Human studies on the effectiveness of steroids for COM have shown effectiveness with short term use, especially when combined with antimicrobials.13,14 In a prospective double blind randomized trial of steroids with antibiotics vs. antibiotics alone, Mandel et al15 showed a significant resolution of OME at 14 days with steroids and antibiotics over antibiotics alone, but saw no difference between the two treatment groups at 4 weeks. Use of oral steroids (Prednisolone) in addition to antibiotics for acute otorrhea via tympanostomy tubes has also been shown to be efficacious.16 It appears from the human OM literature that steroids are more effective in the acute OM setting than for long term resolution of COM.
Two drawbacks exist to human steroid use for otitis media. Firstly, meta-analyses of studies of steroids and antimicrobials for COM have shown decreased effect of steroids after several weeks.17 Also, there is reluctance to give prolonged oral steroid courses to patients (especially children) because of the risks associated with long-term steroid use, such as behavioral changes, appetite changes and adrenal suppression. However, topical combination steroid/antibiotic containing preparations for middle ear infections accessible via a tympanostomy tube or eardrum perforation are widely accepted and shown to have increased efficacy over topical antibiotic preparations alone.18 In addition, dexamethasone in combination with antibiotics delivered to the middle ear does not impair the in vitro antibacterial efficacy of the antibiotic and in fact serves as an otoprotectant to the inner ear.19 Future development of ways to administer a combined antibiotic/steroid preparation through the intact eardrum to shorten antibiotic exposure (and thus lessen antibiotic resistance) and decrease middle and inner ear inflammation (via steroids) may improve and shorten treatment for otitis media.
Conclusion
Our study shows that the combined immunosuppressive and fluid absorption actions of prednisolone were the most effective in reducing the severity of chronic middle ear disease and improving cochlear inflammation in the COM mouse model. These studies offer insight into the potential steroid control of middle and inner ear disease during chronic otitis media.
Acknowledgements
Supported by NIH-NIDCD R01 DC05593 & DC005593-S1, NIH-NIDCD R21 DC007443, and NIH-NIDCD P30 DC005983.
We wish to acknowledge the statistical assistance of Susan Griest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cureoglu S, Schachern PA, Paparella MM, et al. Cochlear changes in chronic otitis media. Laryngoscope. 2004;114:622–626. doi: 10.1097/00005537-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Schachern PA, Paparella MM, Hybertson R, et al. Bacterial tympanogenic labyrinthitis, meningitis and sensorineural damage. Arch Otolaryngol Head Neck Surg. 1992;118:53–57. doi: 10.1001/archotol.1992.01880010057016. [DOI] [PubMed] [Google Scholar]
- 3.MacArthur CJ, Hefeneider SH, Kempton JB, et al. C3H/HeJ Mouse Model for spontaneous chronic otitis media. Laryngoscope. 2006;116:1071–1079. doi: 10.1097/01.mlg.0000224527.41288.c4. [DOI] [PubMed] [Google Scholar]
- 4.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57/10ScCr mice: Mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 5.MacArthur CJ, Pillars D, DeGagne J, et al. Gram-negative Pathogen Klebsiella oxytoca is Associated with Spontaneous Chronic Otitis Media in Toll-Like Receptor 4 Deficient C3H/HeJ Mice. Acta Otolaryngol. 2007 doi: 10.1080/00016480701387124. in press. [DOI] [PubMed] [Google Scholar]
- 6.Trune DR, Kempton JB, Gross ND. Mineralocorticoid receptor mediates glucocorticoid treatment effects in the autoimmune mouse ear. Hear Res. 2006;212:22–32. doi: 10.1016/j.heares.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Choi JY, Son EJ, Kim JL, et al. ENaC- and CFTR-dependent ion and fluid transport in human middle ear epithelial cells. Hear Res. 2006;211:26–32. doi: 10.1016/j.heares.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Li J-P, Kania R, Lecain E, et al. In vivo demonstration of the absorptive function of the middle ear epithelium. Hear Res. 2005;210:1–8. doi: 10.1016/j.heares.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell CR, Kempton JB, Creedon TA, et al. Using a 56-stimulus train for the rapid acquisition of auditory brainstem responses. Audiol Neurootol. 1999;4:80–87. doi: 10.1159/000013824. [DOI] [PubMed] [Google Scholar]
- 10.Herman P, Tan CT, van den Abbeele T, et al. Glucocorticosteroids increase sodium transport in middle ear epithelium. Am J Physiol. 1997;272:C184–90. doi: 10.1152/ajpcell.1997.272.1.C184. [DOI] [PubMed] [Google Scholar]
- 11.Portier F, Kania R, Planes C, et al. Enhanced sodium absorption in middle ear epithelial cells cultured at air-liquid interface. Acta Otolaryngol. 2005;125:16–22. doi: 10.1080/00016480410015749. [DOI] [PubMed] [Google Scholar]
- 12.Mattsson C, Stierna P, Hellstrom S. Treatment with dexamethasone arrests the development of myringosclerosis after myringotomy. Am J Otol. 2000;21:804–808. [PubMed] [Google Scholar]
- 13.Rosenfeld RM, Mandel EM, Bluestone CD. Systemic steroids for otitis media with effusion in children. Arch Otolaryngol Head Neck Surg. 1991;17:984–989. doi: 10.1001/archotol.1991.01870210056008. [DOI] [PubMed] [Google Scholar]
- 14.Butler CC, van der Voort JH. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2002;4:CD001935. doi: 10.1002/14651858.CD001935. [DOI] [PubMed] [Google Scholar]
- 15.Mandel EM, Casselbrant ML, Rockette HE, et al. Systemic steroid for chronic otitis media with effusion in children. Pediatrics. 2002;110(6):1071–1080. doi: 10.1542/peds.110.6.1071. [DOI] [PubMed] [Google Scholar]
- 16.Ruohola A, Heikkinen T, Jero J, et al. Oral prednisolone is an effective adjuvant therapy for acute otitis media with discharge through tympanostomy tubes. J Pediatr. 1999;134:459–463. doi: 10.1016/s0022-3476(99)70204-0. [DOI] [PubMed] [Google Scholar]
- 17.Stool SE, Berg AO, Berman S, et al. Clinical Practice Guideline, Number 12. Agency for Health Care Policy and research, Public Health Service, US Department of Health and Human Services; Rockville, MD: 1994. Otitis media with effusion in young children. AHCPR Publication No. 94-0622. [Google Scholar]
- 18.Alper CM, Dohar JE, Gulhan M, et al. Treatment of chronic suppurative otitis media with topical tobramycin and dexamethasone. Arch Otolaryngol Head Neck Surg. 2000;126:165–173. doi: 10.1001/archotol.126.2.165. [DOI] [PubMed] [Google Scholar]
- 19.Drobbin MT, Phelan ST, Antonelli PJ. Dexamethasone does not alter in vitro antibacterial efficacy of gentamicin. Otolaryngol Head Neck Surg. 2007;136:769–772. doi: 10.1016/j.otohns.2006.11.015. [DOI] [PubMed] [Google Scholar]